Introduction
Osteosarcoma is a common adolescent cancer, which
comprises ~20% of all forms of primary bone cancer (1). The survival rate for osteosarcoma has
remained relatively poor for the last 30 years and is ~60%
worldwide (2,3). The identification of novel oncogenic
targets and the underlying molecular events that promote the
malignant growth, invasion and metastasis of osteosarcoma may aid
the development of successful treatment strategies for patients
with the disease (4–6).
Cancer/testis (CT) antigens are proteins that are
typically expressed in the human germ line. A number of studies
have demonstrated that these proteins are upregulated in various
forms of cancer, and may therefore be targeted by therapeutic
cancer vaccines (7).
Sperm-associated antigen 9 (SPAG9) was originally
identified as a novel member of the CT antigen family, and
functions as a scaffolding protein involved in the activation of
the c-Jun NH2-terminal kinase (JNK) signaling pathway (8–12).
Previous studies have reported that SPAG9 is overexpressed in
various types of human cancer, including breast, thyroid and
cervical cancer, and colorectal carcinoma (13–17). SPAG9
small interfering RNA (siRNA) treatment in cancer cells was
reported to inhibit tumor cell growth and invasion. In addition,
SPAG9 protein expression in tumors was observed to be associated
with circulating SPAG9 antibodies in the serum of patients with
early-stage breast and cervical cancer (13,14),
indicating its potential application in early cancer detection. To
date, the expression pattern and biological function of SPAG9 in
human osteosarcoma remains unknown.
In the present study, the expression pattern of
SPAG9 in 58 osteosarcoma specimens was investigated. In addition,
the potential effects of SPAG9 on cell proliferation and invasion
were examined in U2OS and MG63 osteosarcoma cell lines.
Materials and methods
Patients and specimens
The study protocol was approved by the Institutional
Review Board of Shengjing Hospital of China Medical University
(Shenyang, China). Primary tumor specimens were obtained from 58
patients diagnosed with osteosarcoma who underwent resection at the
First Affiliated Hospital of China Medical University (Shenyang,
China) and Shengjing Hospital of China Medical University between
March 2000 and May 2014. Normal bone specimens were obtained from
normal tissues adjacent to the osteosarcoma tissues. Tissues were
formalin-fixed and embedded in paraffin prior to use. Tissue
sections were stained with hematoxylin and eosin, and the
histological diagnosis was performed according to the World Health
Organization classification guidelines (18). Clinical and histopathological data
were obtained from medical records. According to the Enneking
scoring system, the tumors were classified as highly malignant
intracompartmental osteogenic sarcomas (IIA) or as
extracompartmental lesions (IIB).
Cell culture and transfection
U2OS and MG63 cell lines were obtained from the
American Type Culture Collection (Manassas, VA, USA). The cells
were cultured for 2 days at 37°C in minimum essential medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal calf serum, 100 IU/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich, St. Louis, MO, USA).
Smartpool siRNA targeting SPAG9 was purchased from
Dharmacon (GE Healthcare Dharmacon Inc., Lafayette, CO, USA).
DharmaFECT 2 Transfection Reagent (GE Healthcare Dharmacon Inc.)
was used for siRNA transfection according to the manufacturer's
protocol. Protein level was assessed 48 h later by western
blotting.
Immunohistochemistry
Paraffin-embedded osteosarcoma tissues were cut into
4-µm thick sections prior to immunostaining. Immunostaining was
performed using the EliVision™ Plus Immunohistochemical Detection
kit (Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China). The sections
were deparaffinized in xylene, rehydrated with graded alcohol and
subsequently boiled in citrate buffer (pH 6.0) for 2 min with an
autoclave. Endogenous peroxidase was blocked by 3% hydrogen
peroxide, and normal animal serum (Pierce Biotechnology, Inc.,
Rockford, IL, USA) was used to reduce non-specific binding. Tissue
sections were incubated with polyclonal rabbit anti-SPAG9 antibody
(dilution, 1:300; #ab12331; Abcam, Cambridge, MA, USA) overnight at
4°C. Rabbit immunoglobulin (dilution, 1:300; #ab150062; Abcam) was
used (at the same concentration as the antigen-specific antibody)
as a negative control. Primary staining was followed by incubation
with biotinylated anti-horseradish peroxidase secondary antibodies
(dilution, 1:2,000; #ab34885; Abcam). The peroxidase reaction was
developed with 3,3′-diaminobenzidine (Fuzhou Maixin Biotech Co.,
Ltd.). Counterstaining with hematoxylin was performed and the
sections were dehydrated in ethanol prior to mounting.
Immunostaining of SPAG9 was scored on a
semiquantitative scale by evaluating the percentage and intensity
of tumor cells according to a previous study (19). A total of 400 tumor cells were counted
by microscope and the percentage of positively-stained cells was
calculated. The intensity of SPAG9 staining was scored as follows:
0, negative/weak; 1, moderate; and 2, strong. Percentage scores
were assigned as follows: 1, 1–25%; 2, 26–50%; 3, 51–75%; and 4,
76–100%. The scores of each tumor sample were multiplied to give a
final score between 0–8, and a tumor sample score of ≥4 was
considered to indicate overexpression.
Quantitative polymerase chain reaction
(qPCR)
Template DNA was obtained from U2OS and MG63 cells.
qPCR was performed using the SYBR® Green PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) in a total
volume of 20 µl on a 7500 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The cycling conditions were as
follows: 95°C for 30 sec; 40 cycles of 95°C for 30 sec and 60°C for
30 sec. A dissociation step was performed to generate melting
curves to confirm the specificity of the amplification. PCR was
repeated three times. Expression levels of the analyzed genes were
normalized to the expression of β-actin. The fold change of gene
expression was calculated by the 2−ΔΔCq method (20). The sequences of the primer pairs were
as follows: SPAG9 forward, 5′-GCTTTTGATCGCAATACAGAATCTC-3′; and
reverse, 5′-AACTTCCCGACCCATTCCTAGT-3′; and β-actin forward,
5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and reverse,
5′-CACCTTCTACAATGAGCTGCGTGTG-3′. The primers were designed by Oligo
7 software (www.oligo.net).
Western blot analysis
Total protein was extracted from cells using cell
lysis buffer (Pierce Biotechnology, Inc.) and quantified using the
Bradford method. Samples were separated by 10% sodium dodecyl
sulfate polyacrylamide gel electrophoresis and were transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA) where they were incubated overnight at 4°C with antibodies
against SPAG9 (dilution, 1:1,000; #ab12331; Abcam), cyclin D1
(dilution, 1:1,000; #2978; Cell Signaling Technology, Inc.,
Danvers, MA, USA), phosphorylated (p)-JNK (dilution, 1:1,000;
#4668; Cell Signaling Technology, Inc.), JNK (dilution, 1:1,000;
#9252; Cell Signaling Technology, Inc.), JunD (dilution, 1:1,000;
#5000; Cell Signaling Technology, Inc.) and glyceraldehyde
3-phosphate dehydrogenase (dilution, 1:1,000; #2118; Cell Signaling
Technology, Inc.). Following incubation with peroxidase-coupled
anti-mouse/rabbit immunoglobulin G (Cell Signaling Technology,
Inc.) at 37°C for 2 h, bound proteins were visualized using ECL
Western Blotting Substrate (Pierce Biotechnology, Inc.) and
detected using a BioImaging system (UVP Inc., Upland, CA, USA).
Relative protein levels were quantified using β-actin as a loading
control and ImageJ 1.48v software (National Institutes of Health,
Bethesda, MD, USA).
Transwell invasion and MTT assays
Cell invasion was performed in a 24-well Transwell
chamber (8-µm pore size; Costar, Corning, NY, USA). The insert was
coated with 50 ml Matrigel (dilution, 1:3; BD Bioscience, Franklin
Lakes, NJ, USA). U2OS cells were trypsinized following transfection
with negative control or SPAG9 siRNA for 48 h at 37°C, and were
subsequently transferred to the upper chamber in 100 ml serum-free
medium containing 1×105 cells and incubated for 24 h.
The lower chamber was filled with medium supplemented with 10%
fetal bovine serum (Pierce Biotechnology, Inc.) as chemoattractant.
The membranes were fixed and stained using 0.1% crystal violet. The
number of invading cells were counted in five randomly selected
high-power fields (magnification, ×400) under a microscope. All
experiments were performed in triplicate.
For the MTT assay, the cells were plated in 96-well
plates at a concentration of ~2,000 cells per well and cultured for
3 days. For quantification of cell viability, 20 µl 5 mg/ml MTT
(thiazolyl blue) solution was added to each well and incubated for
4 h at 37°C. The medium was removed from each well and the
resulting MTT formazan was solubilized in 200 µl dimethyl
sulfoxide. The solution was measured spectrophotometrically at 490
nm.
Statistical analysis
SPSS version 11.5 (SPSS, Inc., Chicago, IL, USA) was
used for all statistical analyses. The χ2 test was used
to examine associations between SPAG9 expression and
clinicopathological factors, whilst Student's t-test was used to
compare invasive cells and colony numbers between control and SPAG9
depletion cells. All P-values are based on two-sided statistical
analysis, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression pattern of SPAG9 in
osteosarcoma tissues
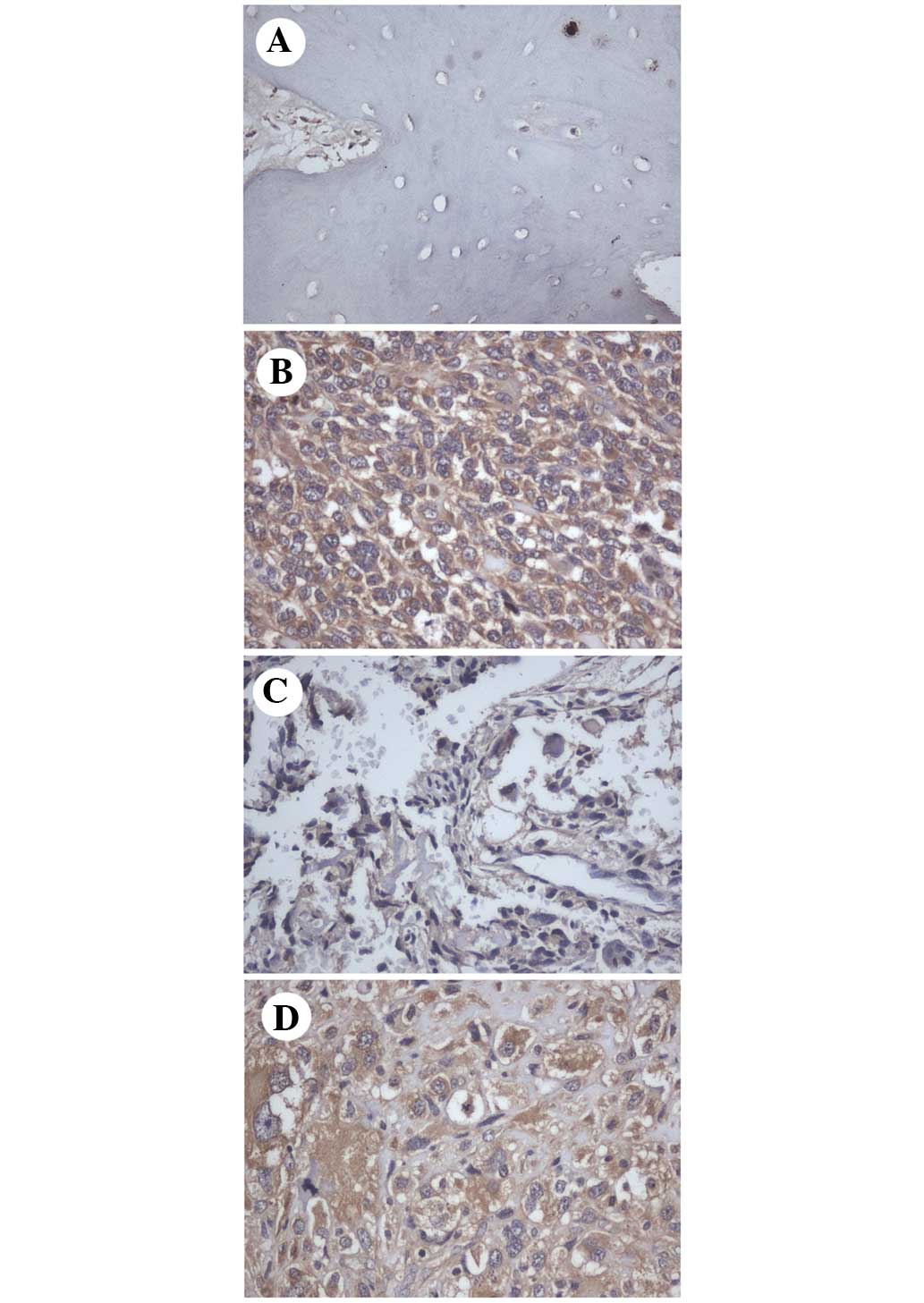
In the normal bone tissues, SPAG9 staining was
negative (Fig. 1A). By contrast,
SPAG9 overexpression was observed in 37 out of 58 (63.8%) human
osteosarcoma tissues (Fig. 1B-D),
while no staining was observed in sections from the same specimens
incubated with non-immune rabbit immunoglobulin. SPAG9 was
primarily localized in the cytoplasm of the osteosarcoma cells. The
association between SPAG9 protein expression and
clinicopathological factors was analyzed, and no association was
identified between SPAG9 expression and age, gender, pathological
classification or location (Table
I).
 | Table I.Association between SPAG9 and
clinicopathological features. |
Table I.
Association between SPAG9 and
clinicopathological features.
|
|
| SPAG9 |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | n | Negative | Positive | P-value |
|---|
| Age, years |
|
|
<18 | 28 | 10 | 18 | 0.9399 |
|
≥18 | 30 | 11 | 19 |
|
| Gender |
|
|
Male | 36 | 12 | 24 | 0.5602 |
|
Female | 22 | 9 | 13 |
|
| Pathological
classification |
|
|
IIA | 10 | 4 | 6 | 0.7838 |
|
IIB | 48 | 17 | 31 |
|
| Location |
|
|
Extremities | 47 | 16 | 31 | 0.4784 |
|
Axial | 11 | 5 | 6 |
|
Biological functions of SPAG9 in
osteosarcoma cells
To determine the biological functions of SPAG9 in
osteosarcoma cell lines, the present study initially examined SPAG9
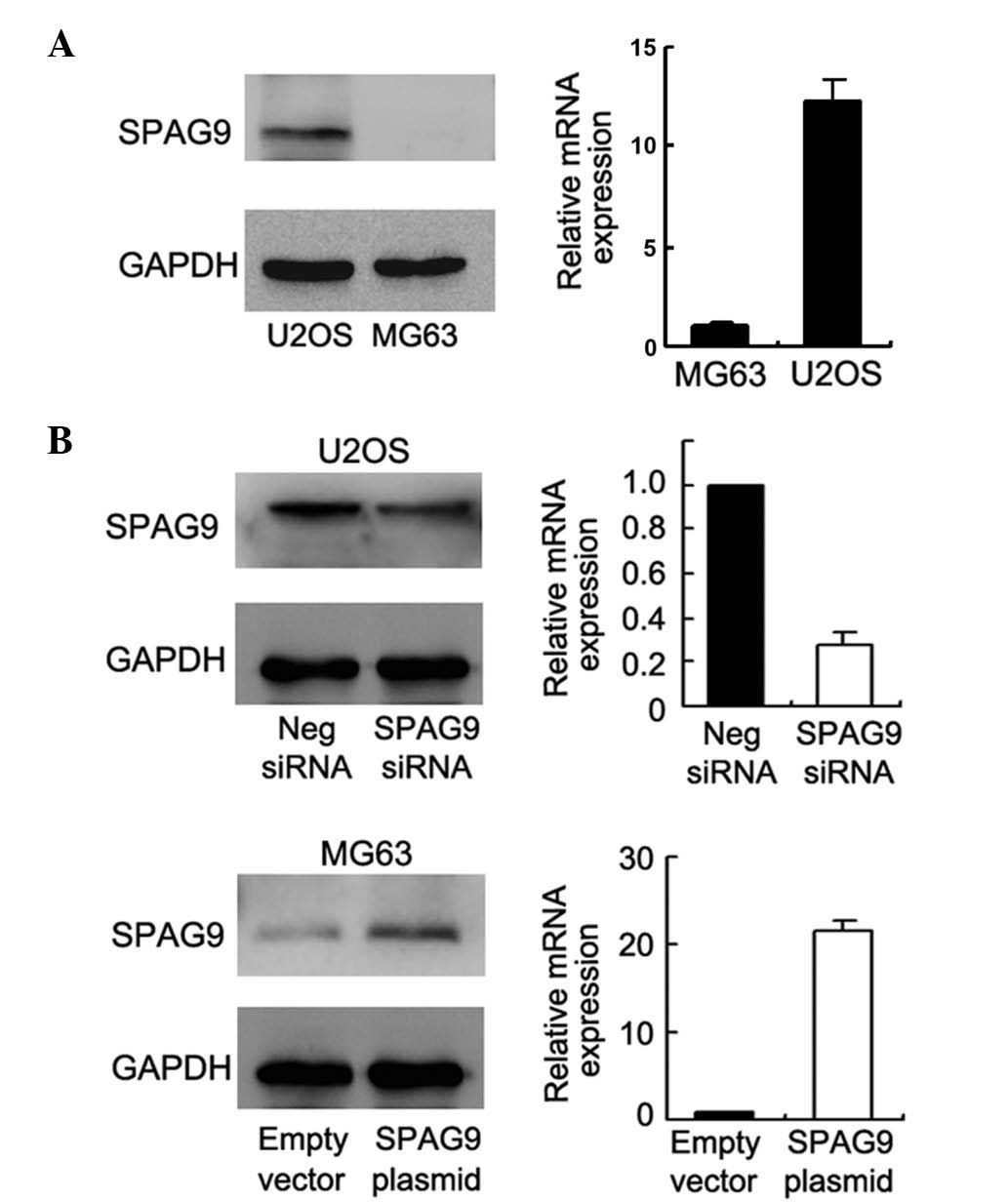
protein expression in the U2OS and MG63 cell lines. It was observed
that SPAG9 expression was potent in the U2OS cells and weak in the
MG63 cells (Fig. 2A). To examine the
effect of SPAG9 on cell proliferation and invasion, siRNA-mediated
SPAG9-knockdown was performed in the U2OS cell line and plasmid
transfection was performed in the MG63 cell line. As presented in
Fig. 2B, SPAG9 siRNA decreased SPAG9
protein and mRNA expression in the U2OS cells, and plasmid
transfection upregulated SPAG9 expression in the MG63 cells. The
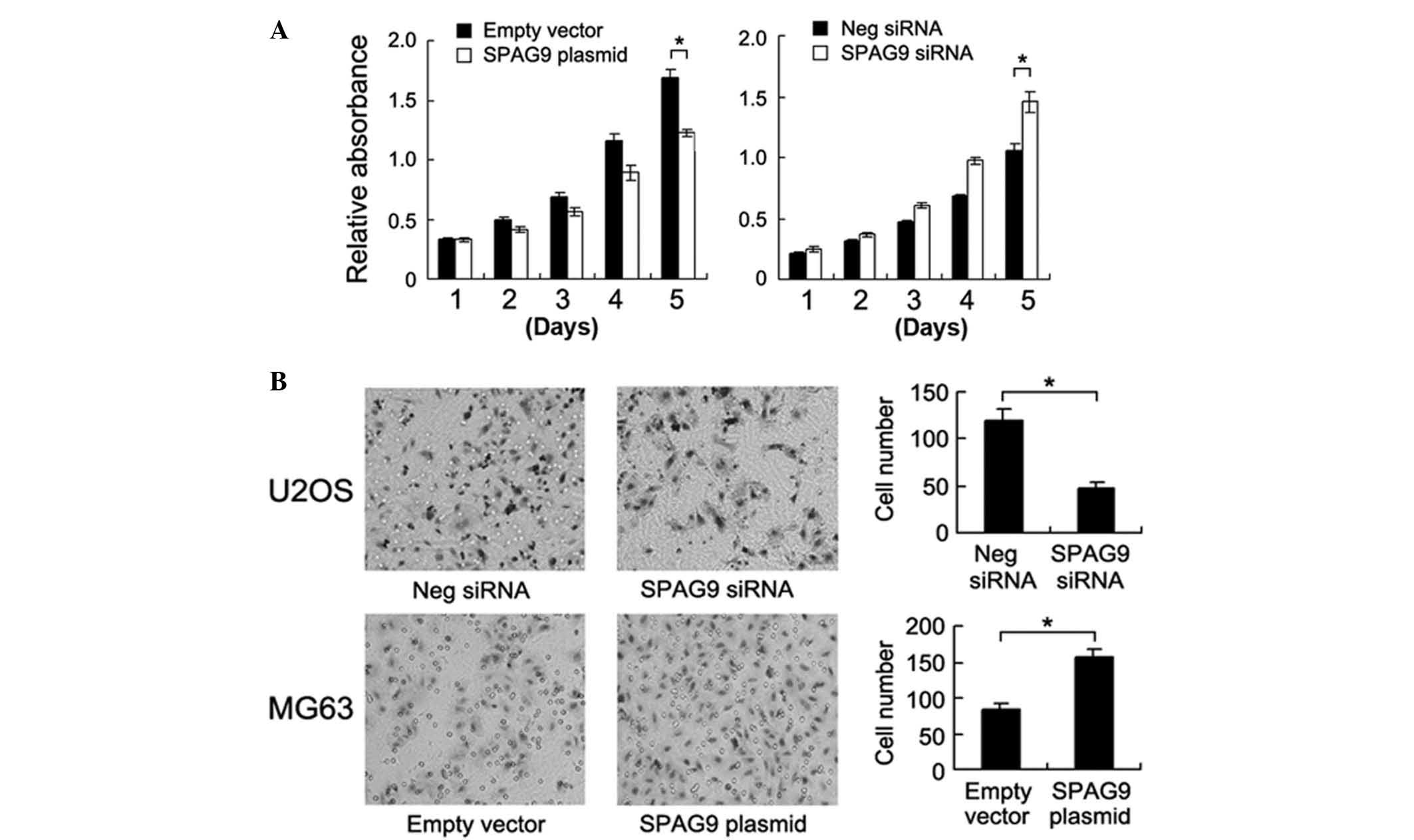
MTT assay was employed to examine the role of SPAG9 on cell
proliferation. The results demonstrated that SPAG9 siRNA inhibited
the cell growth rate in the U2OS cells, and SPAG9 transfection
upregulated cell proliferation in the MG63 cells (Fig. 3A). A Transwell assay was employed to
characterize the effect of SPAG9 on cell invasion. As presented in
Fig. 3B, SPAG9 depletion
significantly inhibited cell invasion in the U2OS cell line
(control vs. siRNA, 119±12 vs. 48±6; P<0.05) and its
transfection accelerated cell invasion in the MG63 cell line (empty
vector vs. plasmid, 83±9 vs. 156±12; P<0.05).
SPAG9 regulates JunD expression in
osteosarcoma cells
Consistent with a previous study (19), the results of the present study
indicated that SPAG9 is a regulator of osteosarcoma cell
proliferation and invasion. It has previously been reported that
SPAG9 is involved in JNK signaling (21). To investigate the mechanisms
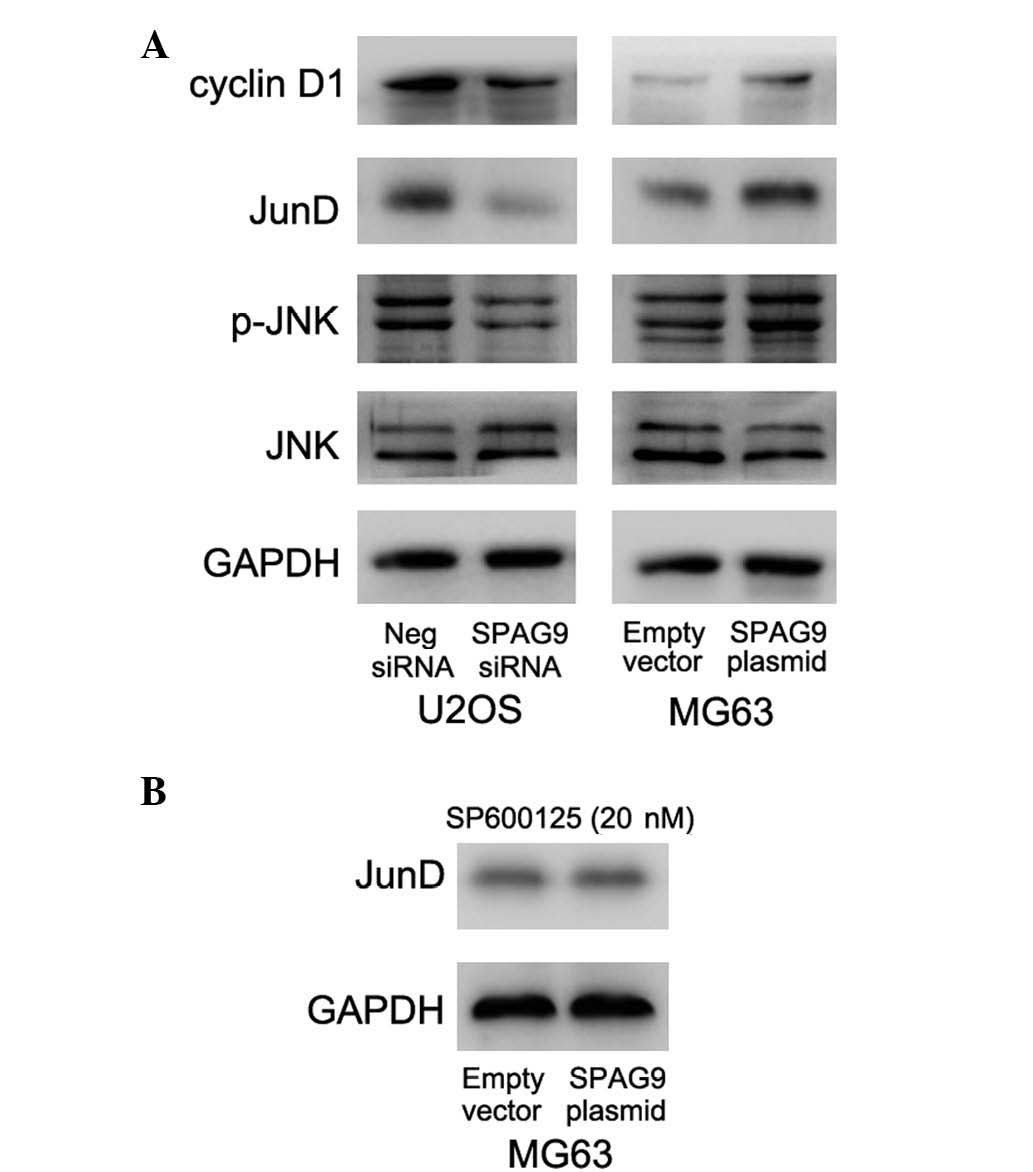
underlying this association, the current study examined the protein
level of JunD following SPAG9-knockdown and transfection. As
presented in Fig. 4A, SPAG9 depletion
decreased cyclin D1 and JunD expression, and JNK phosphorylation in
the U2OS cell line. In the MG63 cells, SPAG9 transfection increased
JNK phosphorylation, and cyclin D1 and JunD protein expression
(Fig. 4A). Treatment with the JNK
inhibitor, SP600125 (20 nM, 3 h post-transfection), abolished the
upregulatory effect of SPAG9 on JunD (Fig. 4B), indicating that SPAG9 may regulate
the malignant biological behavior of osteosarcoma cells through
JNK-JunD regulation.
Discussion
In the present study, it was observed that the level
of SPAG9 expression was elevated in osteosarcoma tissues compared
with that in normal bone specimens. In vitro experiments in
osteosarcoma cell lines demonstrated that SPAG9 depletion inhibited
proliferation and invasion, while SPAG9 transfection accelerated
proliferation and invasion. In addition, it was observed that SPAG9
was able to regulate JunD expression.
SPAG9 has previously been identified to be
overexpressed in various types of cancer, including lung, breast,
renal, hepatocellular and colorectal cancer (13,17,19,21,22).
The current study observed negative SPAG9 staining in normal bone
tissues, and positive cytoplasmic SPAG9 expression in 63.8% (37/58)
of osteosarcoma tissues, which was consistent with previous studies
(19,. A number of studies have described the role of SPAG9 in
cancer cell proliferation and provided accumulating evidence that
characterizes SPAG9 as an oncoprotein regulating cell invasion. It
was reported that SPAG9 siRNA knockdown inhibited cell invasion and
proliferation in renal, lung and colorectal carcinoma (13,21,23–25).
To gain insight into the function of SPAG9 in osteosarcoma
progression, SPAG9 expression was knocked down in the U2OS cell
line and upregulated in the MG63 cell line in the present study.
Consistent with a former study (19,26,27), it
was demonstrated that SPAG9 depletion significantly inhibited cell
proliferation and invasion, whilst SPAG9 plasmid transfection
upregulated proliferation and invasion. These results suggested
that SPAG9 serves an important role in osteosarcoma progression and
may function as a biomarker for malignant behavior.
In the current study, the molecular pathway through
which SPAG9 promotes osteosarcoma invasion and proliferation was
also investigated. It was observed that SPAG9 was involved in JNK
signaling; thus, JNK activation and its downstream target JunD,
which serves as a link between JNK and cancer progression, was
examined. Using western blot analysis, it was demonstrated that
SPAG9 siRNA treatment downregulated p-JNK and JunD protein
expression, whilst SPAG9 plasmid transfection upregulated the level
of p-JNK and JunD. JunD is a component of the activator protein-1
transcription factor complex, which is important in cancer
progression (28). A previous study
reported that JunD activated cell proliferation via upregulation of
cyclin D1 proteins (29), and was
also demonstrated to activate matrix metalloproteinase (MMP)-7 and
MMP-9 transcription (30,31). MMP-7 and MMP-9 have been suggested to
be critical for the metastatic and invasive potential in human
malignancies, including osteosarcoma (32–35). Thus,
when combined, these results suggest that SPAG9 may induce
osteosarcoma cell proliferation and invasion through JNK-JunD
signaling. Furthermore, a previous study reported that inhibition
of JNK signaling facilitates flavonoid-induced apoptosis in
osteosarcoma cells via downregulation of JunD, indicating that JunD
functions as an oncoprotein during osteosarcoma progression
(36).
In conclusion, the present study established that
SPAG9 is overexpressed in human osteosarcoma tissues. The results
demonstrated that SPAG9 facilitated osteosarcoma cell proliferation
and invasion, possibly through regulation of JNK-JunD signaling.
Based on these findings, it may be concluded that SPAG9 is an
important oncogene and may function as a potential therapeutic
target in human osteosarcoma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81271995).
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heare T, Hensley MA and Dell'Orfano S:
Bone tumors: Osteosarcoma and Ewing's sarcoma. Curr Opin Pediatr.
21:365–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levings PP, McGarry SV, Currie TP,
Nickerson DM, McClellan S, Ghivizzani SC, Steindler DA and Gibbs
CP: Expression of an exogenous human Oct-4 promoter identifies
tumor-initiating cells in osteosarcoma. Cancer Res. 69:5648–5655.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujiwara M, Kashima TG, Kunita A, Kii I,
Komura D, Grigoriadis AE, Kudo A, Aburatani H and Fukayama M:
Stable knockdown of S100A4 suppresses cell migration and metastasis
of osteosarcoma. Tumour Biol. 32:611–622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hua Y, Jia X, Sun M, Zheng L, Yin L, Zhang
L and Cai Z: Plasma membrane proteomic analysis of human
osteosarcoma and osteoblastic cells: Revealing NDRG1 as a marker
for osteosarcoma. Tumour Biol. 32:1013–1021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharili AS, Allen S, Smith K, Hargreaves
J, Price J and McGonnell I: Expression of Snail2 in long bone
osteosarcomas correlates with tumour malignancy. Tumour Biol.
32:515–526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simpson AJ, Caballero OL, Jungbluth A,
Chen YT and Old LJ: Cancer/testis antigens, gametogenesis and
cancer. Nat Rev Cancer. 5:615–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ando K, Uemura K, Kuzuya A, Maesako M,
Asada-Utsugi M, Kubota M, Aoyagi N, Yoshioka K, Okawa K, Inoue H,
et al: N-cadherin regulates p38 MAPK signaling via association with
JNK-associated leucine zipper protein: Implications for
neurodegeneration in Alzheimer disease. J Biol Chem. 286:7619–7628.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kashef K, Lee CM, Ha JH, Reddy EP and
Dhanasekaran DN: JNK-interacting leucine zipper protein is a novel
scaffolding protein in the Galpha13 signaling pathway.
Biochemistry. 44:14090–14096. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee CM, Onésime D, Reddy CD, Dhanasekaran
N and Reddy EP: JLP: A scaffolding protein that tethers JNK/p38MAPK
signaling modules and transcription factors. Proc Natl Acad Sci
USA. 99:14189–14194. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nguyen Q, Lee CM, Le A and Reddy EP: JLP
associates with kinesin light chain 1 through a novel leucine
zipper-like domain. J Biol Chem. 280:30185–30191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takaesu G, Kang JS, Bae GU, Yi MJ, Lee CM,
Reddy EP and Krauss RS: Activation of p38alpha/beta MAPK in
myogenesis via binding of the scaffold protein JLP to the cell
surface protein Cdo. J Cell Biol. 175:383–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanojia D, Garg M, Gupta S, Gupta A and
Suri A: Sperm-associated antigen 9, a novel biomarker for early
detection of breast cancer. Cancer Epidemiol Biomarkers Prev.
18:630–639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garg M, Kanojia D, Suri S and Suri A:
Small interfering RNA-mediated down-regulation of SPAG9 inhibits
cervical tumor growth. Cancer. 115:5688–5699. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garg M, Kanojia D, Salhan S, Suri S, Gupta
A, Lohiya NK and Suri A: Sperm-associated antigen 9 is a biomarker
for early cervical carcinoma. Cancer. 115:2671–2683. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garg M, Kanojia D, Suri S, Gupta S, Gupta
A and Suri A: Sperm-associated antigen 9: A novel diagnostic marker
for thyroid cancer. J Clin Endocrinol Metab. 94:4613–4618. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanojia D, Garg M, Gupta S, Gupta A and
Suri A: Sperm-associated antigen 9 is a novel biomarker for
colorectal cancer and is involved in tumor growth and
tumorigenicity. Am J Pathol. 178:1009–1020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schajowicz F, Sissons HA and Sobin LH: The
World Health Organization's histologic classification of bone
tumors. A commentary on the second edition. Cancer. 75:1208–1214.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Dong Q, Miao Y, Fu L, Lin X and
Wang E: Clinical significance and biological roles of SPAG9
overexpression in non-small cell lung cancer. Lung Cancer.
81:266–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garg M, Kanojia D, Khosla A, Dudha N, Sati
S, Chaurasiya D, Jagadish N, Seth A, Kumar R, Gupta S, et al:
Sperm-associated antigen 9 is associated with tumor growth,
migration and invasion in renal cell carcinoma. Cancer Res.
68:8240–8248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie C, Fu L, Liu N and Li Q:
Overexpression of SPAG9 correlates with poor prognosis and tumor
progression in hepatocellular carcinoma. Tumour Biol. 35:7685–7691.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jagadish N, Rana R, Selvi R, Mishra D,
Garg M, Yadav S, Herr JC, Okumura K, Hasegawa A, Koyama K and Suri
A: Characterization of a novel human sperm-associated antigen 9
(SPAG9) having structural homology with c-Jun N-terminal
kinase-interacting protein. Biochem J. 389:73–82. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gantulga D, Tuvshintugs B, Endo Y, Takino
T, Sato H, Murakami S and Yoshioka K: The scaffold protein c-Jun
NH2-terminal kinase-associated leucine zipper protein regulates
cell migration through interaction with the G protein G (alpha 13).
J Biochem. 144:693–700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kashef K, Radhakrishnan R, Lee CM, Reddy
EP and Dhanasekaran DN: Neoplastic transformation induced by the
gep oncogenes involves the scaffold protein JNK-interacting leucine
zipper protein. Neoplasia. 13:358–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miao ZF, Wang ZN, Zhao TT, Xu YY, Wu JH,
Liu XY, Xu H, You Y and Xu HM: Overexpression of SPAG9 in human
gastric cancer is correlated with poor prognosis. Virchows Arch.
467:525–533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Peng Y, Niu H, Wu B, Zhang Y, Zhang
Y, Bai X and He P: SPAG9 is overexpressed in human prostate cancer
and promotes cancer cell proliferation. Tumour Biol. 35:6949–6954.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mehraein-Ghomi F, Kegel SJ, Church DR,
Schmidt JS, Reuter QR, Saphner EL, Basu HS and Wilding G: Targeting
androgen receptor and JunD interaction for prevention of prostate
cancer progression. Prostate. 74:792–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ouafik L, Berenguer-Daize C and Berthois
Y: Adrenomedullin promotes cell cycle transit and up-regulates
cyclin D1 protein level in human glioblastoma cells through the
activation of c-Jun/JNK/AP-1 signal transduction pathway. Cell
Signal. 21:597–608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakachi S, Nakazato T, Ishikawa C, Kimura
R, Mann DA, Senba M, Masuzaki H and Mori N: Human T-cell leukemia
virus type 1 tax transactivates the matrix metalloproteinase 7 gene
via JunD/AP-1 signaling. Biochim Biophys Acta. 1813:731–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Byun HJ, Hong IK, Kim E, Jin YJ, Jeoung
DI, Hahn JH, Kim YM, Park SH and Lee H: A splice variant of CD99
increases motility and MMP-9 expression of human breast cancer
cells through the AKT-, ERK- and JNK-dependent AP-1 activation
signaling pathways. J Biol Chem. 281:34833–34847. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim MS, Park MJ, Kim SJ, Lee CH, Yoo H,
Shin SH, Song ES and Lee SH: Emodin suppresses hyaluronic
acid-induced MMP-9 secretion and invasion of glioma cells. Int J
Oncol. 27:839–846. 2005.PubMed/NCBI
|
|
33
|
Dong QZ, Wang Y, Tang ZP, Fu L, Li QC,
Wang ED and Wang EH: Derlin-1 is overexpressed in non-small cell
lung cancer and promotes cancer cell invasion via EGFR-ERK-mediated
up-regulation of MMP-2 and MMP-9. Am J Pathol. 182:954–964. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Shi Q, Yuan TX, Song QL, Zhang Y,
Wei Q, Zhou L, Luo J, Zuo G, Tang M, et al: Matrix
metalloproteinase 9 (MMP-9) in osteosarcoma: Review and
meta-analysis. Clin Chim Acta. 433:225–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim SM, Lee H, Park YS, Lee Y and Seo SW:
ERK5 regulates invasiveness of osteosarcoma by inducing MMP-9. J
Orthop Res. 30:1040–1044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kook SH, Son YO, Jang YS, Lee KY, Lee SA,
Kim BS, Lee HJ and Lee JC: Inhibition of c-Jun N-terminal kinase
sensitizes tumor cells to flavonoid-induced apoptosis through
down-regulation of JunD. Toxicol Appl Pharmacol. 227:468–476. 2008.
View Article : Google Scholar : PubMed/NCBI
|