Introduction
Cancer is a major public health issue in developed
countries (1). In Japan, the
incidence of colorectal cancer (CRC) has significantly increased in
recent years (2). The most critical
factor causing mortality of patients with CRC is metastasis
(3). Intensive follow-up and adjuvant
therapy is urgently required for patients that undergo curative
resection for CRC, in order to prevent recurrence and metastasis
(4–6).
According to previous studies, CRC metastasis to distant organs,
including the liver and lung, is resectable (7–11), and a
successful resection clearly results in an increased survival time.
Long-term survival has been reported in association with surgical
resection for solitary adrenal metastasis resulting from CRC
(12–22). However, isolated adrenal metastasis is
rare; adrenal lesions usually occur in the presence of multiple
synchronous metastases and are detected in the terminal phase of
cancer (23–25). Therefore, surgical resection is not
generally considered as a treatment for adrenal metastasis.
However, several studies have reported that there is a favorable
prognosis and notable benefit to patients following surgical
resection for adrenal metastasis. To associate the features of
adrenal metastasis and the clinical outcome of patients with
adrenal metastasis resulting from CRC, the present study reports
the cases of 3 CRC patients that experienced a long-term survival
following surgical resection for adrenal metastasis with local and
other metastatic sites, including the liver, lung and distant lymph
nodes.
Case report
Between 1998 and 2002, 3 patients underwent surgery
for adrenal metastasis at the Department of Gastroenterological
Surgery at Osaka University (Suita, Osaka, Japan). All resected
specimens were diagnosed according to the tumor-node-metastasis
(TNM) classification (26,27). Following surgery, the patients were
followed-up using serological examinations, including serum
carcinoembryonic antigen (CEA) and cancer antigen 125, and imaging
modalities, such as abdominal ultrasonography, computed tomography
(CT) and chest X-ray, every 3–6 months. The therapies the patients
received were administered according to the Japanese guidelines for
CRC treatment (7). Table I summarizes the clinical data of the 3
patients, who currently remain within the standard follow-up period
following curative surgery (5 years).
 | Table I.Clinical characteristics of the
patients. |
Table I.
Clinical characteristics of the
patients.
|
|
|
| Primary tumor | Previous
metastasis | Adrenal
metastasis | Following
adrenalectomy |
|---|
|
|
|
|
|
|
|
|
|---|
| Case | Age, years | Gender | Location | History | Stage | Site | Interval | Treatment | SSM | Interval | Side | Size, mm | CEA, ng/ml | Recurrence | Interval | Outcome |
|---|
| 1 | 63 | M | Rectum | Well | T3N1 IIIa | Local | 22 | RT | None | 44 | Left | 35 | 16.0 | None | NA | Alive at 114 m |
| 2 | 62 | M | Rectum | Well | T3N0 II | Local+lung | 5 | CT+RT | Lunga | 25 | Left | 45 |
4.2 | None | NA | Alive at 103 m |
| 3 | 57 | F | Sigmoid | Mod | T3N0 II | Liver | 11 | CT+resection | PAN | 58 | Right | 15 |
7.0 | Local+liver | 78, 125 | Alive at 86 m |
Case 1
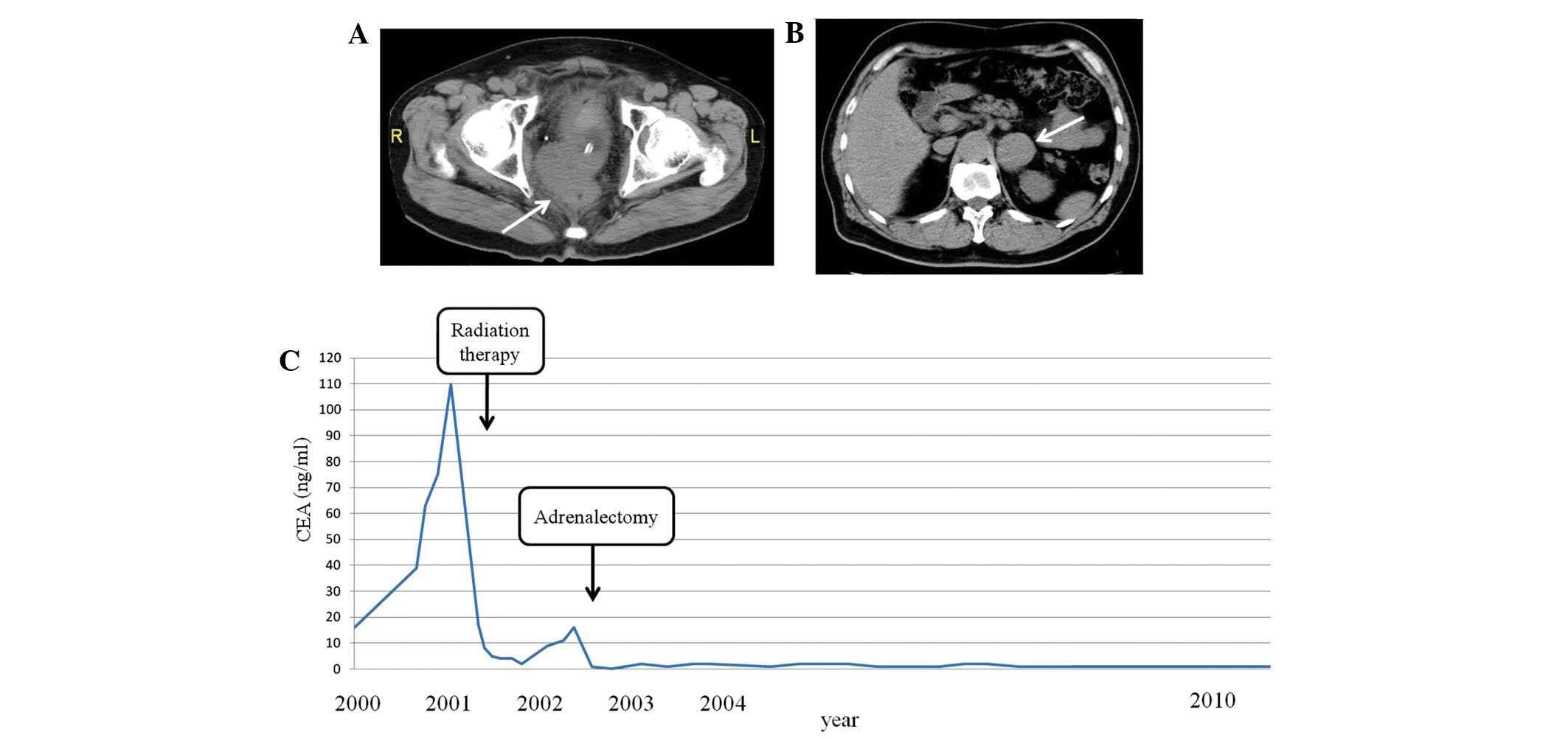
In May 2000, a 63-year-old man presented with
locally recurrent rectal cancer subsequent to an anterior
peritoneal resection conducted in July 1998. According to the TNM
classification (26,27), the pathological staging of the tumor
in was stage IIIa [well-differentiated adenocarcinoma; pT3pN1M0;
residual tumor (R) 0]. In May 2000, the CEA level of the patient
had increased to 16 ng/ml (normal range, 0.0–4.0 ng/ml). An
abdominal CT scan (Discovery CT750 HD; GE Healthcare, Piscataway,
NJ, USA) identified the presence of local recurrence, which was
posterior to the bladder. The patient refused treatment. The
patient presented to hospital 1 year later with symptoms of anal
pain and hematuria. The CEA level of the patient had increased to
110 ng/ml and the previously observed local recurrence had
increased in size to a 60-mm diameter from an original size of
20-mm diameter (Fig. 1A). Beginning
in July 2001, 40 Gy of external radiation in 20 fractions and 30 Gy
of interstitial radiation therapy in 5 fractions was administered
to the recurrent tumor. The CEA level of the patient (8 ng/ml) had
decreased by October 2001. However, in August 2002, the CEA level
of the patient increased to 11 ng/ml and an abdominal CT scan
revealed left adrenal metastasis (Fig.
1B). The patient underwent laparoscopic left adrenalectomy. A
pathological examination of the tumor demonstrated a
well-differentiated adenocarcinoma consistent with primary CRC. The
patient was followed up without adjuvant chemotherapy following
adrenalectomy, and was alive and well at the last follow-up in
April 2012, with no evidence of recurrence observed using positron
emission tomography (PET)-CT (HEADTOME/set. 2400W; Shimadzu Co.,
Kyoto, Japan) and serum CEA, 9.5 years subsequent to the resection
of the adrenal metastasis (Fig.
1C).
Case 2
A 62-year-old man underwent low anterior resection
for rectal cancer in July 2000. According to the TNM classification
(26,27), the post-operative staging of the tumor
was stage II (well-differentiated adenocarcinoma; pT3pN0M0; R0).
Subsequent to 5 months, abdominal and chest CT (Discovery CT750 HD;
GE Healthcare) revealed the presence of a 30-mm mass near the
anastomotic region in the abdominal cavity and a 10-mm mass in the
lung, consistent with pulmonary metastasis. A total of 4 cycles of
chemotherapy [750 mg 5-fluorouracil (5-FU) with 400 mg leucovorin
(LV)] were administered and the pulmonary metastasis decreased in
size. However, the local recurrence increased to 50 mm in diameter
(Fig. 2A). Therefore, 50 Gy of
external radiation in 25 fractions was initiated, which resulted in
no alteration in the local recurrence. Additional chemotherapy [400
mg tegaful/uracil (UFT) with 180 mg camptothecin-11 (CPT-11)] was
administered for 5 cycles; subsequently, the local recurrence
appeared as scar tissue on CT scans and the serum CEA level of the
patient gradually decreased from 19.1 to 1.3 ng/ml, which was
within the normal range (≤3.0 ng/ml). Abdominal and chest CT were
performed 10 months later and a solitary pulmonary metastasis 18 mm
in size was revealed in the right lower lobe and the presence of
left adrenal metastasis (Fig. 2B and
C). Left adrenalectomy and partial right lower lobe
pneumonectomy were performed in September 2003. A pathological
examination confirmed that the adrenal gland and pulmonary nodules
were well-differentiated adenocarcinoma consistent with metastatic
CRC. A total of 3 cycles of 5-FU with LV were administered as
adjuvant chemotherapy following the second surgery. At the last
follow-up in May 2012, the patient was alive and without evidence
of metastasis observed using PET-CT (HEADTOME/set. 2400W; Shimadzu
Co.) and with a normal CEA level for almost 8.6 years following the
second surgical resection of adrenal and pulmonary metastases
(Fig. 2D).
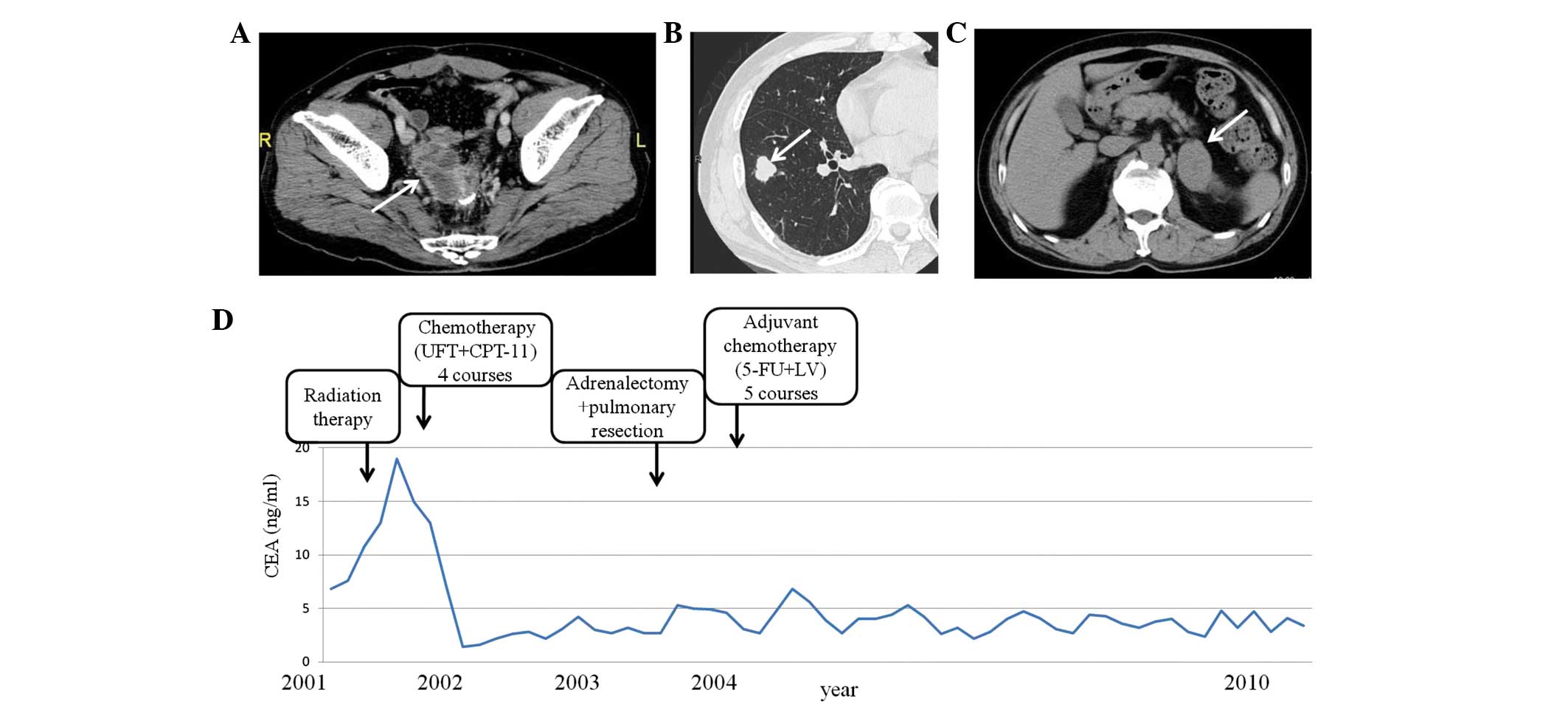 | Figure 2.Patient 2. (A) Abdominal CT of local
recurrence near the anastomotic lesion, indicated by an arrow. (B)
Chest CT of the right lung nodule, which was 18 mm in diameter,
indicated by an arrow. (C) Abdominal CT of an enlarged left adrenal
gland, indicated by an arrow. (D) Alterations in the serum CEA
levels of the patient. CT, computed tomography; CEA, serum
carcinoembryonic antigen; UFT, tegaful/uracil; CPT-11,
camptothecin-11; F-FU, 5-fluorouracil; LV, leucovorin. |
Case 3
A 57-year-old woman underwent a sigmoidectomy in
January 2002 for CRC. According to the TNM classification (26,27), the
pathological staging of the tumor was II (moderately-differentiated
adenocarcinoma; pT3pN0M0; R0). Eleven months after sigmoidectomy,
abdominal CT (Discovery CT750 HD; GE Healthcare) revealed liver
metastasis [segment (S) 2 and S7], and 4 cycles of chemotherapy
(400 mg UFT with 240 mg CPT-11) were administered to the patient
for 5 months. After 4 cycles of chemotherapy, the liver metastasis
decreased in size and new lesions were not detected. A partial
liver resection (S2 and S7) was performed in July 2003, and 3
additional cycles of chemotherapy (400 mg UFT with 180 mg CPT-11)
were administered. Forty months subsequent to the second surgery,
PET-CT (HEADTOME/set. 2400W; Shimadzu Co.) revealed the presence of
adrenal metastasis and para-aortic lymph node recurrences (Fig. 3A-D). In total, 4 cycles of the
modified folinic acid, 5-FU and oxaliplatin (mFOLFOX6) regimen
(5-FU with LV and oxaliplatin) were administered to the patient as
pre-operative chemotherapy. In each course, there were no cycles,
but simply one rapid infusion and one continuous infusion for 46 h.
The adrenal metastasis and lymph node recurrence decreased in size,
and a right adrenalectomy and para-aortic lymph node dissection was
performed in July 2007. A pathological examination of the adrenal
gland confirmed the diagnosis of moderately-differentiated
adenocarcinoma consistent with metastatic CRC. Metastasis was
detected in 1 out of 13 dissected para-aortic lymph nodes. mFOLFOX6
was administered as post-operative chemotherapy for 4 cycles
following the third surgery. Subsequently, the CEA level of the
patient was elevated to 8 ng/ml and PET-CT revealed a local
recurrence in the right adrenal gland. Therefore, mFOLFOX6 with
bevacizumab was administered to the patient for 23 cycles and
discontinued following the development of adverse reactions,
including peripheral neuropathy (grade 2), general fatigue and
nausea. Following mFOLFOX and bevacizumab treatment for the local
adrenal recurrence, the patient was well and no evidence of an
additional recurrence was observed using PET-CT (Fig. 3E). However, 79 months subsequent to
the initial resection of adrenal metastasis, the patient underwent
a curative resection for recurrence of liver metastases and
recurrence in the right adrenal gland. At the last follow-up in
February 2015, the patient was alive and no evidence of metastasis
was observed using PET-CT and the patient had a normal CEA level
for 12 months following the second surgical resection of adrenal
and liver metastases.
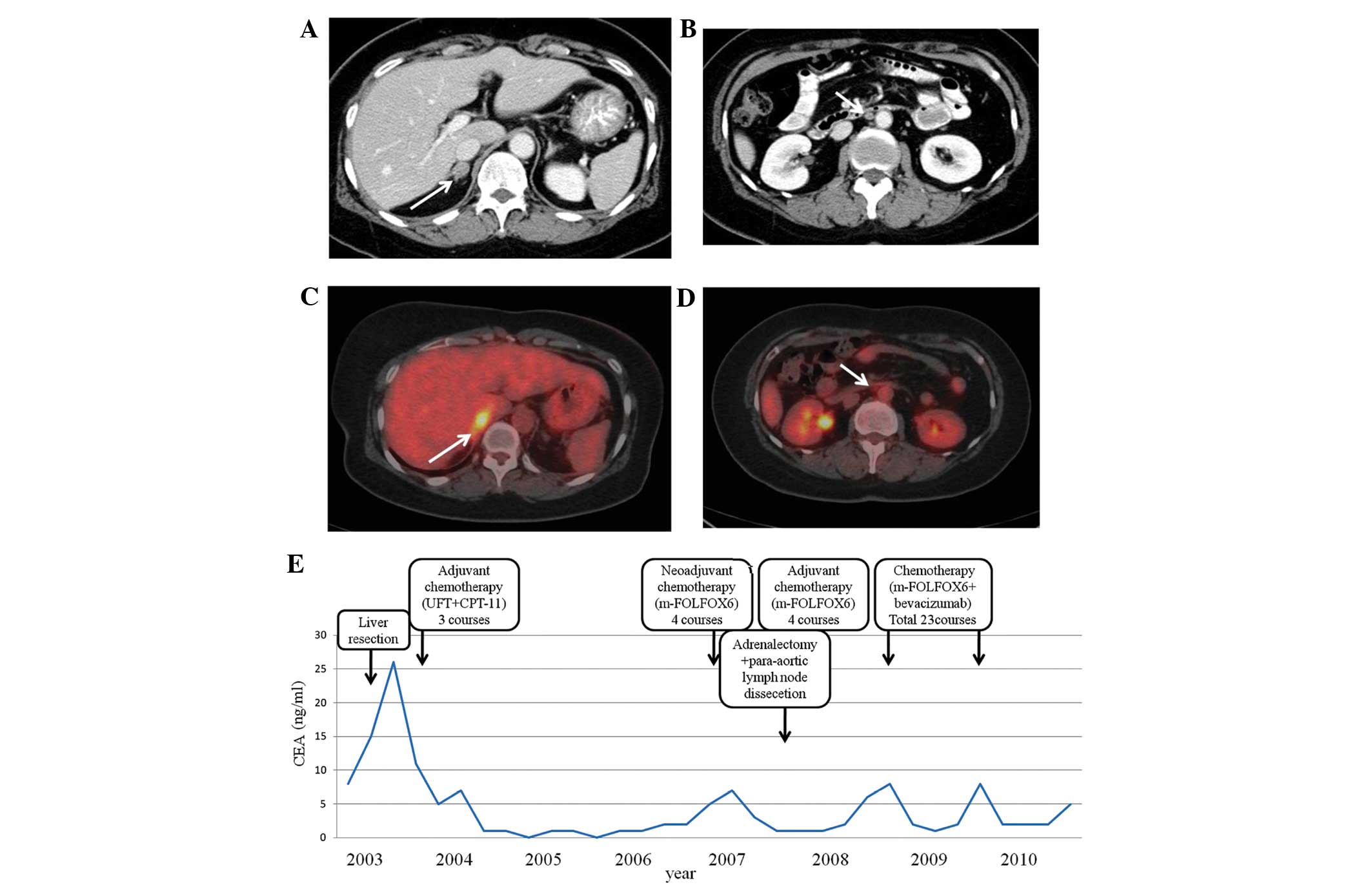 | Figure 3.Patient 3. (A) Abdominal CT of an
enlarged right adrenal gland, indicated by an arrow. (B) Abdominal
CT of para-aortic lymph node swelling, indicated by an arrow. (C)
Abdominal PET-CT with FDG uptake of right adrenal gland, indicated
by an arrow. (D) Abdominal PET-CT of the para-aortic lymph node
with FDG uptake, indicated by an arrow. (E) Alterations in the
serum CEA levels of the patient. CT, computed tomography; PET,
positron emission tomography; FDG,
[18F]-2-fluoro-2-deoxy-D-glucose; CEA, serum carcinoembryonic
antigen; UFT, tegaful/uracil; CPT-11, camptothecin-11; mFOLFOX6,
modified FOLFOX6 regimen (5-fluorouracil with leucovorin and
oxaliplatin). |
Discussion
According to the results from several studies,
metastasis to the adrenal glands is a relatively frequent
observation at autopsy in cancer patients (23–25). The
most common neoplasms to metastasize to the adrenal glands are lung
cancer, breast cancer and renal cell carcinoma (23–25). The
incidence of adrenal metastasis observed at autopsy is 8.6–27.0% of
all malignancies (23–25). The range of the incidence of adrenal
metastasis resulting from CRC is 4.2–14.4% (23–25).
Cedermark et al reviewed the autopsy records of 457 patients
that succumbed to CRC and observed that the frequency of metastasis
to the liver and lung was 48 and 38%, respectively, whereas
metastasis to the adrenal gland was 14% (25).
It is widely accepted that the serum CEA level is
important in patients with CRC, as it may affect tumor diagnostic
procedures (28). In addition, the
serum CEA level is reportedly useful for indicating the presence of
adrenal metastasis following surgical resection for CRC (13,19,20). In
the present study, the post-operative recurrence was predicted from
the alterations in the serum CEA level, and recurrence of the
tumors was suspected when the CEA level became elevated. In
patients 1 and 2, the serum CEA level was within normal limits for
>5 years following the first surgery, and in patient 3,
recurrence was suspected following the third surgical resections
when the CEA level increased. Therefore, a serial post-operative
determination of the serum CEA level is a useful and effective
guide for determining the status of patients with recurrent
CRC.
All patients in the present study were asymptomatic,
and did not present with abdominal pain or adrenal insufficiency,
which has been reported in a previous study (22). It has been estimated that 80–90% of
the adrenal gland must be replaced or destroyed by tumor cells
prior to adrenal insufficiency being detected; therefore, symptoms
rarely appear as an initial sign of adrenal metastasis resulting
from CRC (22). The rate of detecting
clinically silent adrenal masses has increased due to the
widespread use of abdominal imaging modalities, including
ultrasonography, CT, magnetic resonance imaging (MRI) and PET
(29,30). Adrenal incidentaloma is defined as an
adrenal mass detected during abdominal imaging performed for
reasons not associated with the adrenal glands (31,32).
Certain adrenal incidentalomas are metastatic adrenal tumors from a
different primary cancer, usually the lung or kidney. Among
patients undergoing adrenalectomy for metastatic cancer, 13–17% of
patients possessed metastasis resulting from CRC (22,23).
Candel et al conducted a study on fine-needle aspiration
biopsies of adrenal masses in various malignancies, which revealed
that 5 out of 39 cases (12.8%) were derived from CRC (33). Frilling et al concluded that
MRI and PET provided an accurate diagnosis in patients with
indeterminate adrenal tumors (29).
Even though the incidence of adrenal metastasis varies (33,34), it is
possible that adrenal metastasis from CRC is not unusual. Several
studies identified that the majority of autopsy cases, where
metastasis to the adrenal glands was observed, possessed several
other metastases (23–25).
By contrast, a solitary adrenal metastasis is
extremely unusual. When a solitary adrenal metastasis is observed,
surgical resection of the involved adrenal glands is associated
with a good prognosis for patients (1,2,4–7,12–16,35–37).
Muth et al reported that the factors associated with a
longer survival time in patients with CRC, renal cell carcinoma,
non-small-cell lung cancer and malignant melanoma were the tumor
type, of which CRC demonstrated the best prognosis, no prior
surgery or metastases, a long disease-free interval and potentially
curative adrenalectomy at the time of surgery (32). Therefore, it is important to consider
the possibility of adrenal metastasis resulting from CRC during
follow-up subsequent to a primary surgery, since the early
detection of a solitary adrenal metastasis may result in a second
curative surgery and improve the long-term survival time of
patients. Although adrenal metastasis is usually observed in
combination with widespread metastasis (23–25,38,39),
it is considered feasible to resect a solitary adrenal metastasis
in patients with CRC, which leads to an improved prognosis
(12–22).
Recently, metastasis to the adrenal glands has been
more frequently recognized during follow-up subsequent to surgery
for cancer, due to an improvement in high-resolution imaging
modalities (22). However, metastasis
to the adrenal glands usually occurs in combination with multiple
synchronous metastases at other sites (13–26,38,39).
In the present study, 2 out of 3 patients that possessed multiple
metastases, underwent adrenalectomy for metastatic CRC and were
alive without chemotherapy 8 years and 6 years subsequent to the
last surgery, respectively. The third patient that underwent
adrenalectomy was alive and undergoing mFOLFOX6 with bevacizumab
treatment almost 3 years subsequent to the last surgery. The
present results suggest that aggressive surgical resection for
adrenal metastasis resulting from CRC may lead to a survival
benefit in patients for whom curative treatment is performed at
other metastatic sites. Surgical resection for metastasis to
distant organs, including the liver and lung, from CRC is
recommended, due to the numerous studies regarding these metastases
(7–11). Similarly, adrenal metastasis resulting
from CRC should be aggressively resected if distant metastasis may
also be curatively treated.
In conclusion, aggressive surgical resection for
adrenal metastasis resulting from CRC should be performed in
certain patients, for whom curative treatment is performed at other
metastatic sites.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kohno SI, Luo C, Nawa A, Fujimoto Y,
Watanabe D, Goshima F, Tsurumi T and Nishiyama Y: Oncolytic
virotherapy with an HSV amplicon vector expressing
granulocyte-macrophage colony-stimulating factor using the
replication-competent HSV type 1 mutant HF10 as a helper virus.
Cancer Gene Ther. 14:918–926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolpin BM and Mayer RJ: Systemic treatment
of colorectal cancer. Gastroenterology. 134:1296–1310. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kornmann M, Formentini A, Ette C,
Henne-Bruns D, Kron M, Sander S, Baumann W, Kreuser ED, Staib L and
Link KH: Prognostic factors influencing the survival of patients
with colon cancer receiving adjuvant 5-FU treatment. Eur J Surg
Oncol. 34:1316–1321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bathe OF, Dowden S, Sutherland F, Dixon E,
Butts C, Bigam D, Walley B, Ruether D and Ernst S: Phase II study
of neoadjuvant 5-FU + leucovorin + CPT-11 in patients with
resectable liver metastases from colorectal adenocarcinoma. BMC
Cancer. 4:322004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watanabe T1, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese Society for Cancer of the Colon and Rectum: Japanese
Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010
for the treatment of colorectal cancer. Int J Clin Oncol. 17:1–29.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mori M, Tomoda H, Ishida T, Kido A,
Shimono R, Matsushima T, Kuwano H and Sugimachi K: Surgical
resection of pulmonary metastases from colorectal adenocarcinoma.
Special reference to repeated pulmonary resections. Arch Surg.
126:1297–1302. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Regnard JF, Nicolosi M, Coggia M,
Spaggiari L, Fourquier P, Levi JF and Levasseur P: Results of
surgical treatment of lung metastases from colorectal cancers.
Gastroenterol Clin Biol. 19:378–384. 1995.(In French). PubMed/NCBI
|
|
10
|
van Ginkel RJ, de Jong KP, Peeters PM, de
Vries EG and Slooff MJ: Good results with liver resection for
colorectal liver metastases. Ned Tijdschr Geneeskd. 139:1546–1550.
1995.(In Dutch). PubMed/NCBI
|
|
11
|
Ambiru S, Miyazaki M, Ito H, Nakagawa K,
Shimizu H, Kato A, Nakamura S, Omoto H and Nakajima N: Resection of
hepatic and pulmonary metastases in patients with colorectal
carcinoma. Cancer. 82:274–278. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanjo T, Albertini M and Weber S:
Long-term disease-free survival after adrenalectomy for isolated
colorectal metastases. Asian J Surg. 29:291–293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katayama A, Mafune K and Makuuchi M:
Adrenalectomy for solitary adrenal metastasis from colorectal
carcinoma. Jpn J Clin Oncol. 30:414–416. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagakura S, Shirai Y, Nomura T and
Hatakeyama K: Long-term survival after resection of colonic
adenocarcinoma with synchronous metastases to the liver, adrenal
gland, and aortic-caval lymph nodes: Report of a case. Dis Colon
Rectum. 45:1679–1680. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mourra N, Hoeffel C, Duvillard P, Guettier
C, Flejou JF and Tiret E: Adrenalectomy for clinically isolated
metastasis from colorectal carcinoma: Report of eight cases. Dis
Colon Rectum. 51:1846–1849. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kosmidis C, Efthimiadis C, Anthimidis G,
Levva S, Ioannidou G, Zaramboukas T, Emmanouilides C, Baka S,
Kosmidou M, Basdanis G and Fachantidis E: Adrenalectomy for
solitary adrenal metastasis from colorectal cancer: A case report.
Cases J. 1:492008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shoji Y, Dohke M, Masuda T, Nakamura F,
Yano T, Niizeki H, Kashimura N and Matsunami O: Solitary adrenal
metastasis in a patient with sigmoid colon cancer; report of a
case. Int J Gastrointest Cancer. 37:120–123. 2006.PubMed/NCBI
|
|
18
|
Murakami S, Terakado M, Hashimoto T, Tsuji
Y, Okubo K and Hirayama R: Adrenal metastasis from rectal cancer:
Report of a case. Surg Today. 33:126–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watatani M, Ooshima M, Wada T, Terashita
H, Matsuda T, Shindo K and Yasutomi M: Adrenal metastasis from
carcinoma of the colon and rectum: A report of three cases. Surg
Today. 23:444–448. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujita K, Kameyama S and Kawamura M:
Surgically removed adrenal metastasis from cancer of the rectum.
Report of a case. Dis Colon Rectum. 31:141–143. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim SH, Brennan MF, Russo P, Burt ME and
Coit DG: The role of surgery in the treatment of clinically
isolated adrenal metastasis. Cancer. 82:389–394. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lam KY and Lo CY: Metastatic tumours of
the adrenal glands: A 30-year experience in a teaching hospital.
Clin Endocrinol (Oxf). 56:95–101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abrams HL, Spiro R and Goldstein N:
Metastasis in carcinoma; analysis of 1000 autopsied cases. Cancer.
3:74–85. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bullock WKH and Hirst AE Jr: Metastatic
carcinoma of the adrenal. Am J Med Sci. 226:521–524. 1953.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cedermark BJ, Blumenson LE, Pickren JW,
Holyoke DE and Elias EG: Ths significance of metastases to the
adrenal glands in adenocarcinoma of the colon and rectum. Surg
Gynecol Obstet. 144:537–546. 1977.PubMed/NCBI
|
|
26
|
Eriksen MT, Wibe A, Norstein J, Haffner J
and Wiig JN: Norwegian Rectal Cancer Group: Anastomotic leakage
following routine mesorectal excision for rectal cancer in a
national cohort of patients. Colorectal Dis. 7:51–57. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ueno H, Mochizuki H, Hashiguchi Y,
Ishiguro M, Miyoshi M, Kajiwara Y, Sato T, Shimazaki H and Hase K:
Extramural cancer deposits without nodal structure in colorectal
cancer: Optimal categorization for prognostic staging. Am J Clin
Pathol. 127:287–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan E, Gouvas N, Nicholls RJ, Ziprin P,
Xynos E and Tekkis PP: Diagnostic precision of carcinoembryonic
antigen in the detection of recurrence of colorectal cancer. Surg
Oncol. 18:15–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Frilling A, Tecklenborg K, Weber F, Kühl
H, Müller S, Stamatis G and Broelsch C: Importance of adrenal
incidentaloma in patients with a history of malignancy. Surgery.
136:1289–1296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harrison J, Ali A, Bonomi P and Prinz R:
The role of positron emission tomography in selecting patients with
metastatic cancer for adrenalectomy. Am Surg. 66:432–436;
discussion 436–437. 2000.PubMed/NCBI
|
|
31
|
Wade TP, Longo WE, Virgo KS and Johnson
FE: A comparison of adrenalectomy with other resections for
metastatic cancers. Am J Surg. 175:183–186. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muth A, Persson F, Jansson S, Johanson V,
Ahlman H and Wängberg B: Prognostic factors for survival after
surgery for adrenal metastasis. Eur J Surg Oncol. 36:699–704. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Candel AG, Gattuso P, Reyes CV, Prinz RA
and Castelli MJ: Fine-needle aspiration biopsy of adrenal masses in
patients with extraadrenal malignancy. Surgery. 114:1132–1136;
discussion 1136–1137. 1993.PubMed/NCBI
|
|
34
|
Sirén JE, Haapiainen RK, Huikuri KT and
Sivula AH: Incidentalomas of the adrenal gland: 36 operated
patients and review of literature. World J Surg. 17:634–639. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Strong VE, D'Angelica M, Tang L, Prete F,
Gönen M, Coit D, Touijer KA, Fong Y and Brennan MF: Laparoscopic
adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol.
14:3392–3400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sebag F, Calzolari F, Harding J, Sierra M,
Palazzo FF and Henry JF: Isolated adrenal metastasis: The role of
laparoscopic surgery. World J Surg. 30:888–892. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sarela AI, Murphy I, Coit DG and Conlon
KC: Metastasis to the adrenal gland: The emerging role of
laparoscopic surgery. Ann Surg Oncol. 10:1191–1196. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lo CY, van Heerden JA, Soreide JA, Grant
CS, Thompson GB, Lloyd RV and Harmsen WS: Adrenalectomy for
metastatic disease to the adrenal glands. Br J Surg. 83:528–531.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wade TP, Virgo KS, Li MJ, Callander PW,
Longo WE and Johnson FE: Outcomes after detection of metastatic
carcinoma of the colon and rectum in a national hospital system. J
Am Coll Surg. 182:353–361. 1996.PubMed/NCBI
|