Introduction
Gastric cancer is the fourth most common cancer and
the second most common cause of cancer-related mortalities
(1). Delayed diagnosis is the
principal cause of increased mortality and morbidity associated
with this type of cancer. At the time of diagnosis, only 25% of
patients are able to undergo surgical resection. The 5-year
survival rate is only 10–15% in individuals with advanced disease
(1). Therefore, early diagnosis is
crucial, especially given that early symptoms (dyspepsia, mild
epigastric pain, nausea, and anorexia) are not very specific.
Biomarkers including, CEA and CA 19-9, have been tested. However,
these biomarkers have a low diagnostic ability to detect gastric
cancer (2). Therefore, identification
of novel biomarkers for the diagnosis and follow-up of gastric
cancer is essential.
Platelets play an important and multifaceted role in
cancer progression (3). Previous
findings suggested that platelets accelerate the natural course of
cancer by promoting neoangiogenesis, degradation of the
extracellular matrix, release of adhesion molecules, and growth
factors, thus providing essential components for tumor growth and
metastatic spread (4). The presence
of platelets is increased by proinflammatory cytokines released by
cancer cells through the promotion of megakaryocyte proliferation
(5). Given the relationship between
platelet and cancer, platelet-based markers are potential
candidates for the diagnosis and follow-up of gastric cancer.
Elevated mean platelet volume (MPV) of peripheral blood has been
identified in various types of cancer, including hepatocellular
carcinoma (6), ovarian (7), colon (8)
lung and breast (9) cancer. In the
present study, we examined whether MPV is suitable as a diagnostic
and prognostic marker for the detection of resectable gastric
cancer.
Materials and methods
Patients
The study was conducted as a retrospective study of
patients with gastric cancer who had been referred to the First
Affiliated Hospital of Soochow University between January, 2007 and
January, 2010. Approval for the study was granted by the Medical
Ethics Committees of the First Affiliated Hospital of Soochow
University (Jiangsu, China). Patients with hypertension,
hematological and renal disease, heart failure, chronic infection,
hepatic disorder and other cancer types were excluded from the
study. In total, 168 patients with resectable gastric cancer were
recruited in this study. Patient characteristics are presented in
Table I. The mean age (range) of
study patients was 56.5 (31–82) years. The staging of cancer was
determined according to the tumor-node-metastasis (TNM)
classification, using the American Joint Committee on Cancer (AJCC)
recommendations (10). The patients
were followed regularly for 60 months. Thirty age- and
gender-matched healthy individuals were also included in the
present study.
 | Table I.Relationship between pre-operative MPV
and demographic and clinical parameters. |
Table I.
Relationship between pre-operative MPV
and demographic and clinical parameters.
| Parameters | No. of patients | Low MPV (<10.51),
no. of patients | High MPV (≥10.51),
no. of patients | χ2 | P-value |
|---|
| Gender |
|
| Male | 116 | 62 | 54 | 1.7825 | 0.1818 |
|
Female | 52 | 22 | 30 |
|
|
| Age (years) |
|
|
<65 | 96 | 45 | 51 | 0.8750 | 0.3496 |
| ≥65 | 72 | 39 | 33 |
|
|
| Tumor size (cm) |
|
|
<5 | 108 | 51 | 57 | 0.9333 | 0.3340 |
| ≥5 | 60 | 33 | 27 |
|
|
| Lauren type |
|
|
Intestinal | 97 | 50 | 47 | 0.2195 | 0.6394 |
|
Diffuse | 71 | 34 | 37 |
|
|
| Depth of
invasion |
|
| T1,
T2 | 66 | 15 | 51 | 32.3422 |
<0.001a |
| T3,
T4 | 102 | 69 | 33 |
|
|
| Lymph node
metastases |
|
| N0,
N1 | 54 | 12 | 42 | 24.5614 |
<0.001a |
| N2,
N3 | 114 | 72 | 42 |
|
|
| Degree of
differentiation |
|
| Highly
differentiated | 50 | 23 | 27 | 0.4556 | 0.4997 |
|
Moderately or poorly
differentiated | 118 | 61 | 57 |
|
|
| AJCC stage |
|
| I,
II | 57 | 12 | 45 | 28.9161 |
<0.001a |
| III,
IV | 111 | 72 | 39 |
|
|
Blood analysis
Peripheral venous blood (5–7 ml) was collected into
sterile EDTA tubes. Blood specimens were obtained in the morning
between 06:30 and 07:30 a.m. to minimize the impact of circulating
hormones (circadian rhythm) on the number and subtype distribution
of white blood cells. Haematological parameters were analyzed
within 30 min after blood collection using a haematology analyser
Sysmex XE-2100 (Sysmex, Kobe, Japan). MPV was thus obtained and
used in subsequent analyses.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
software (SPSS, Inc., Chicago, IL, USA). Measurement data were
presented as mean ± standard variation. The association between MPV
and clinicopathological features were tested using the Chi-square
test. For the analysis of survival data, Kaplan-Meier curves were
constructed, and statistical analysis was carried out using the
log-rank test. The prognostic analyses were performed as
disease-free survival (DFS) and overall survival (OS). OS was
defined as the time from the diagnosed date to death from any
cause. DFS was defined as the time from the primary operation to
the relapse of tumor. The multivariate Cox regression was performed
for each outcome parameter, using a backwards elimination technique
to derive a potentially suitable set of predictors. P<0.05 was
considered to indicate statistically significant results.
Results
Pre-operative MPV is higher in
patients with gastric cancer patients compared with healthy
controls
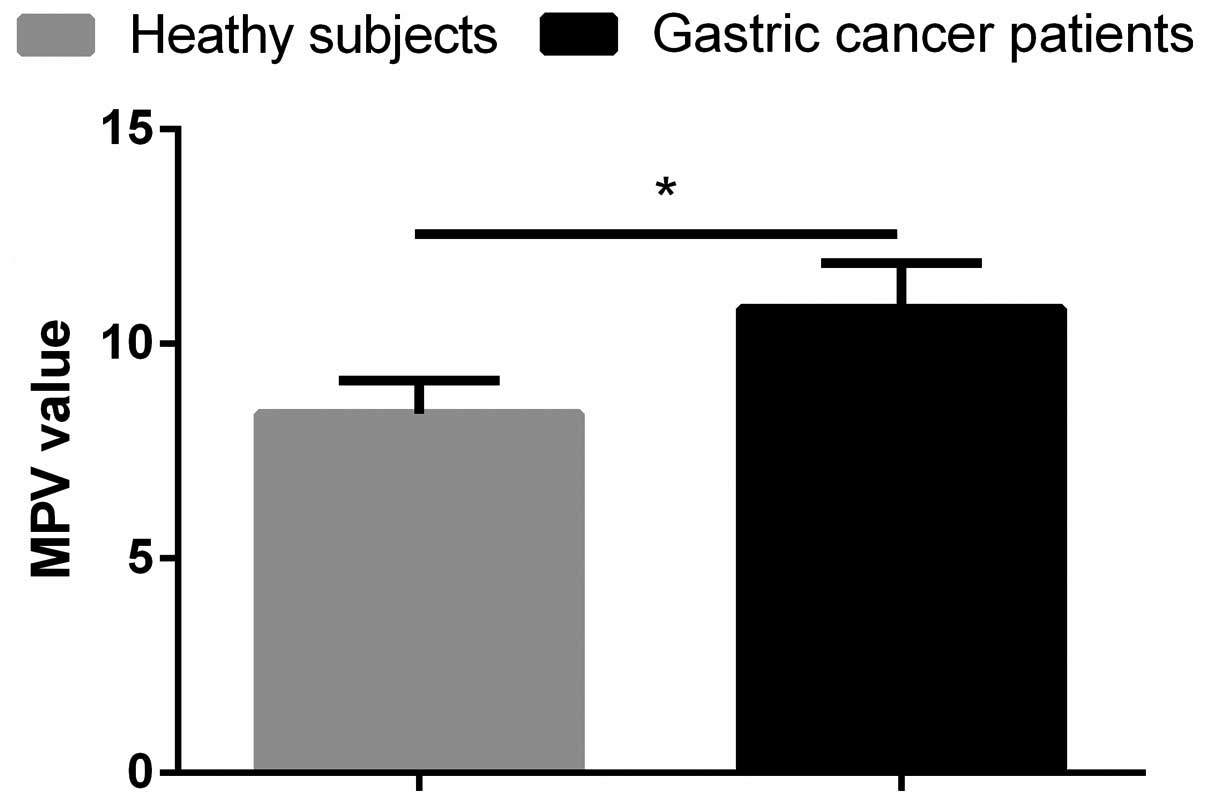
The mean pre-operative MPV in the study patients was
10.82±1.06, which was significantly higher than that in the healthy
individuals (8.37±0.78, p<0.001; Fig.
1). This result indicated that MPV is useful in the early
diagnosis of gastric cancer.
Low pre-operative MPV level predicts
better outcomes
As shown in Table I,
pre-operative MPV levels inversely correlated with
clinicopathological parameters, including depth of invasion,
lymphonodus metastasis and the AJCC stage.
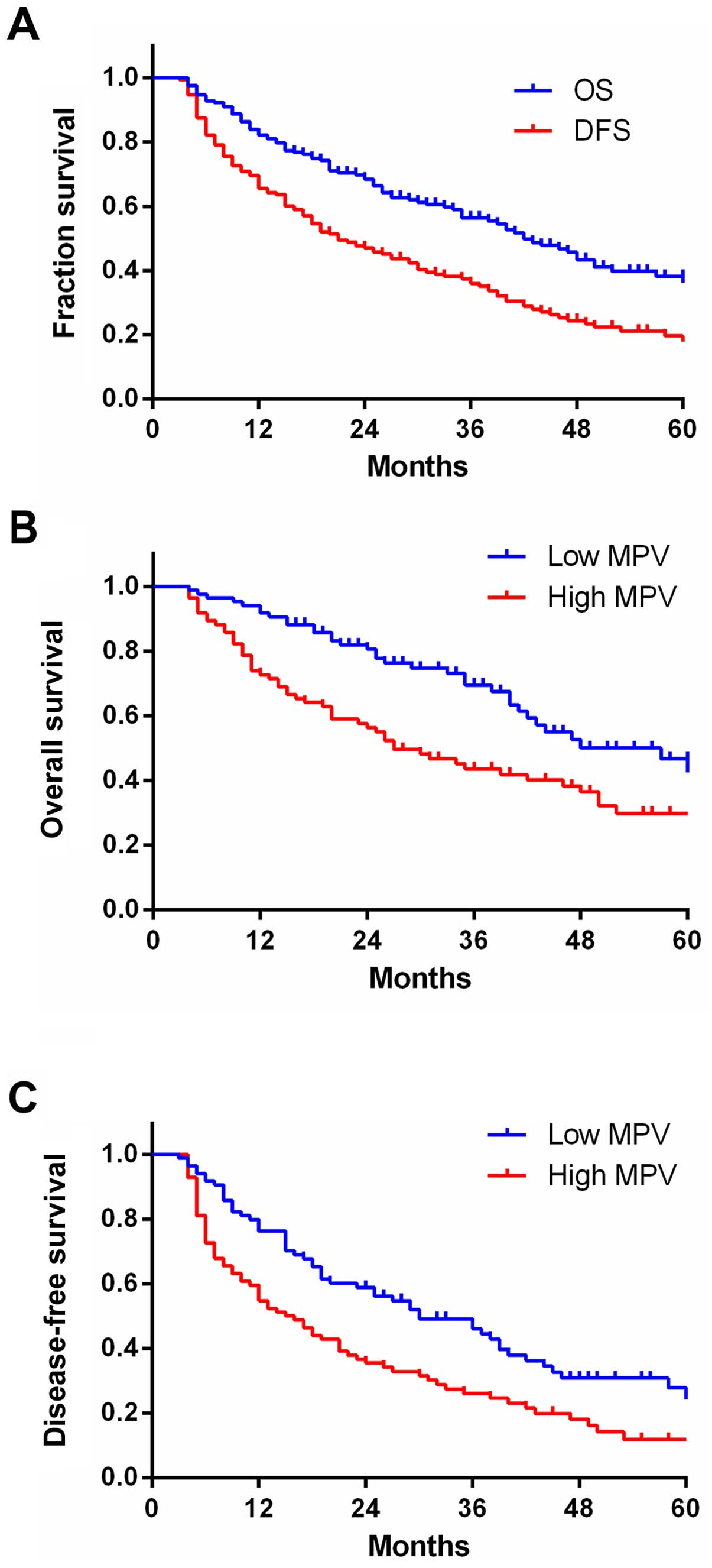
Median OS for all the patients was 57 months,
whereas the median was DFS 27 months (Fig. 2A).
The patients were separated into two groups
according to median pre-operative MPV: low (<10.51) and high
(≥10.51) MPV. The Kaplan-Meier plots showed the association between
pre-operative MPV and OS and PFS (Fig. 2B
and C). The OS and PFS rates of high pre-operative MPV were
29.8 and 11.9%, respectively, and were significantly different from
corresponding rates in the low pre-operative MPV group (46.7 and
24.3%, respectively, both p<0.01). This result demonstrated that
patients with higher pre-operative MPV had decreased survival
rates.
MPV changes before and after surgery
predict improved outcomes
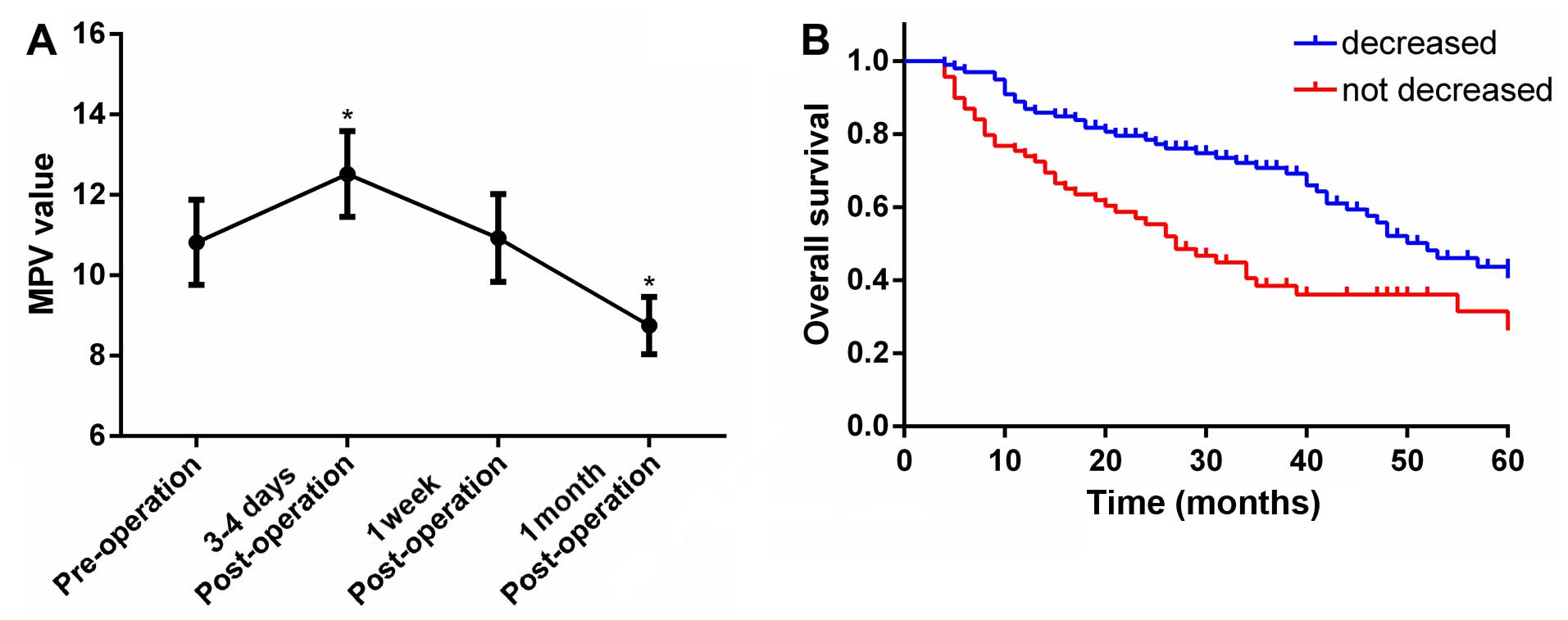
MPV increased significantly 3–4 days following
surgery and returned to pre-operation levels one week after the
surgery (Fig. 3A). Furthermore,
surgical tumor resection led to a significant decrease in the
average MPV one month after surgery (Fig.
3A).
When individual MPV changes were evaluated, it was
observed that a decrease was present in 99 patients and absent in
the remaining 69 patients. The Kaplan-Meier plots indicating an
association between MPV values and OS are shown in Fig. 3B. It was evident that OS was improved
in patients whose MPV decreased after surgery, compared with those
without any change (40.5 vs. 28.9%, p<0.0037; Fig. 3B).
Univariate and multivariate analysis
of risk factors for OS and DFS
Univariate and multivariate analyses were performed
to identify the risk factors associated with OS and DFS. As shown
in Table II, univariate analysis
revealed that 5 of 10 risk factors affected OS and DFS. These
factors included depth of invasion, lymphonodus metastasis, AJCC
stage, pre-operatic MPV, and changes in MPV after surgery.
Multivariate analysis further confirmed that depth of invasion,
lymphonodus metastasis, AJCC stage, and changes in MPV following
surgery were the factors associated with OS. Furthermore, AJCC
stage and pre-operative MPV were the prognostic factors for
DFS.
 | Table II.Univariate and multivariate analysis
of risk factors for OS and DFS. |
Table II.
Univariate and multivariate analysis
of risk factors for OS and DFS.
|
| OS | DFS |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Risk factors | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Gender |
|
| Male or
female | 0.75
(0.46–1.72) | 0.806 | – | – | 0.81
(0.44–1.60) | 0.835 | – | – |
| Age (years) |
|
| <65
or ≥65 | 1.16
(0.69–1.82) | 0.629 | – | – | 1.22
(0.68–2.03) | 0.825 | – | – |
| Tumor size
(cm) |
|
| <5
or ≥5 | 1.37
(0.68–2.73) | 0.227 | – | – | 1.27
(0.65–2.42) | 0.237 | – | – |
| Lauren type |
|
|
Intestinal or diffuse | 1.29
(0.73–2.25) | 0.605 | – | – | 1.89
(1.33–2.40) | 0.454 | – | – |
| Depth of
invasion |
|
| T1, T2
or T3, T4 | 2.61
(1.53–3.02) | 0.036 | 2.53
(1.64–3.05) | 0.028 | 2.60
(1.42–3.31) | 0.040 | – | – |
| Degree of
differentiation |
|
| Highly
or moderately/poorly differentiated | 1.46
(0.72–1.85) | 0.722 | – | – | 1.38
(0.74–1.91) | 0.757 | – | – |
| Lymph node
metastases |
|
| N0, N1
or N2, N3 | 3.54
(2.47–6.80) | 0.022 | 3.64
(2.49–6.85) | 0.021 | 2.92
(1.86–4.53) | 0.037 | – | – |
| AJCC stage |
|
| I, II
or III, IV | 4.82
(3.15–7.89) | 0.002 | 4.32
(2.70–6.83) | 0.003 | 4.39
(2.98–6.97) | 0.002 | 4.25
(2.80–6.51) | 0.003 |
| MPV |
|
| Low
(<10.51) or High (≥10.51) | 2.56
(1.42–3.37) | 0.004 | – | – | 2.78
(1.67–3.78) | 0.003 | 2.41
(1.36–3.52) | 0.001 |
| Changes in MPV
after operation |
|
|
Decreased or not
decreased | 3.52
(2.27–6.04) | 0.001 | 3.57
(2.49–5.92) | 0.001 | 3.55
(2.16–5.83) | 0.001 | – | – |
Discussion
The involvement of platelets and coagulation factors
in hematogenous tumor metastasis are well known. Elevated
thrombocytosis and platelet counts are associated with advanced,
often metastatic, stages of cancer and to be negative prognostic
markers for various types of cancer, including endometrial
carcinoma, cervical, ovarian, gastric, and esophageal cancer
(11).
Platelets participate in multiple steps of
hematogenous metastasis. Covered with platelets, circulating cancer
cells can transport more easily in the bloodstream and overcome
countering effects of immune cells and physical factors such as
shear force and mechanical trauma due to passage through
microvasculature (12,13). Platelets can also promote tumor growth
by increasing angiogenesis via the cytokine vascular endothelial
growth factor (VEGF). The platelet content of VEGF is significantly
elevated in cancer patients, and there is a direct correlation
between the number of circulating platelets and the level of serum
VEGF (3).
The cancer-promoting effects of platelets are
amplified by stimulation of activation and aggregation of platelets
caused by proinflammatory cytokines released by cancer cells
(4). This involves proliferation and
differentiation of early progenitor cells such as megakaryocyte
progenitors. In addition, malignant cells possess the ability of
aggregating platelets through a process known as tumor cell-induced
platelet aggregation (TCIPA) (12).
Therefore, the close involvement of the platelet with metastasizing
cancer makes this cell type a promising candidate for early cancer
diagnosis and treatment (11).
Large platelets are more reactive than their smaller
counterparts, and are more likely to aggregate, leading to
thrombosis. Large platelets are an independent risk factor for
myocardial infarction, as platelet size is a predictor of recurrent
myocardial infarction and death (14). The MPV tested in our study can be
easily evaluated by hematological analyzers, which makes it a
convenient marker of platelet functions and activation (3). Elevated MPV may indicate a tendency
towards thrombosis, as it has been demonstrated for myocardial
infarction and cerebrovascular embolus (15). Previous studies suggested that MPV is
a potential biomarker for the diagnosis and follow-up of types of
cancer (6–9).
Elevated MPV values may be a consequence of systemic
inflammatory response (16), which is
believed to play a critical role in the development and progression
of different cancers by promoting cancer cell proliferation and
survival, angiogenesis, cancer metastasis and modulating cancer
cell response to therapies (3).
Numerous types of cancer release proinflammatory cytokines, such as
interleukin (IL)-1, IL-3, and IL-6, which promote the proliferation
of megakaryocytes, resulting in platelet activation and aggregation
(4). In ovarian cancer, elevated
levels of IL-6 in ascites and cyst fluids have been associated with
thrombocytosis. Furthermore, administration of recombinant IL-6 has
been associated with increased platelet count (5). Elevated IL-6 is significantly higher in
individuals with gastric cancer, as well as in patients with
prostate cancer (17,18).
The close interplay between inflammation,
coagulation, and cancer progression ignited intensive studies in
this field (4). For example,
long-term use of non-steroid anti-inflammatory drugs such as
aspirin is associated with a reduced risk of esophageal cancer
(1). Clinical and epidemiological
studies demonstrated an association between chronic inflammation
and gastric cancer (19–21). Based on these considerations, we
postulated that elevated MPV values in patients with gastric cancer
may be a consequence of systemic inflammatory response.
Correspondingly, a decrease of MPV values after surgery may be due
to a decreased systemic inflammatory response. Therefore, patients
whose MPV values did not decrease after surgical resection of the
cancer may continue to harbor untamed systemic inflammatory
response, leading to an unfavourable prognosis.
In conclusion, the present study indicates an
association of the pre-operative MPV level and changes between pre-
and post-operative levels with the diagnosis and prognosis of
gastric cancer. Although MPV is also a non-specific marker, this
non-invasive, convenient and inexpensive biomarker may be a
complement to the present biomarkers, and a benefit to the early
detection and prognosis evaluation of gastric cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81472296, 81101867,
81272542, 81200369 and 81372443), the China International Medical
Foundation (grant no. CIMF-F-H001-057), the Special Foundation of
Clinical Medicine of Jiangsu Provincial Bureau of Science and
Technology (grant no. BL2014039), the Scientific Research Project
of Jiangsu Provincial Bureau of Traditional Chinese Medicine (grant
no. L213236), the Medical Scientific Research Project of Jiangsu
Provincial Bureau of Health (grant no. Z201206), the Special
Foundation of Wu Jieping Medical Foundation for Clinical Scientific
Research (grant nos. 320.6753.1225 and 320.6750.12242), the Science
and Education for Health Foundation of Suzhou for Youth (grant nos.
SWKQ1003 and SWKQ1011), the Science and Technology Project
Foundation of Suzhou (grant nos. SYS201112, SYSD2012137 and
SYS201335), the Science and Technology Foundation of Suzhou
Xiangcheng (grant nos. SZXC2012-70 and XJ201451), and a Project
Founded by the Priority Academic Program Development of Jiangsu
Higher Education Institutions.
References
|
1
|
Wang WH, Huang JQ, Zheng GF, Lam SK,
Karlberg J and Wong BC: Non-steroidal anti-inflammatory drug use
and the risk of gastric cancer: a systematic review and
meta-analysis. J Natl Cancer Inst. 95:1784–1791. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mroczko B, Groblewska M, Łukaszewicz-Zajac
M, Bandurski R, Kedra B and Szmitkowski M: Pre-treatment serum and
plasma levels of matrix metalloproteinase 9 (MMP-9) and tissue
inhibitor of matrix metalloproteinases 1 (TIMP-1) in gastric cancer
patients. Clin Chem Lab Med. 47:1133–1139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kemal Y, Yucel I, Ekiz K, Demirag G,
Yilmaz B, Teker F and Ozdemir M: Elevated serum neutrophil to
lymphocyte and platelet to lymphocyte ratios could be useful in
lung cancer diagnosis. Asian Pac J Cancer Prev. 15:2651–2654. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seretis C, Seretis F, Lagoudianakis E,
Politou M, Gemenetzis G and Salemis NS: Enhancing the accuracy of
platelet to lymphocyte ratio after adjustment for large platelet
count: a pilot study in breast cancer patients. Int J Surg Oncol.
2012:6536082012.doi: 10.1155/2012/653608. PubMed/NCBI
|
|
5
|
Heras P, Hatzopoulos A, Kritikos N and
Kritikos K: Platelet count and tumor progression in gastric cancer
patients. Scand J Gastroenterol. 45:1005–1006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho SY, Yang JJ, You E, Kim BH, Shim J,
Lee HJ, Lee WI, Suh JT and Park TS: Mean platelet volume/platelet
count ratio in hepatocellular carcinoma. Platelets. 24:375–377.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kemal Y, Demirağ G, Ekiz K and Yücel I:
Mean platelet volume could be a useful biomarker for monitoring
epithelial ovarian cancer. J Obstet Gynaecol. 34:515–518. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mutlu H, Berk V, Karaca H, Erden A, Aslan
T and Akca Z: Treatment regimen with bevacizumab decreases mean
platelet volume in patients with metastatic colon cancer. Clin Appl
Thromb Hemost. 18:546–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aksoy S, Kilickap S, Hayran M,
Harputluoglu H, Koca E, Dede DS, Erman M and Turker A: Platelet
size has diagnostic predictive value for bone marrow metastasis in
patients with solid tumors. Int J Lab Hematol. 30:214–219. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Erpenbeck L and Schön MP: Deadly allies:
the fatal interplay between platelets and metastasizing cancer
cells. Blood. 115:3427–3436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lian L, Li W, Li ZY, Mao YX, Zhang YT,
Zhao YM, Chen K, Duan WM and Tao M: Inhibition of MCF-7 breast
cancer cell-induced platelet aggregation using a combination of
antiplatelet drugs. Oncol Lett. 5:675–680. 2013.PubMed/NCBI
|
|
13
|
Shou LM, Zhang QY, Li W, Xie X, Chen K,
Lian L, Li ZY, Gong FR, Dai KS, Mao YX, et al: Cantharidin and
norcantharidin inhibit the ability of MCF-7 cells to adhere to
platelets via protein kinase C pathway-dependent downregulation of
α2 integrin. Oncol Rep. 30:1059–1066. 2013.PubMed/NCBI
|
|
14
|
Karagöz B, Bilgi O, Alacacioğlu A, Ozgün
A, Sayan O, Erikçi AA and Kandemir EG: Mean platelet volume
increase after tamoxifen, but not after anastrazole in adjuvant
therapy of breast cancer. Med Oncol. 27:199–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mutlu H, Artis TA, Erden A and Akca Z:
Alteration in mean platelet volume and platicrit values in patients
with cancer that developed thrombosis. Clin Appl Thromb Hemost.
19:331–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McMillan DC: Systemic inflammation,
nutritional status and survival in patients with cancer. Curr Opin
Clin Nutr Metab Care. 12:223–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lukaszewicz-Zając M, Mroczko B, Gryko M,
Kędra B and Szmitkowski M: Comparison between clinical significance
of serum proinflammatory proteins (IL-6and CRP) and classic tumor
markers (CEAand CA 19-9) in gastric cancer. Clin Exp Med. 11:89–96.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shariat SF, Andrews B, Kattan MW, Kim J,
Wheeler TM and Slawin KM: Plasma levels of interleukin-6 and its
soluble receptor are associated with prostate cancer progression
and metastasis. Urology. 58:1008–1015. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ilhan N, Ilhan N, Ilhan Y, Akbulut H and
Kucuksu M: C-reactive protein, procalcitonin, interleukin-6,
vascular endothelial growth factor and oxidative metabolites in
diagnosis of infection and staging in patients with gastric cancer.
World J Gastroenterol. 10:1115–1120. 2004.PubMed/NCBI
|
|
20
|
Lochhead P and El-Omar EM: Gastric cancer.
Br Med Bull. 85:87–100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hussain SP and Harris CC: Inflammation and
cancer: an ancient link with novel potentials. Int J Cancer.
121:2373–2380. 2007. View Article : Google Scholar : PubMed/NCBI
|