Introduction
Cervical cancer is the fourth most common cancer in
women following breast, lung and colorectal cancer with an
incidence rate of 6% (1), and it is
one of the leading causes of cancer-related mortality in women with
a mortality rate of 3% in the USA (2). Significant progress has been achieved in
cervical cancer treatment with the emergence of novel therapeutic
drugs and treatment approaches (3).
However, for patients in the advanced stages of disease, tumor
relapse and resistance to radiation and available therapeutic
approaches is inevitable. Therefore, investigation of the
mechanisms of tumor relapse and drug resistance is a matter of
great urgency.
In recent years, the cancer stem cell (CSC) theory
has become widely accepted; it was found that CSCs, which represent
a small fraction of tumor cells in the bulk of the tumor, are
characterized by self-renewal, infinite proliferation and multiple
differentiation potentials, and may be the root cause of tumor
relapse and drug resistance (4,5). At
present, the sorting methods of CSCs include flow cytometry based
on special cell surface markers, the side population cell sorting
technique, tumor sphere culturing and the ALDEFLUOR™ assay
(6–9).
Previous studies have identified the CSCs from colon, breast and
glioblastoma cancer, as well as other cancer cell lines, using the
sphere culturing method (10–12). Tumor cell sphere culturing may be a
convenient method for generating cancer stem-like cells to be used
in the research of malignant behavior.
The present study attempted to identify a population
with CSC properties from the CaSki cell line and performed a
preliminary evaluation of the role of CSCs in tumor relapse and
drug resistance.
Materials and methods
Cell lines and cell culture
The human cervical carcinoma epithelioid CaSki cell
line was maintained in the State Key Laboratory of Molecular
Oncology, Cancer Institute/Hospital, Peking Union Medical College
and Chinese Academy of Medical Sciences (Beijing, China), and were
purchased from Cell Resource Center, Institute of Basic Medical
Science, Peking Union Medical College, Chinese Academy of Medical
Sciences (Beijing, China). The cells were routinely grown in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum, penicillin (100 U/ml) and streptomycin (100
mg/ml) at 37°C in a 5% CO2 standard incubator.
Antibodies
The following antibodies were used: Anti-SRY-box 2
(Sox2) rabbit anti-mouse polyclonal antibody (1:1,000 dilution;
catalogue no. ab59776; Abcam, Cambridge, UK), anti-POU class 5
homeobox 1 (Oct4) rabbit anti-mouse polyclonal antibody (1:1,000
dilution; catalogue no. ab18976; Abcam), anti-β-actin mouse
anti-rabbit monoclonal antibody (1:5,000 dilution; catalogue no.
A5316; Sigma-Aldrich, St. Louis, MO, USA), anti-ATP binding
cassette subfamily G member 2 (ABCG2) rabbit anti-human polyclonal
antibody (1:1,000 dilution; catalogue no. BS3482; Bioworld
Technology Inc., Nanjing, China) and anti-poly (ADP-ribose)
polymerase (PARP) rabbit anti-human polyclonal antibody (1:1,000
dilution; catalogue no. ab6079; Abcam).
Tumor sphere culturing
The CaSki cells were cultured in an incubator at
37°C with 5% CO2, and were digested into single cells
suspension and replanted into 6-well plates (500 cells/well) in
serum-free DMEM-F12 (Gibco; Thermo Fisher Scientific Inc., Waltham,
MA, USA), supplemented with 10 ng/ml basic fibroblast growth factor
(PeproTech Inc., Rocky Hill, NJ, USA), 20 ng/ml epidermal growth
factor (PeproTech Inc.), 25 ng/ml insulin (Novo Nordisk, Bagsværd,
Denmark), 4 µg/ml heparin sodium (Changzhou Qianhong Bio-Pharma
Co., Ltd., Changzhou, China), 20 nM/l progestin (Amresco, Solon,
OH, USA), 30 nM/l sodium selenite (Amresco), 100 µg/ml
apo-Transferrin (Sigma-Aldrich), 4 mg/ml bovine serum albumin
(Sigma-Aldrich), 4 mg/ml glucose (Amresco) and 2 mM/l L-glutamine
(Amresco). After 5–7 days, the tumor spheres were collected by
centrifugation at 157 × g, digested with Accutase (Sigma-Aldrich)
to generate single cells and passaged every 5–7 days when the
spheres reached a diameter of 100 µm.
Western blot assay of stemness
markers
The normal CaSki cells and the CaSki tumor-forming
cells were lysated with radioimmunoprecipitation assay buffer
(catalogue no. P0013C; Beyotime Institute of Biotechnology, Haimen,
China). Protein concentrations were determined using the Bio-Rad
Protein assay (NanoDrop 2000c; Bio-Rad Laboratories Inc., Hercules,
CA, USA) and 40 µg protein was loaded per lane. Western blot
analysis was performed according to standard procedures.
Polyvinylidene difluoride (PVDF; EMD Millipore, Billerica, MA, USA)
and 10% sodium dodecyl sulfate polyacrylamide gel (Applygen
Technologies Inc., Beijing, China) membranes were used for protein
electrophoresis and transfer. Rabbit anti-mouse polyclonal Sox2
(catalogue no. ab59776; Abcam) and Oct4 (catalogue no. ab18976)
antibodies were diluted at 1:1,000 and incubated with the membrane
overnight at 4°C. The blotted membranes were visualized using the
ImageQuant™ LAS 4000 mini system (GE Healthcare Life Sciences,
Chalfont, UK). Grey-scale values of the bands were analyzed
quantitatively by Image Quant TL software (GE Healthcare Life
Sciences). The levels of the stemness indicators, Sox2 and Oct4,
the drug resistance marker, ABCG2, and the apoptosis marker, PARP,
were determined.
Telomerase activity assay
The telomerase activity of the CaSki sphere-forming
cells and the normal CaSki cells was measured using the TRAPeze RT
Telomerase Detection kit (catalogue no. S7710; EMD Millipore). The
experimental process was performed in accordance with the
manufacturer's protocols. Reactions were set up in triplicate.
Samples were subjected to the following cycling parameters using an
Applied Biosystems 7300 Real-Time PCR system: 1 cycle of 30 min at
30°C (extension of telomerase substrate) and 1 cycle of 2 min at
95°C, followed by 45 cycles of 15 sec at 94°C, 1 min at 59°C and 30
sec at 45°C (PCR amplification of extended telomerase substrate).
The calculation of the products (amount of DNA amplification
produced by whole protein containing telomerase per mg/min) was
performed according to the formula described previously (13). This experiment was repeated twice.
Cell growth assay
To determine the difference in the drug resistance
between the normal CaSki cells and the CaSki tumor-forming cells,
cells planted in 96-well plates were treated with different
concentrations (0.5, 1, 2 and 5 ng/ml) of cisplatin. The
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay was used to detect the inhibition of cell proliferation
subsequent to 72 h of incubation at 37°C. The optical density
values of each well were measured with a microplate reader (model
no. 680; Bio-Rad Laboratories Inc.) at 490 nm. The half maximal
inhibitory concentration (IC50) was calculated using the
SPSS software version 19 (IBM SPSS, Armonk, NY, USA). This
experiment was repeated twice.
Cell invasion assay
The cell invasion assay was performed using
Transwell chambers (Corning Inc., Corning, NY, USA) coated with 50
µl Matrigel polycarbonate membrane, as described in the
manufacturer's protocols. The CaSki tumor-forming cells and normal
CaSki cells, which grew at the exponential growth phase, were
resuspended in serum-free DMEM and stem cell culturing medium at a
concentration of 5×105 cells/ml. The single-cell
suspension was added into the upper chamber (200 µl/well) and the
lower chambers were loaded with complete medium (500 µl/well).
After 10 h of incubation at 37°C, the cells that did not penetrate
through the polycarbonate membrane were removed with wet cotton
swabs, while the cells that had moved to the other side of the
polycarbonate membrane were stained with 0.2% crystal violet dye
for 30 min. Subsequently, the cell numbers from four randomly
selected fields were counted using a microscope (Nikon SE; Nikon
Corporation, Tokyo, Japan). This experiment was repeated twice.
Statistical analysis
All statistical analyses were performed using SPSS
19.0 statistical software. Statistically significant differences
between groups were determined using an unpaired Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
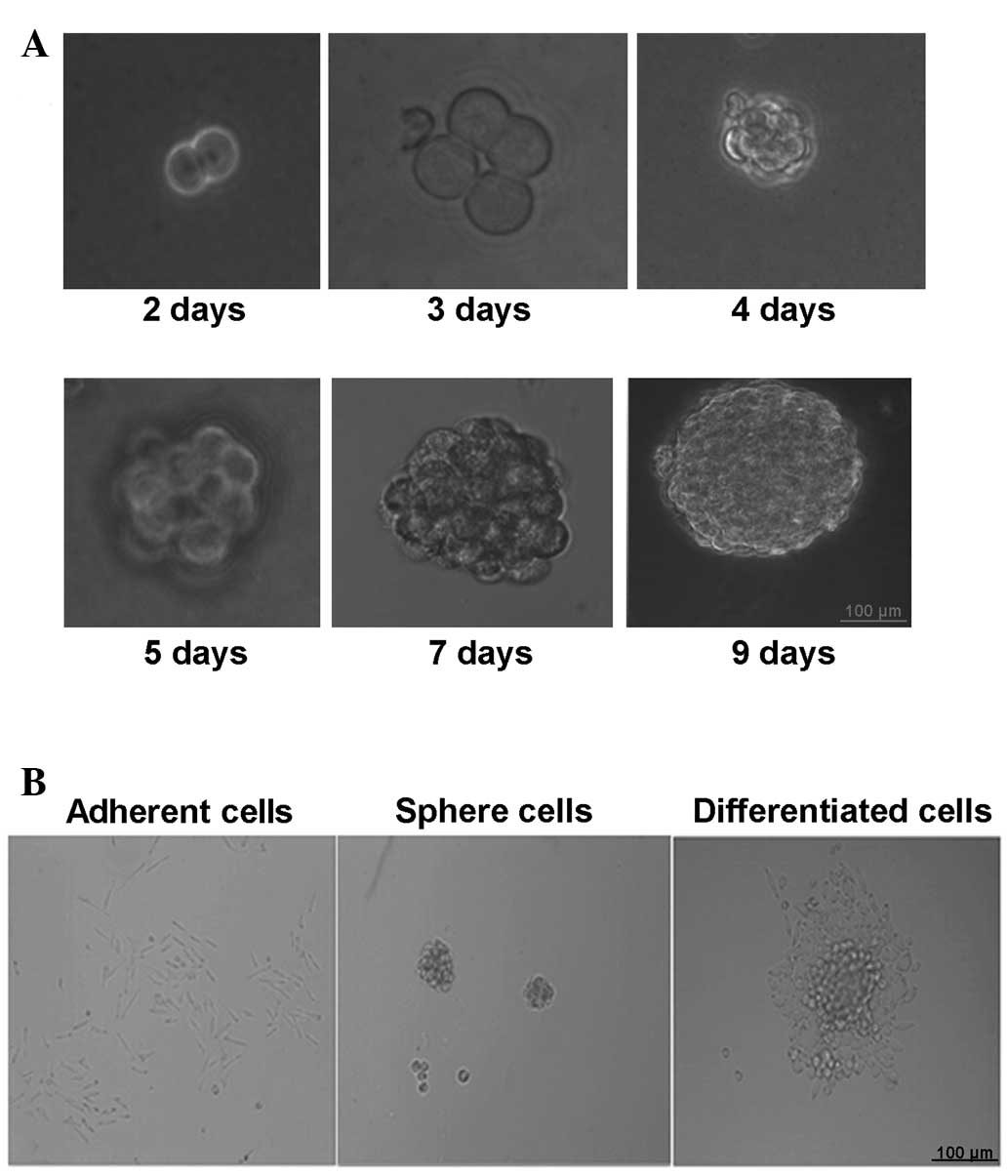
Cell culture of cervical CSCs
Cervical CSCs were selectively expanded using the
stem cell medium. After 24–48 h, a proportion of the cells in stem
cell medium were observed to proliferate and grow into spheres. The
bigger CaSki cell spheres ~100 µm in diameter were produced after
5–7 days of culturing and the rate of sphere formation was
0.15–0.2% (Fig. 1A). The cells in the
spheres grew in a compacted pattern. When re-seeded in medium with
FBS, the CaSki stem cell spheres were adherent to the bottom of the
dish and started to differentiate (Fig.
1B).
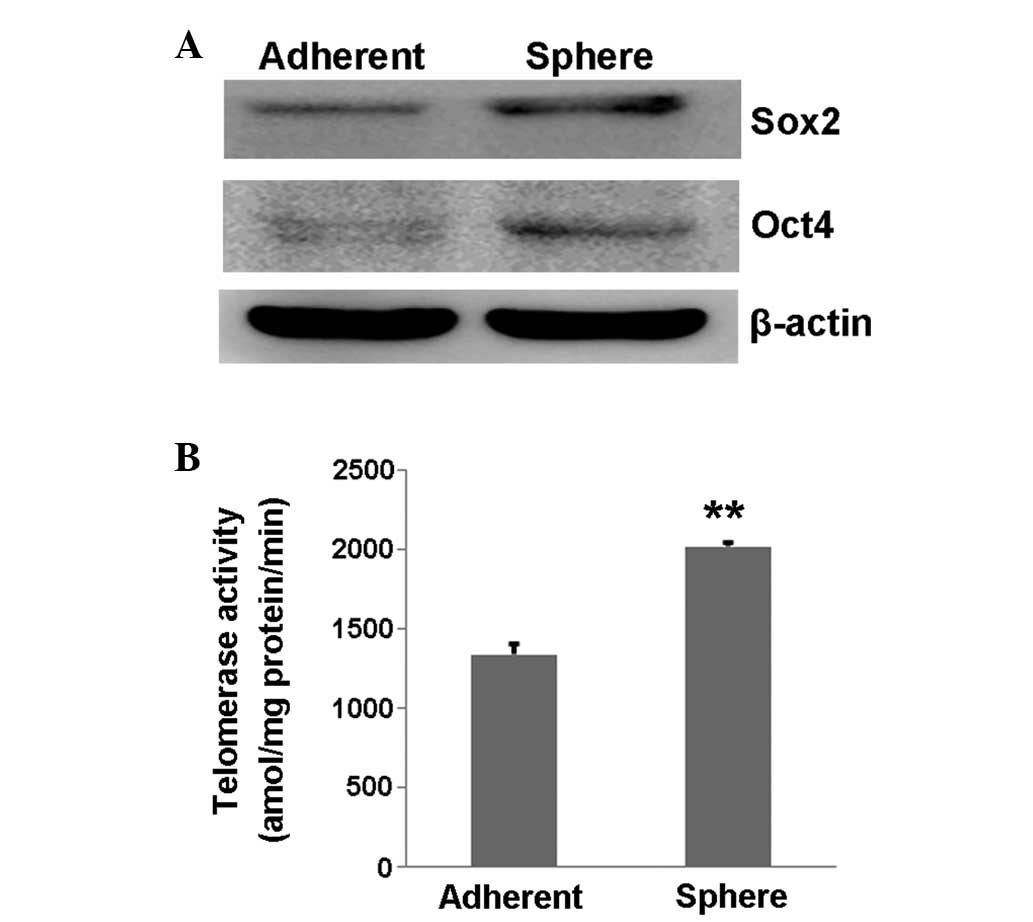
Stemness of cervical cancer cells from
spheres
First, the expression of stemness markers was tested
using western blot and the expression of Oct4 and Sox2 was found to
be increased in the CaSki sphere-forming cells compared with the
normal control CaSki cells (Fig. 2A).
Next, the telomerase activity was measured in a PCR-based assay
that permitted precise quantitation of enzymatic activity with each
prepared cell extract. The result showed that the telomerase
activity in the CaSki sphere-forming cells was significantly higher
than that in the control CaSki cells (P=0.002; Fig. 2B), suggesting that the CaSki
sphere-forming cells had a higher proliferative ability.
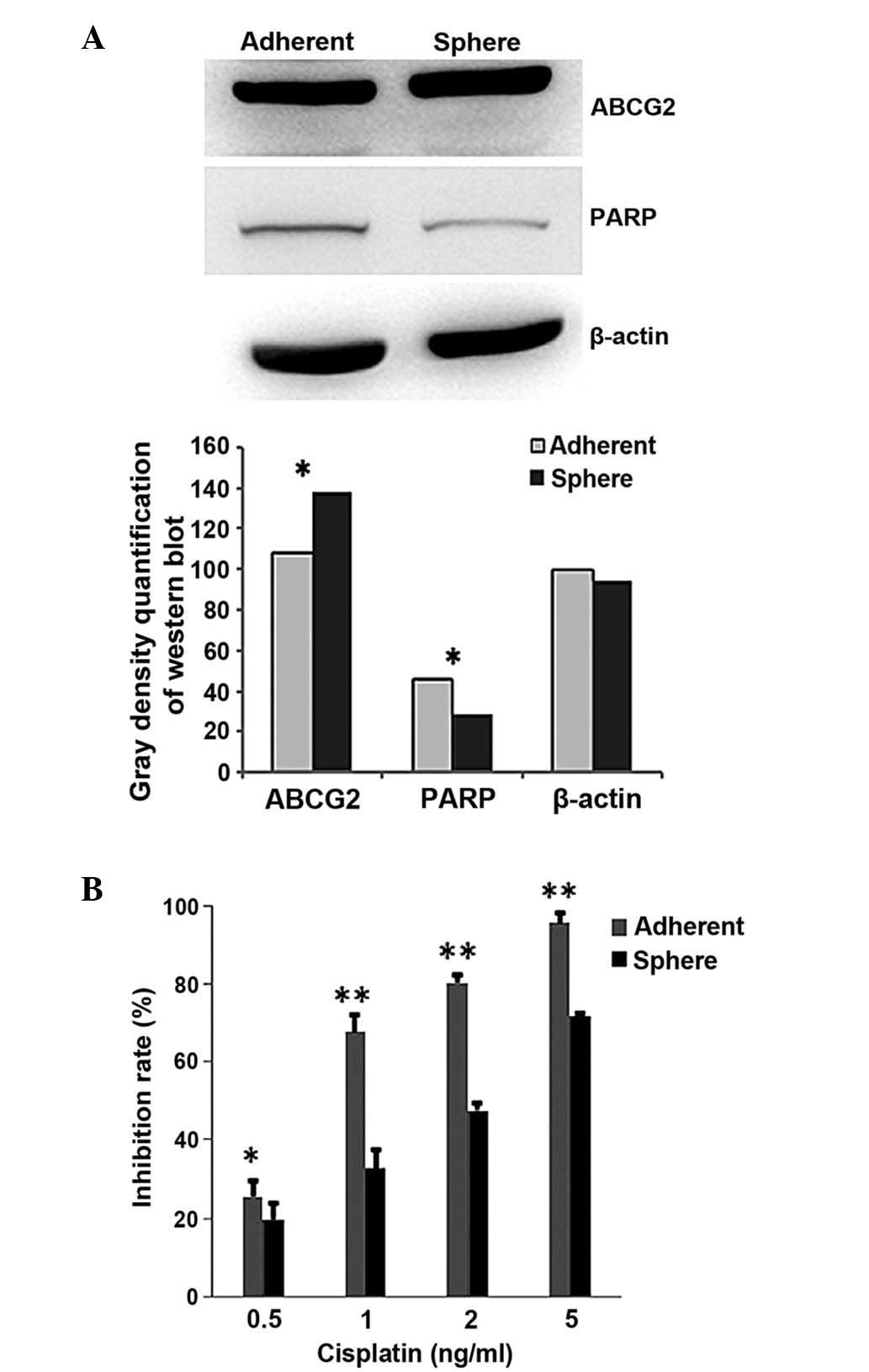
Drug resistance of cervical tumor
sphere-forming cells
To demonstrate whether chemotherapeutics have a less
suppressive effect on cervical CSCs than on cervical cancer cells,
western blot and MTS assays were performed. The western blot
results showed that the protein levels of ABCG2 and PARP were
different between the CaSki sphere-forming cells and the CaSki
cells (Fig. 3A). ABCG2, the drug
transportation-related protein, was expressed at a higher level in
the CaSki sphere-forming cells, while PARP, the apoptosis-related
protein, was expressed at a lower level, when compared with the
CaSki cells (Fig. 3A). The MTS
results showed that cisplatin (0.5, 1, 2 and 5 ng/ml) induced
significantly less proliferation inhibition in the CaSki
sphere-forming cells than in the CaSki cells (P=0.04, 0.002, 0.008
and 0.002, respectively; Fig. 3B).
The IC50 of cisplatin in the CaSki cells was 0.81 µg/ml,
while the IC50 in the CaSki sphere-forming cells was
2.06 µg/ml. This line of evidence indicated that the CaSki
sphere-forming cells are more resistant to chemotherapeutics than
CaSki cells.
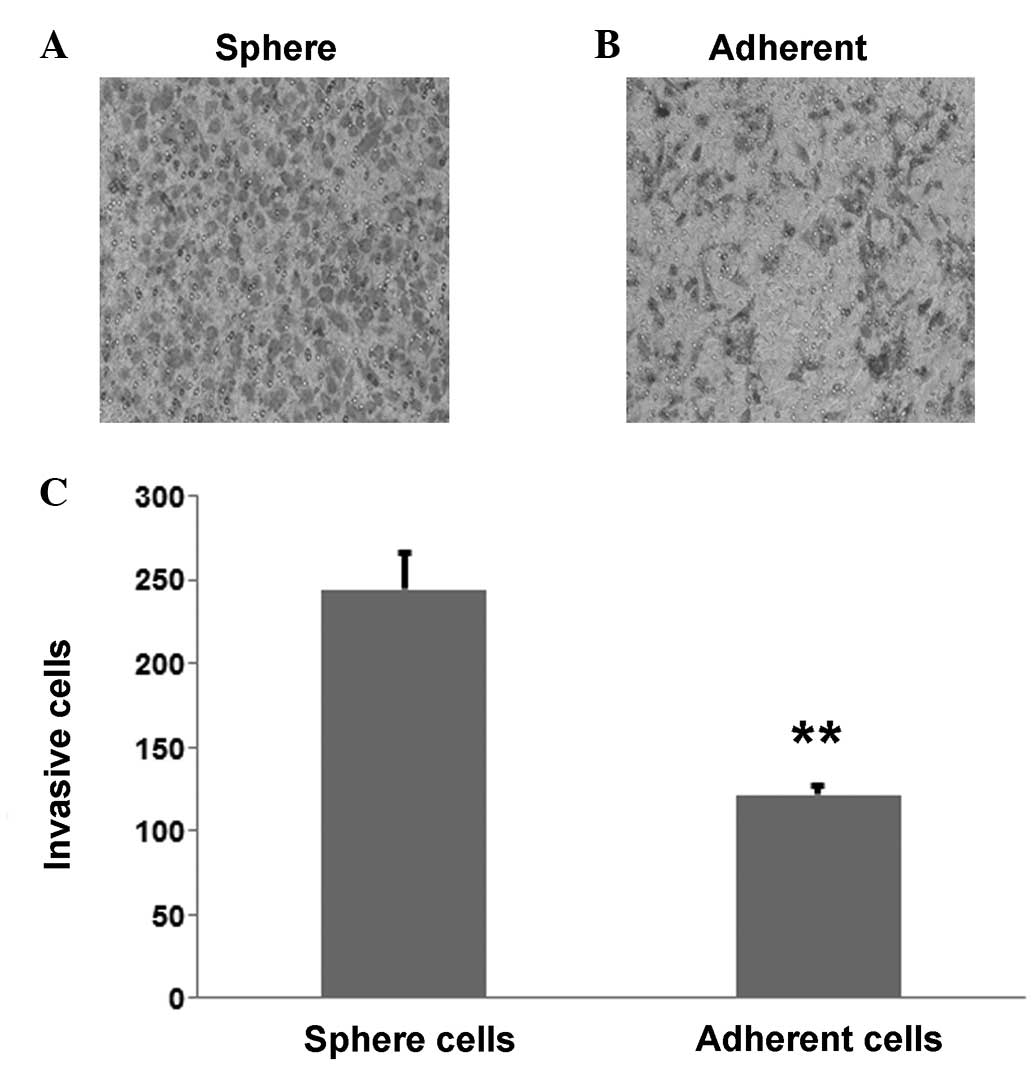
High invasive ability of cervical
cancer cells
To investigate the difference in invasive ability
between the CaSki sphere-forming cells and the CaSki cells, a
Transwell assay was performed; the results showed that the CaSki
sphere-forming cells (Fig. 4A) were
more invasive than the CaSki cells (Fig.
4B). The number of invasive CaSki sphere-forming cells was
244±21.1 compared with 121±5.5 CaSki cells, which was determined to
be statistically significant (P=0.003; Fig. 4C).
Discussion
Cervical cancer is a health threat to women that
requires an urgent solution. Tumor relapse and drug resistance are
the most troubling problems faced by patients and should be further
illustrated mechanically to provide treatment strategies.
The present study performed selective enrichment of
CaSki stem-like cells and conducted a telomerase activity assay,
the results of which suggested that the CaSki sphere-forming cells
not only highly expressed stemness markers, but also exhibited
higher proliferative ability than CaSki cells. The MTS and cell
invasion assays showed that the CaSki sphere-forming cells were
more resistant to chemotherapy drugs (cisplatin) and more invasive
than the CaSki cells.
Increasing evidence has highlighted the existence of
CSCs and their role in tumor relapse and drug resistance (14,15). The
first successful isolation of CSCs from a solid tumor occurred in
2003 (16), demonstrating the
existence of CSCs in cancers. In the present study, CaSki
sphere-forming cells were isolated and shown to express the
stemness markers, Oct4 and Sox2. It was also found that the CaSki
sphere-forming cells exhibited higher telomerase activity than the
CaSki cells. These results indicated the presence of CSCs in
cervical cancer cells and their stronger proliferative ability than
normal cells. Therefore, CSCs in cervical cancer may be the root
cause of tumor recurrence and treatment failure (17).
After years of study on CSCs, it was found that
serum-free medium with cytokines may be beneficial for the
amplification of stem cells in vitro, maintaining the
undifferentiated state and the potential of multi-directional
differentiation. In the present study, it was shown that, following
serum supplementation, the CaSki sphere-forming cells showed signs
of differentiation. Therefore, this method is convenient for
researchers to use to enrich the CSCs in the study of tumor
phenotypes.
The drug resistance of tumor cells is the major
cause of cancer treatment failure. The mechanisms of drug
resistance involve, but are not limited to, the following aspects:
The impairment of the DNA repair ability (18), the enrichment of P-glycoprotein
(19), the glutathione transferase
promotion of the metabolism of anticancer medicine (20) and the existence of CSCs (21). In the present study, the existence of
CSCs in drug resistance was preliminarily evaluated.
Highly-expressed stemness markers and the ability to form tumor
spheres are considered as the hallmarks of CSCs, which are
resistant to anticancer drugs. ABCG2, which is an efflux
transporter on the cell membrane and a stem cell marker, is
considered to confer drug resistance by expelling chemotherapeutic
agents out of the cells (22). PARP,
which is a DNA-repairing enzyme, is considered to be an important
index of cell apoptosis and to play a crucial role DNA damage
repair (23). In the present study,
the expression of these two proteins was determined and the CaSki
sphere-forming cells were shown to express a higher ABCG2 protein
level and a lower PARP protein level than the CaSki cells. Although
cisplatin is an effective anticancer drug in cervical cancer, it is
unable to prevent cancer relapse. We hypothesized that CSCs may
play a crucial role in drug resistance. Using the MTS assay, the
difference in drug-resistance between the CaSki sphere-forming
cells and CaSki cells was markedly illustrated, indicating that the
CaSki sphere-forming cells were more resistant to cisplatin,
demonstrating the stem cell characteristics as a potential
mechanism for drug resistance.
Tumor metastasis, which is a fatal step in the
progression of tumor disease, is a crucial sign for a poor
prognosis. It has previously been suggested that CSCs may be at the
center of this step (24). In the
present study, in order to demonstrate the invasive ability of
CaSki sphere-forming cells and CaSki cells, a Transwell invasion
assay was performed, which found that the CaSki sphere-forming
cells possessed higher invasive ability than the CaSki cells. This
indicated that CSCs have a greater chance to metastasize than
non-CSCs.
In conclusion, the present study demonstrated the
presence of CSCs in cervical cancer that could be enriched by
sphere-forming culturing. Under optimal conditions, the CaSki tumor
spheres showed self-renewal, drug resistance and a strong invasion
potential, which may lead to the recurrence of cervical cancer.
This in vitro study described a suitable model for cervical
cancer research on the mechanism of tumor relapse and
metastasis.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81071773, 30973447 and
81372158), the Natural Science Foundation of Beijing City (no.
7132071), the New Teacher Foundation for the Doctoral Program of
Higher Education (no. 20101107120011), the Talents Project of
Beijing (no. 2010D003034000043), the Open Issue of State Key
Laboratory of Molecular Oncology (no. SKL-KF-2013-08) and the
Independent Issue of State Key Laboratory of Molecular Oncology
(no. SKL-2013-12).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leisching GR, Loos B, Botha MH and
Engelbrecht AM: The role of mTOR during cisplatin treatment in an
in vitro and ex vivo model of cervical cancer. Toxicology.
335:72–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan HC, Fang LS, Xu J, Qiu YY, Lin XM,
Huang HX and Han QY: The identification of the biological
characteristics of human ovarian cancer stem cells. Eur Rev Med
Pharmacol Sci. 18:3497–3503. 2014.PubMed/NCBI
|
|
7
|
Yang CH, Wang HL, Lin YS, Kumar KP, Lin
HC, Chang CJ, Lu CC, Huang TT, Martel J, Ojcius DM, et al:
Identification of CD24 as a cancer stem cell marker in human
nasopharyngeal carcinoma. PLoS One. 9:e994122014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen YK, Huang AH and Lin LM:
Sphere-forming-like cells (squamospheres) with cancer stem-like
cell traits from VX2 rabbit buccal squamous cell carcinoma. Int J
Oral Sci. 6:212–738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ueda K, Ogasawara S, Akiba J, Nakayama M,
Todoroki K, Ueda K, Sanada S, Suekane S, Noguchi M and Matsuoka K:
Aldehyde dehydrogenase 1 identifies cells with cancer stem
cell-like properties in a human renal cell carcinoma cell line.
PLoS One. 8:e754632013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Batsaikhan BE, Yoshikawa K, Kurita N,
Iwata T, Takasu C, Kashihara H and Shimada M: Cyclopamine decreased
the expression of sonic hedgehog and its downstream genes in colon
cancer stem cells. Anticancer Res. 34:6339–6344. 2014.PubMed/NCBI
|
|
11
|
Peleg R, Romzova M, Kogan-Zviagin I, Apte
RN and Priel E: Modification of topoisomerases in mammospheres
derived from breast cancer cell line: Clinical implications for
combined treatments with tyrosine kinase inhibitors. BMC Cancer.
14:9102014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sassi Fde A, Caesar L, Jaeger M, Nör C,
Abujamra AL, Schwartsmann G, de Farias CB, Brunetto AL, Lopez PL
and Roesler R: Inhibitory activities of trichostatin a in U87
glioblastoma cells and tumor sphere-derived cells. J Mol Neurosci.
54:27–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benko AL, Olsen NJ and Kovacs WJ: Estrogen
and telomerase in human peripheral blood mononuclear cells. Mol
Cell Endocrinol. 364:83–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Castillo V, Valenzuela R, Huidobro C,
Contreras HR and Castellon EA: Functional characteristics of cancer
stem cells and their role in drug resistance of prostate cancer.
Int J Oncol. 45:985–994. 2014.PubMed/NCBI
|
|
15
|
Huang Y, Ju B, Tian J, Liu F, Yu H, Xiao
H, Liu X, Liu W, Yao Z and Hao Q: Ovarian cancer stem cell-specific
gene expression profiling and targeted drug prescreening. Oncol
Rep. 31:1235–1248. 2014.PubMed/NCBI
|
|
16
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mannino M, Gomez-Roman N, Hochegger H and
Chalmers AJ: Differential sensitivity of glioma stem cells to
aurora kinase a inhibitors: Implications for stem cell mitosis and
centrosome dynamics. Stem Cell Res. 13:135–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Grady S, Finn SP, Cuffe S, Richard DJ,
O'Byrne KJ and Barr MP: The role of DNA repair pathways in
cisplatin resistant lung cancer. Cancer Treat Rev. 40:1161–1170.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chufan EE, Sim HM and Ambudkar SV:
Molecular basis of the polyspecificity of P-glycoprotein (ABCB1):
Recent biochemical and structural studies. Adv Cancer Res.
125:71–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Backos DS, Franklin CC and Reigan P: The
role of glutathione in brain tumor drug resistance. Biochem
Pharmacol. 83:1005–1012. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JK, Jeon HY and Kim H: The molecular
mechanisms underlying the therapeutic resistance of cancer stem
cells. Arch Pharm Res. 38:389–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zinzi L, Contino M, Cantore M, Capparelli
E, Leopoldo M and Colabufo NA: ABC transporters in CSCs membranes
as a novel target for treating tumor relapse. Front Pharmacol.
5:1632014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wielgos M and Yang ES: Discussion of PARP
inhibitors in cancer therapy. Pharm Pat Anal. 2:755–766. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen T, Yang K, Yu J, Meng W, Yuan D, Bi
F, Liu F, Liu J, Dai B and Chen X: Identification and expansion of
cancer stem cells in tumor tissues and peripheral blood derived
from gastric adenocarcinoma patients. Cell Res. 22:248–258. 2012.
View Article : Google Scholar : PubMed/NCBI
|