Introduction
Uterine cervical carcinoma is very frequent and
aggressive in human immunodeficiency virus (HIV)/human papilloma
virus (HPV) doubly infected women, compared with the general
population (1). Although this is
considered to depend on HIV-promoted immune deficiency favouring
high-risk (HR)-HPV persistence in infected women (1), a direct pathogenic link may exist
between HIV and uterine cervical carcinoma. In particular, it was
previously shown that transfection of HR-HPV+ human
epithelial cells with HIV-1 Tat DNA is followed by an increase in
the expression of HPV genes, including that encoding for the
oncoprotein E6 (2). In this context,
other studies indicated that degradation of the cellular
oncosuppressor protein p53 by HPV-E6 prevents the differentiation,
senescence and apoptosis of HPV-infected cells of the cervical
epithelium basal layer (3).
Transfection of HIV DNA mimics HIV infection;
however, one should consider that among the cell types present in
the uterine cervix, only lymphocytes and macrophages permit HIV-1
productive infection (4), and
consequently, the efficient expression of HIV-1 genes such as
Tat.
Notably, previous studies have highlighted that i)
HIV-infected lymphocytes release the HIV-1 Tat protein in a
biologically active form that is able to enter neighboring,
HIV-infected or uninfected cells (5);
ii) extracellular Tat protein plays a key role in the pathogenesis
of HIV-associated malignancies, including Kaposi's sarcoma and
non-Hodgkin lymphoma (5); and iii)
HIV-infected leukocytes infiltrate the uterine cervix (6).
Based on these findings, one could hypothesise that
upon its release by HIV-infected leukocytes infiltrating the
uterine cervix, biologically active HIV-1 Tat protein is taken up
by HR-HPV+ cervical epithelial cells, and that this
could accelerate the progression of uterine cervical carcinoma.
Thus, the aim of the present study was to evaluate
whether extracellular HIV-1 Tat protein enters human uterine
cervical carcinoma cells, and whether this is followed by an
alteration of HPV-E6 or p53 expression in these cells. In addition,
the effect of Tat on the HPV-E7 oncogene and the HPV-linked
p16inhibitor of cyclin-dependent kinase 4a (ink4a) cell
cycle inhibitor (7) was also
evaluated.
Materials and methods
Reagents
Recombinant Tat protein from HIV-1 (subtype B) was
expressed, purified, handled and tested for biological activity as
described earlier (8). The human
cervical cancer cell line SiHa harbouring HR-HPV16 DNA was cultured
according to the supplier's protocol (American Type Culture
Collection, Manassas, VA, USA). Growth medium (Dulbecco's modified
Eagle's medium), supplements, phosphate-buffered saline (PBS)
solution and the monoclonal antibody directed against the E7
protein of HPV16 were obtained from Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Monoclonal antibodies raised
against the E6 protein of HPV16, human p53 (clone DO-1) and human
p16ink4a were acquired from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Anti-β-actin monoclonal antibodies, human
plasma fibronectin (FN), bovine serum albumin (BSA, fraction V),
doxorubicin hydrochloride and the chemicals employed for protein
extraction were purchased from Sigma-Aldrich (St. Louis, MO, USA).
The primers and probes employed for RNA analyses were purchased
from Applied Biosystems (Thermo Fisher Scientific, Inc.).
Cell adhesion, growth and viability
assays
For the adhesion assays, 96-well, flat-bottom,
non-tissue culture-treated polystyrene plates (Invitrogen; Thermo
Fisher Scientific, Inc.) were coated overnight at 4°C with Tat, FN
or BSA. Plates were then rinsed and incubated for 3 h at room
temperature with PBS-1% BSA. A total of 100 µl of the cell
suspension (5×105 cells/ml in serum-free medium) was
added to the wells (in quadruplicate). Plates were incubated for 1
h at 37°C in a 5% CO2 atmosphere and washed with PBS.
The adherent cells were then fixed with 4% paraformaldehyde and
stained with 1% toluidine blue (Sigma-Aldrich). Cell adherence was
quantitated using a microtiter plate reader (Victor 1420;
PerkinElmer, Inc., Waltham, MA, USA) set at 570 nM.
Cell growth or viability assays were performed by
the cell counting method (9) or the
trypan blue cell exclusion test (10), respectively.
Fluorescence microscopy
SiHa cells were plated on chamber slides (Thermo
Fisher Scientific, Inc.), starved in serum-free medium, and then
cultured in complete medium containing Tat or its suspension buffer
(PBS-0.1% BSA). After 30, 60 or 120 min of Tat addition to the
growth medium, the cells were fixed in 2% paraformaldehyde, treated
with permeabilizing solution (BD Biosciences, Franklin Lakes, NJ,
USA), incubated first with rabbit polyclonal affinity-purified
anti-Tat antibodies (dilution, 1:100; catalog no., ANT0041;
Diatheva s.r.l., Fano, Italy) or rabbit immunoglobulin (Ig)G
control antibodies (dilution, 1:100; catalog no., I-5006;
Sigma-Aldrich), then with anti-rabbit fluorescein
isothiocyanate-labeled antibodies (dilution, 1:100; catalog no.,
F-1262; Sigma-Aldrich) and finally stained with
4′,6-diamidino-2-phenylindole (Sigma-Aldrich). Tat uptake and
intracellular distribution were observed and photographed using
fluorescence microscopy.
Intracellular Tat staining and flow
cytometry
SiHa cells were suspended by trypsinization,
incubated for 30 min at 37°C in complete medium containing Tat or
its buffer, washed with cold medium, treated for 5 min with
trypsin-ethylenediaminetetraacetic acid solution (Invitrogen;
Thermo Fisher Scientific, Inc.) to remove cell surface-bound Tat,
fixed for 10 min at 4°C with BD FACS™ lysing solution (BD
Biosciences), exposed for 30 min at 4°C to permeabilizing solution
(BD Biosciences), stained with rabbit polyclonal affinity-purified
anti-Tat antibodies or rabbit IgG control antibodies, and analyzed
by flow cytometry (8).
Polymerase chain reaction (PCR)
Total RNA was extracted from the cells, purified and
used to synthesise complementary (c)DNA as previously described
(9). The reverse-transcribed (RT)
cDNA from repeated, independent experiments was used for
quantitative (q)PCR analysis of HPV-E6 or HPV-E7, according the
TaqMan™ technique (Thermo Fisher Scientific, Inc.).
The primers for HPV16-E6 were: Forward,
5′-AATGTTTCAGGACCCACAGG-3′ and reverse, 5′-TTGTTTGCAGCTCTGTGCAT-3′.
The probe for HPV16-E6 was 5′-AGCGACCCAGAAAGTTACCA-3′. The primers
for HPV16-E7 were: Forward, 5′-CAAGTGTGACTCTACGCTTCGG-3′ and
reverse, 5′-GTGGCCCATTAACAGGTCTTCCAA-3′. The probe for HPV16-E7 was
5′-TGCGTACAAAGCACACACGTAGACATTCGT-3′.
The RT reaction was normalized by amplifying samples
for β-actin. The primers for β-actin were: Forward,
5′-AAGAGCTACGAGCTGCCTGA-3′ and reverse,
5′-TGGAGTTGAAGGTAGTTTCGTG-3′. The probe for β-actin was
5′-CATCACCATTGGCAATGAGCGGT-3′.
Amplification consisted of 20 sec at 50°C, 10 min at
95°C, 15 sec at 95°C and 1 min at 58°C for 45 cycles. The complexes
formed by PCR products and related probes were quantified by the
2-ΔΔCq method (11).
In other experiments, cDNA was amplified using the
SYBR™ Green technique (Thermo Fisher Scientific, Inc.), and the
following oligonucleotide primers derived from the p53 cDNA
sequence: Forward, 5′-TCTGACTGTACCACCATCCACTA-3′ and reverse,
5′CAAACACGCACCTCAAAGC-3′. qPCR was performed using the QuantiFast
SYBR Green PCR kit (Qiagen, Inc., Valencia, CA, USA). Amplification
consisted of 20 sec at 95°C, and 3 sec at 95°C followed by 30 sec
at 60°C for 40 cycles. The reaction was normalized by amplifying
samples for glyceraldehyde-3-phosphate dehydrogenase, which served
as housekeeping gene (9).
PCR data were analyzed with the 7500 Fast System SDS
software version 2.0.5 (Applied Biosystems; Thermo Fisher
Scientific, Inc.).
Western blot analysis
Total proteins were extracted from the cells,
quantified, separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto membranes, which were probed
with the corresponding primary antibody [monoclonal mouse
anti-HPV-E7 (dilution, 1:200; catalog no., MA5-15439; Invitrogen;
Thermo Fisher Scientific, Inc.); monoclonal mouse anti-HPV-E6
(dilution, 1:200; catalog no., sc-460; Santa Cruz Biotechnology,
Inc.); monoclonal mouse anti-p53 (clone DO-1; dilution, 1:100;
catalog no., sc-126; Santa Cruz Biotechnology, Inc.); monoclonal
mouse anti-p16ink4a (dilution, 1:100; catalog no.,
sc-9968; Santa Cruz Biotechnology, Inc.); monoclonal mouse anti-β
actin (dilution, 1:500; catalog no., A5316; Sigma-Aldrich)] and a
specific horseradish peroxidase-conjugated goat anti-mouse
secondary antibody (dilution, 1:2,000; catalog no., sc-2031; Santa
Cruz Biotechnology, Inc.), as described earlier (9). Filters were developed with the use of
the LiteAblot® Plus Enhanced Chemiluminescent Substrate
Sample (EuroClone S.p.A., Pero, Italy), and the intensity of the
bands was quantified by employing the ChemiDoc XRS+ imaging system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean + standard deviation
from 3–4 experiments. Statistical analysis was performed using the
SPSS 15.0 software (SPSS Inc., Chicago, IL, USA). P-values were
determined with the Student's t-test. P<0.05 was considered to
indicate a statistical significant difference.
Results
Initial experiments evaluated whether the HIV-1 Tat
protein could promote the adhesion of human cervical cancer cells,
thus implying a contact between the surface of these tumor cell
types and extracellular Tat. Specifically, SiHa cells were seeded
onto plates coated with recombinant Tat protein, immobilised at
0.1, 1 or 10 µg/ml. The pro-adhesive extracellular matrix molecule
FN was employed as the positive control (12), whereas BSA was used as the negative
control. The results indicated that, when immobilised at 10 µg/ml,
Tat promoted SiHa cell adhesion to the same extent as FN, while 0.1
or 1 µg/ml of Tat had no significant effect (Fig. 1A).
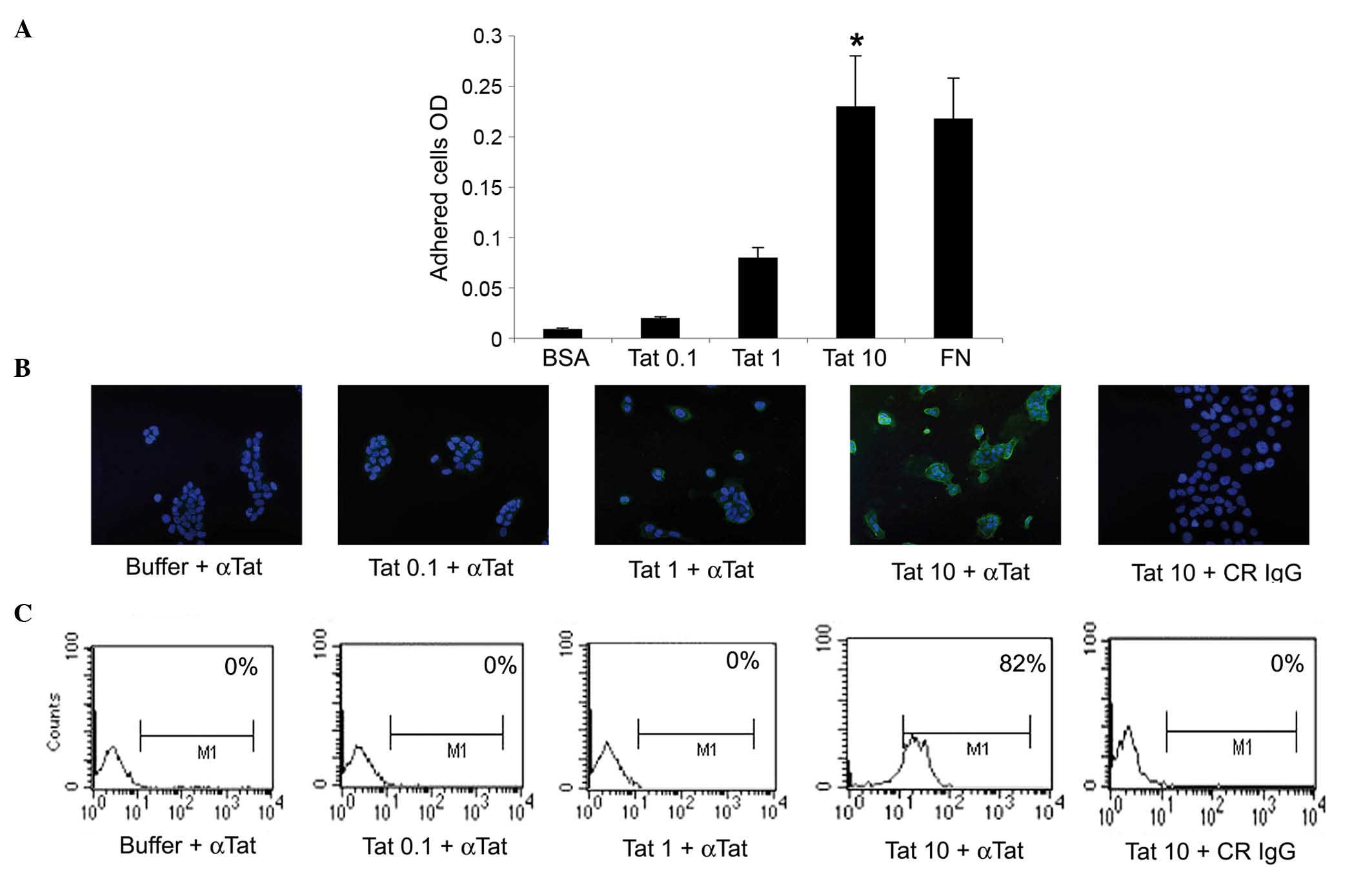 | Figure 1.SiHa cells take up micromolar amounts
of HIV-1 Tat protein. (A) SiHa cells were seeded on plates coated
with 0.1, 1 or 10 µg/ml Tat. BSA or FN concentrations equimolar to
10 µg/ml Tat were employed as the negative or positive control,
respectively. Adherent cells were quantified as described in the
Materials and methods section. Results are expressed as mean
optical density values from three experiments (*P<0.05). (B and
C) SiHa cells were incubated for 30 min in medium containing 0.1, 1
or 10 µg/ml biologically active Tat or its suspension buffer
(phosphate-buffered saline containing 0.1% BSA, which served as the
negative control), and stained with anti-Tat or control antibodies.
(B) Tat intracellular localization was visualized by fluorescence
microscopy and photographed. Blue color corresponds to SiHa cell
nuclei stained with 4′,6-diamidino-2-phenylindole, while green
color indicated intracellular Tat protein, which was revealed as
described in the Materials and methods section. Magnification, ×20.
(C) The intracellular Tat content was evaluated by intracellular
staining and flow cytometry. The percentage of positive cells
(compared with isotype-stained samples) is reported in the boxes.
The data in panels B and C correspond to a representative
experiment. Repeated experiments produced similar results. OD,
optical density; BSA, bovine serum albumin; FN, fibronectin; αTat,
anti-Tat antibody; CR, control; IgG, immunoglublin G; M1, marker
1. |
Considering that cells can internalize the
extracellular-bound molecules onto which they are adhered (13), additional experiments evaluated
whether SiHa cells could take up extracellular HIV-1 Tat protein.
To this end, SiHa cells were exposed to 0.1, 1 or 10 µg/ml of
recombinant, biologically active Tat protein, or to its suspension
buffer (PBS-0.1% BSA), which was employed as the negative control.
The uptake of Tat was then visualized at 30, 60 or 120 min by
fluorescence microscopy. In agreement with the results from the
cell adhesion assays, SiHa cells took up Tat protein only when it
was diluted at 10 µg/ml, this being fully appreciable at 30 min
(Fig. 1B and data not shown). These
findings were confirmed by intracellular staining with anti-Tat or
control antibodies and by flow cytometry (Fig. 1C).
Further assays evaluated whether, following its
internalization, the Tat protein could modulate HPV-E6 expression
by SiHa cells. As shown in Fig. 2A,
SiHa cell exposure to 10 µg/ml Tat increased HPV-E6 RNA levels by
1.54-fold (P=0.02), compared with control cells. In addition, Tat
augmented by 1.35-fold (P=0.04) the content of HPV-E6 protein in
SiHa cells (Fig. 2B).
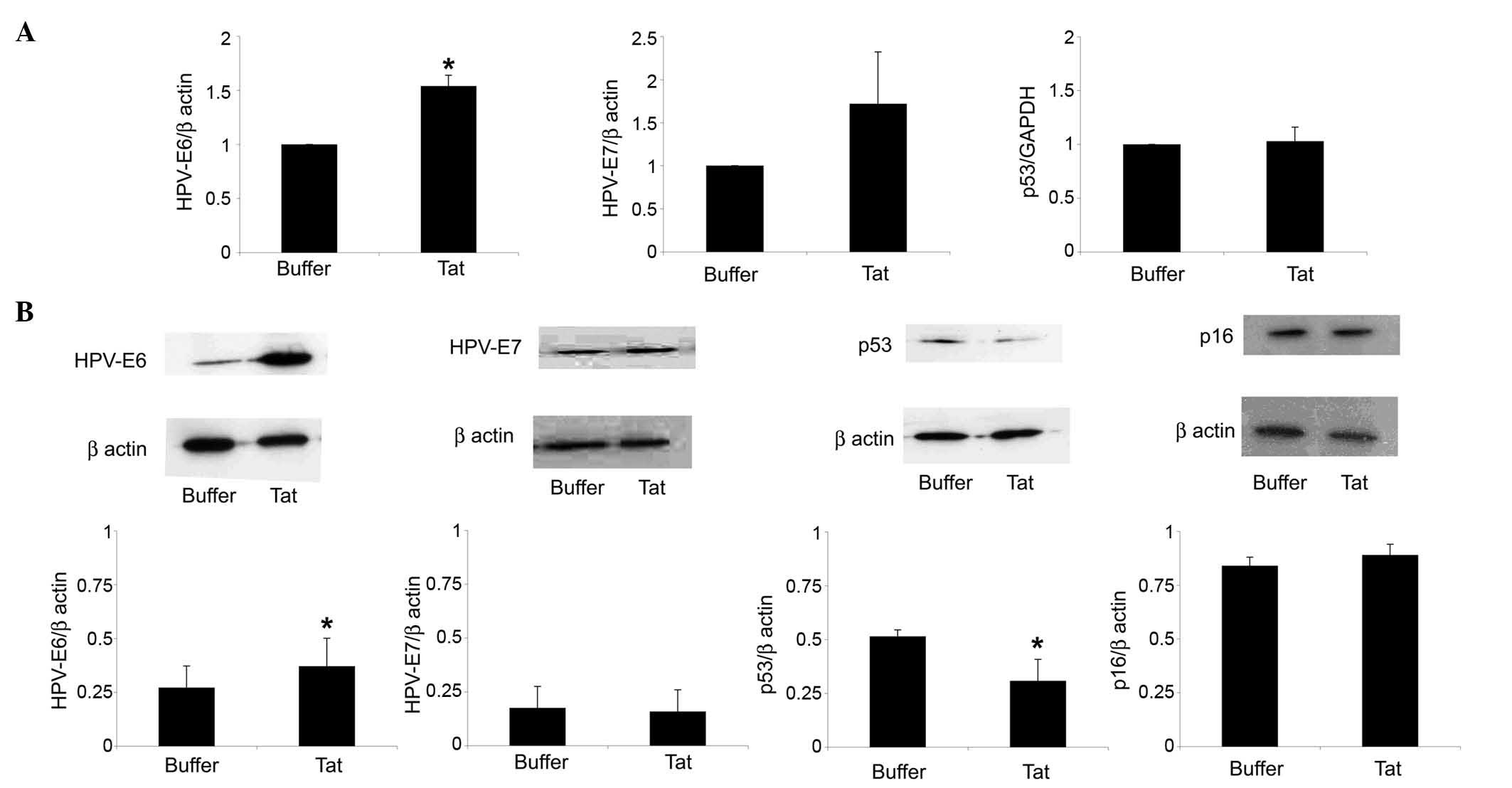 | Figure 2.Uptake of extracellular Tat by SiHa
cells is followed by a variation on HPV-E6 or p53 levels. SiHa
cells were exposed for 48 h to 10 µg/ml Tat or its buffer. (A)
Reverse transcription-quantitative polymerase chain reaction
analysis of HPV-E6 (left panel), HPV-E7 (central panel) or p53
(right panel) RNA levels in SiHa cells cultured in the absence or
presence of Tat. The results refer to the relative HPV-E6, HPV-E7
or p53 expression, normalized to the levels of β-actin or
glyceraldehyde 3-phosphate dehydrogenase, which served as
housekeeping genes. Bars represent the mean ± SD from four
experiments (*P<0.05). (B) Cells were lysed, and equal amounts
of total proteins were electrophoresed and analyzed by western
blotting using a monoclonal antibody directed against the E6 or E7
protein of HPV16, human p53 or human p16ink4a. Blots
were re-probed with anti-β-actin monoclonal antibody to verify
equal loading of protein in each lane. The upper panels are
representative western blots of HPV-E6, HPV-E7, p53,
p16ink4a or β-actin. The lower panels represent the
quantitative (densitometric) analysis of HPV-E6, HPV-E7, p53 or
p16ink4a protein levels (normalized to those of β-actin)
in control or Tat-treated SiHa cells. Bars represent the mean ± SD
from 3–4 experiments (*P<0.05). SD, standard deviation; HPV,
human papilloma virus; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; INK4, inhibitor of cyclin-dependent kinase 4. |
In this context, Tat (compared with its suspension
buffer) upregulated HPV-E7 gene but not protein expression in SiHa
cells; however, these results were not significant (Fig. 2).
The E6 protein of HR-HPV promotes the degradation of
the oncosuppressor protein p53 by engaging the cellular proteasome,
a cytosolic complex of enzymes that regulates the turnover of
intracellular proteins (3). In
agreement with its capability of upregulating E6 expression, Tat
reduced by 40% (P=0.04) the protein content of p53 in SiHa cells,
while it had no effect on p53 gene expression (Fig. 2).
In contrast to the p53 protein, Tat did not modify
the protein levels of the cell cycle inhibitor p16ink4a
in SiHa cells (Fig. 2B). Consistent
with this result, Tat did not increase SiHa cell growth rate,
neither at 48 or 96 h after its addition to the growth medium
(Fig. 3A).
Additional experiments next evaluated whether, in
view of its effect on p53 protein, Tat could reduce SiHa cell
sensitivity to doxorubicin, a drug that promotes the death of
HR-HPV+ cervical cancer cells in a p53-dependent manner
(10).
Preliminary cell viability assays indicated that
doxorubicin was cytostatic at 0.5–1.5 µM, and cytotoxic at 2–5 µM
(data not shown). Based on these data, SiHa cells were first
incubated with Tat protein (using its suspension buffer as
control), and then cultured in the presence or absence of 2 µM
doxorubicin. The results indicated that Tat partially rescued
doxorubicin-promoted SiHa cell death (Fig. 3B, P=0.03).
Discussion
The present study has demonstrated that
extracellular, biologically active HIV-1 Tat protein can enter
human uterine cervical carcinoma cells, and this is followed by an
increase in HPV-E6 protein and RNA levels in the cells. These
findings, which are consistent with previous results obtained with
HIV-1 Tat DNA (2), suggest a possible
link between extracellular Tat protein and the high incidence and
clinical aggressiveness of uterine cervical carcinoma observed in
HIV/HPV doubly infected women (1).
The present results reveal that the upregulation of
HPV-E6 expression promoted by Tat parallels a decrease in the
levels of p53 protein, a potent antagonist of uterine cervical
carcinoma development and progression (3,14). The
lack of any effect on p53 gene expression suggests that Tat reduces
p53 protein levels in SiHa cells only indirectly, via an increase
of E6 which, in turn, will commit p53 to proteasome-mediated
degradation (3).
In addition, it was also observed that, differently
from what occurred for p53, Tat had no effect on the cell cycle
inhibitor p16ink4a. Altogether, these results are
consistent with earlier studies reporting that, in cervical
carcinoma tissues, low p53 and high p16ink4a protein
levels are indicative of HR-HPV intensity of expression and
predictive of tumor clinical progression (15).
Although the in vitro findings described in
the present study were observed at Tat concentrations exceeding
those present in the sera of HIV-infected individuals (5), evidence suggests that in vivo
uterine cervical cells are likely to be exposed to Tat
concentrations higher than its plasma levels. In particular,
earlier studies demonstrated that, in tumor lesions of the uterine
cervix, epithelial cells express intercellular adhesion molecule
(ICAM)-1, a leukocyte-binding cell membrane receptor (16–18). Thus,
due to the presence of ICAM-1 on their surface, uterine cervical
carcinoma cells would closely contact HIV-infected, Tat-releasing
leukocytes.
Notably, inflammation of the uterine cervix is
frequent in HIV-infected women (14,19,20). In
this context, the capability that inflammatory mediators have to
induce ICAM-1 appearance on epithelial cell surface (21,22) could
favour the adhesion of HIV-infected leukocytes to the epithelium of
the uterine cervix, thus facilitating Tat uptake by cervical
epithelial cells prior to their transformation into carcinoma
cells.
The present study has demonstrated that
extracellular HIV-1 Tat protein is able to enter human uterine
cervical carcinoma cells, and that this is followed by upregulation
of tumorigenic HPV-E6 and downregulation of anti-tumorigenic p53.
Future work will evaluate whether the same events occur when
cervical carcinoma cells are co-cultured with HIV-acutely infected,
Tat-releasing lymphocytes. In addition, a clinical-epidemiological
survey will assess whether the presence of anti-Tat antibodies is
associated with a reduced incidence and/or delayed progression of
uterine cervical carcinoma in HIV/HPV doubly infected women.
Acknowledgements
The present study was supported by grants from the
Italian Ministry of Health (Rome, Italy) to B.E. and G.B. (grant
no., OR/70DF), and from the Italian Ministry of University and
Research (Rome, Italy) to G.B. (grant no., RSA/0906). The authors
would like to thank P. Arciero (National Institute of Health, Rome,
Italy) for technical help.
References
|
1
|
Kang M and Cu-Uvin S: Association of HIV
viral load and CD4 cell count with human papillomavirus detection
and clearance in HIV-infected women initiating highly active
antiretroviral therapy. HIV Med. 13:372–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim RH, Yochim JM, Kang MK, Shin KH,
Christensen R and Park NH: HIV-1 Tat enhances replicative potential
of human oral keratinocytes harboring HPV-16 genome. Int J Oncol.
33:777–782. 2008.PubMed/NCBI
|
|
3
|
Pol SB Vande and Klingelhutz AJ:
Papillomavirus E6 oncoproteins. Virology. 445:115–137. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen R, Richter HE and Smith PD: Early
HIV-1 target cells in human vaginal and ectocervical mucosa. Am J
Reprod Immunol. 65:261–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barillari G and Ensoli B: Angiogenic
effects of extracellular HIV-1 Tat protein and its role in the
pathogenesis of AIDS-associated Kaposi's sarcoma. Clin Microbiol
Rev. 15:310–326. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Henning TR, Kissinger P, Lacour N,
Meyaski-Schluter M, Clark R and Amedee AM: Elevated cervical white
blood cell infiltrate is associated with genital HIV detection in a
longitudinal cohort of antiretroviral therapy-adherent women. J
Infect Dis. 202:1543–1552. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calil LN, Edelweiss MI, Meurer L, Igansi
CN and Bozzetti MC: p16 INK4a and Ki67 expression in normal,
dysplastic and neoplastic uterine cervical epithelium and human
papillomavirus (HPV) infection. Pathol Res Pract. 210:482–487.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fanales-Belasio E, Moretti S, Nappi F,
Barillari G, Micheletti F, Cafaro A and Ensoli B: Native HIV-1 Tat
protein targets monocyte-derived dendritic cells and enhances their
maturation, function, and antigen-specific T cell responses. J
Immunol. 168:197–206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barillari G, Iovane A, Bacigalupo I,
Palladino C, Bellino S, Leone P, Monini P and Ensoli B: Ritonavir
or saquinavir impairs the invasion of cervical intraepithelial
neoplasia cells via a reduction of MMP expression and activity.
AIDS. 26:909–919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suh DS, Kim SC, An WG, Lee CH, Choi KU,
Song JM, Jung JS, Lee KS and Yoon MS: Differential apoptotic
response in HPV-infected cancer cells of the uterine cervix after
doxorubicin treatment. Oncol Rep. 23:751–756. 2010.PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bachman H, Nicosia J, Dysart M and Barker
TH: Utilizing fibronectin integrin-binding specificity to control
cellular responses. Adv Wound Care (New Rochelle). 4:501–511. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Engelholm LH, List K, Netzel-Arnett S,
Cukierman E, Mitola DJ, Aaronson H, Kjøller L, Larsen JK, Yamada
KM, Strickland DK, et al: uPARAP/Endo180 is essential for cellular
uptake of collagen and promotes fibroblast collagen adhesion. J
Cell Biol. 160:1009–1015. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mwakigonja AR, Torres LM, Mwakyoma HA and
Kaaya EE: Cervical cytological changes in HIV-infected patients
attending care and treatment clinic at muhimbili national hospital,
Dar es salaam, Tanzania. Infect Agent Cancer. 7:32012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conesa-Zamora P, Doménech-Peris A,
Orantes-Casado FJ, Ortiz-Reina S, Sahuquillo-Frías L, Acosta-Ortega
J, García-Solano J and Pérez-Guillermo M: Effect of human
papillomavirus on cell cycle-related proteins p16, Ki-67, Cyclin
D1, p53 and ProEx C in precursor lesions of cervical carcinoma: A
tissue microarray study. Am J Clin Pathol. 132:378–390. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Coleman N, Greenfield IM, Hare J,
Kruger-Gray H, Chain BM and Stanley MA: Characterization and
functional analysis of the expression of intercellular adhesion
molecule-1 in human papillomavirus-related disease of cervical
keratinocytes. Am J Pathol. 143:355–367. 1993.PubMed/NCBI
|
|
17
|
Guo J, Si L and Wang Y: An in situ study
on immunostimulatory molecules in cancer cells within the cervical
carcinoma tissues. Zhonghua Yi Xue Za Zhi. 80:342–345. 2000.(In
Chinese). PubMed/NCBI
|
|
18
|
Chancey CJ, Khanna KV, Seegers JF, Zhang
GW, Hildreth J, Langan A and Markham RB: Lactobacilli-expressed
single-chain variable fragment (scFv) specific for intercellular
adhesion molecule 1 (ICAM-1) blocks cell-associated HIV-1
transmission across a cervical epithelial monolayer. J Immunol.
176:5627–5636. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nkwanyana NN, Gumbi PP, Roberts L, Denny
L, Hanekom W, Soares A, Allan B, Williamson AL, Coetzee D, Olivier
AJ, et al: Impact of human immunodeficiency virus 1 infection and
inflammation on the composition and yield of cervical mononuclear
cells in the female genital tract. Immunology. 128(Suppl 1):
e746–e757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mitchell C, Balkus JE, McKernan-Mullin J,
Cohn SE, Luque AE, Mwachari C, Cohen CR, Coombs R, Frenkel LM and
Hitti J: Associations between genital tract infections, genital
tract inflammation, and cervical cytobrush HIV-1 DNA in US versus
Kenyan women. J Acquir Immune Defic Syndr. 62:143–148. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie M, Hu A, Luo Y, Sun W, Hu X and Tang
S: Interleukin-4 and melatonin ameliorate high glucose and
interleukin-1β stimulated inflammatory reaction in human retinal
endothelial cells and retinal pigment epithelial cells. Mol Vis.
20:921–928. 2014.PubMed/NCBI
|
|
22
|
Thichanpiang P and Wongprasert K: Green
tea polyphenol epigallocatechin-3-gallate attenuates TNF-α-induced
intercellular adhesion molecule-1 expression and monocyte adhesion
to retinal pigment epithelial cells. Am J Chin Med. 43:103–119.
2015. View Article : Google Scholar : PubMed/NCBI
|

















