Introduction
Melanomas are aggressive malignant melanocytic
tumors, whereas nevi are benign tumors and far more frequent than
melanomas. The main effective therapy of this skin cancer is early
and sufficiently large surgical removal of the primary lesion.
Histopathological examination is the gold standard to discriminate
a malignant melanoma from a benign nevus; however, morphologic
criteria are not always consensual enough to provide a good inter-
and intra-observer reproducibility. As diagnostic failure may pose
medical, psychological and medico-legal problems, there is a
requirement for ancillary diagnostic tools to aid pathologists to
accurately classify melanocytic tumors (1–9).
A major difference between nevi and melanomas is the
presence of numerous and recurrent chromosomal imbalances in
melanomas (10–15), which are rare in nevi, with the
exception of various particular types, including Harvey rat
sarcoma viral oncogene homolog (HRAS) gain (11p15) in certain
Spitz nevi, breast cancer 1 associated protein-1 (ubiquitin
carboxy-terminal hydrolase) (BAP1) loss (3p21.1) in ‘BAPomas’,
neuroblastoma RAS viral oncogene homolog (NRAS) gains
(1p13.2) in certain Spitzoid tumors and heterozygote deletion of
cyclin-dependent kinase inhibitor 2A (CDKN2A) (9p21) in
certain melanocytic dysplastic nevi (10–20).
Molecular cytogenetic methods have been proposed to improve the
distinction between melanoma and nevi using fluorescence in
situ hybridization (FISH) or comparative genomic hybridization
(CGH) and CGH array (21,22).
These two methods can be used with formalin-fixed,
paraffin-embedded samples from routine dermatopathology practice.
FISH requires only a few additional tissue sections compared with
immunohistochemical analysis, and provides a morphological-based
information concerning the copy number of a limited set of
chromosomal loci using an epifluorescence microscope, even in small
samples. By contrast, CGH on chromosomal preparations and CGH array
on DNA microarrays require a larger amount of tissue for DNA
extraction and detection of the copy number changes throughout the
genome, with potential limitation in case of tumor heterogeneity or
small samples, without morphological confrontation.
A so-called ‘melanoma FISH test’ has been proposed
to aid distinguishing between a benign nevus (as a ‘FISH-negative
lesion’) and a malignant melanoma (as a ‘FISH-positive lesion’) on
the basis of a 30 nuclei based-signal count on a single FISH slide
concerning chromosome 6 centromere (CEP6), Ras-responsive
element-binding protein 1 (RREB1) (6p25), MYB (6q23) and
cyclin D1 (CCND1) (11q13) (21,23). In
spite of notable performances, additional studies already argue for
the requirement of additional FISH analyses with other probes, such
as those targeting CDKN2A (9p21) and c-MYC (8q24), to
overcome ‘melanoma FISH test’ false-negative results (24). Melanoma FISH test interpretation
requires complex algorithms and certain level of expertise to avoid
false-positive results, mainly due to polyploidy in nevi, resulting
in copy number gains and FISH so-called ‘favor malignant’ results
(21,24–29).
False-positive results could also result from a biased selection of
only abnormal nuclei, larger than others at different foci in a
lesion, instead of counting all nuclei in a given area, what has
been called ‘cherry picking’ by Busam, who, in a recent review,
mentioned the requirement for novel probe sets testing a broader
number of chromosomal loci to overcome these problems (26).
In the present study, an alternative test to the
classical ‘melanoma FISH test’ is proposed, which is based on the
digital image analysis and detection of intra-chromosomal
imbalances in chromosomes 6, 8, 9 and 11. The present study also
attempted to develop a software-assisted FISH count method and a
semi-quantitative visual approach. In addition, tumor heterogeneity
and its limitation in the detection of chromosomal imbalances is
assessed.
Materials and methods
Case selection
A total of 170 samples from cases analyzed at
Department of Pathology, Brest University Hospital (Brest, France)
were collected between 2010 and 2012. A first set of 62 cases of
non-ambiguous melanoma (47 samples) and nevi (15 samples) with ≥2
mm thickness was selected to be included in tissue microarray (TMA)
blocks, and a second set of 108 other tumors (43 primary melanomas,
27 metastases and 38 nevi) was studied on whole-slide sections on
the basis of digitalized FISH slides. In this second set of tumors,
12 primary melanomas were analyzed in four different areas for each
tumor, and comparisons were made between paired melanoma samples in
10 patients with metastatic melanomas. The pathological data of the
two sets of tumors are summarized in Table I.
 | Table I.Histological subtypes of the two sets
of tumors. |
Table I.
Histological subtypes of the two sets
of tumors.
| Tumor
characteristics | First set (62
TMA-included tumors) | Second set (108
TMA-included tumors) |
|---|
| Primary
melanomas | 47 | 43 |
|
Superficial spreading | 16 | 16 |
|
Nodular | 24 | 8 |
| Acral
lentiginous | 3 | 1 |
| Lentigo
maligna | 4 | 8 |
|
Desmoplastic | 0 | 4 |
|
Mucosal | 0 | 2 |
|
Unclassifiable | 0 | 3 |
| Nevi | 15 | 38 |
|
Junctional | 0 | 8 |
|
Dermal | 5 | 11 |
|
Compound | 7 | 6 |
|
Congenital | 1 | 3 |
|
Reed | 2 | 4 |
|
Spitz | 0 | 1 |
|
Conventional blue | 0 | 3 |
|
Cellular blue | 0 | 2 |
| Metastases | 0 | 27 |
| Lymph
node | 0 | 13 |
|
In-transit metastasis | 0 | 3 |
|
Skin | 0 | 9 |
|
Lung | 0 | 1 |
| Adrenal
gland | 0 | 1 |
All samples were included in a registered tumor
tissue collection, and the study was conducted in compliance with
the Declaration of Helsinki and following approval by the
institutional review board of Brest University Hospital (Brest,
France; approval no. CPP n DC-2008-214), which included written
informed consent obtained from the patients.
TMA
TMA blocks were built using
Tissue-arrayer® (Beecher Instruments Inc., Sun Prairie,
WI, USA). For each case, six tumor cores (0.6-mm diameter) of tumor
were transferred from the selected tumor areas to the recipient
block. Sections of 5 µm were cut on a microtome and transferred to
Superfrost™ glass slides (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The first slides were colored routinely to
attest the presence of tumor cells on a spot. For each tumor
included, only the most tumor cell-rich and best conserved spot was
considered for further FISH analyses. In this manner, the potential
impact of likely intra-tumor heterogeneity was minimized by
analyzing a single and identical tissue area per TMA-included tumor
with the two commercial and bacterial artificial chromosome (BAC)
probes FISH assays.
BAC probes
BAC clones corresponding to the DNA sequences
included in the regions of interest on chromosomes 6, 8, 9 and 11
were selected, according to the information contained in the UCSC
Genome Browser (http://genome.ucsc.edu), and were purchased from the
Children's Hospital Oakland Research Institute (Oakland, CA, USA;
http://bacpac.chori.org). Two BAC clones were
selected for each chromosome, one on the short arm and the other
one on the long arm, and labeled with different fluorochromes to
enable co-hybridization on a single slide. BAC extraction was
conducted as previously described (30). The extracted DNA was labeled with
SpectrumRed™ or SpectrumGreen™ fluorochromes
(Abbott Molecular, Des Plaines, IL, USA) using Nick Translation kit
(Abbott Molecular) following the manufacturer's protocol, and was
precipitated into hybridization buffer. Mapping of each BAC was
validated by FISH analysis on healthy donor metaphases (Table II).
 | Table II.BAC clones used to prepare
fluorescence in situ hybridization probes. |
Table II.
BAC clones used to prepare
fluorescence in situ hybridization probes.
| BAC clone | Chromosomal
locus | Labeling | Probes sets |
|---|
| RP11-61O16 | RREB1 (6p25) | SpectrumRed™ | Chromosome 6 |
| RP11-323N12 | MYB (6q23.3) | SpectrumGreen™ | Chromosome 6 |
| RP11-440N18 | c-MYC (8q24.1) | SpectrumRed™ | Chromosome 8 |
| RP11-1084C20 | POTEA (8p11.1) | SpectrumGreen™ | Chromosome 8 |
| RP11-478M20 | CDKN2A
(9p21.3) | SpectrumRed™ | Chromosome 9 |
| RP11-959B21 | GNAQ (9q21.2) | SpectrumGreen™ | Chromosome 9 |
| RP11-156B3 | CCND1
(11q13.3) | SpectrumRed™ | Chromosome 11 |
| RP11-1007G14 | HRAS (11p15.5) | SpectrumGreen™ | Chromosome 11 |
FISH methods
Five hybridizations were performed in the first set
of TMA-included tumors, one with the Vysis Melanoma FISH Probe kit
(Abbott Molecular) and four with the four BAC probes pairs. The
second set of tumors was studied with BAC probes only. Following
deparaffinization and rehydratation, the slides were pretreated
with Histology FISH Accessory kit (Dako, Glostrup, Denmark)
following the manufacturer's protocol. FISH was performed
with a hybridization automaton (HYBrite; Abbott Molecular). Probes
were placed on the TMA slide, covered with a glass slide and then
sealed with rubber cement (Starkey Chemical Process Co., La Grange,
IL, USA). After co-denaturation at 73°C during 5 min, the probes
and the target DNA were allowed to hybridize at 37°C overnight in a
humid and dark atmosphere. Then, the excess probes and non-specific
hybridizations were eliminated by stringent washing in a bath with
2X saline sodium citrate and NP-40 at 72°C. Slides were assembled
following air-drying in the darkness and counter-coloration with 14
µl 4′,6-diamidino-2-phenylindole (DAPI) solution (Vector
Laboratories Inc., Burlingame, CA, USA). The first set of
TMA-included tumors was read using an epifluorescence microscope at
×1,000 magnification (Zeiss AG, Oberkochen, Germany) with DAPI,
SpectrumGreen™, SpectrumRed™,
SpectrumGold™ and SpectrumAqua™ filters
(Abbott Molecular). The microscope was connected to a
charge-coupled device camera and a software (In Situ Ichtyoplankton
Imaging System version 5.3; MetaSystems Hard & Software GmbH,
Altlußheim, Germany) for analyzing fluorescent signals, either
directly on microscopic examination for the Vysis Melanoma FISH
test or on captured images saved as Joint Photographic Experts
Group files for BAC probes. For the second set of tumors, FISH
slides were scanned using an automated microscope and imaging
software (PathScan® FISH; Excilone, Elancourt, France). Tumor areas
were captured and saved as Tagged Image File Format files using the
PathScan® Vewer software (Excilone). A field of tumor of 0.06
mm2 (equivalent to ×40 magnification) was captured per
slide, with the exception of 12 large primary melanomas, where four
different areas were captured to search for intra-tumor
heterogeneity. FISH analyses, manual and automated, were then
performed on the basis of these images, manually and with the
ImageJ open source image manipulation tool developed by Mr. Wayne
Rasband (National Institutes of Health, Bethesda, MD, USA;
http://rsb.info.nih.gov/ij), which is
widely used for biomedical image processing. Only tumor cell-rich
fields were considered.
Signals counting and FISH
interpretation
Previously developed criteria were used to analyze
the results of Vysis FISH test. A total of 30 melanocytic nuclei
per lesion were directly examined under the microscope. A lesion
was considered to be positive if any of the following criteria was
met: Gain in 6p25 (RREB1, SpectrumRed™) relative to CEP6
(SpectrumAqua™) >55%, gain in 6p25 >29% (>2
signals/nucleus), loss in 6q23 (MYB, SpectrumGold™) relative
to CEP6 >40% or gain in 11q13 (CCND1, SpectrumGreen™)
>38% (21,23).
To analyze BAC-based hybridization, FISH positivity
thresholds were defined on the basis of non-tumor tissue analyses,
and any results beyond these thresholds were considered as
positive. BAC signals were counted independently of the number of
nuclei per picture. Exact signal counts were performed in the first
set of TMA-included tumors and as part of the analyses performed in
the second set of tumors, using the build-in cell counter tool of
ImageJ software to manually point and count every signal within the
image. Additional semi-quantitative visual appreciation of
green/red signal ratios and automated macro-based signal counts
were performed in the second set of tumors and compared with the
reference manual exact count.
Statistical analyses
Statistical analyses were performed using MedCalc
statistical software version 13.2.2 (MedCalc Software bvba, Ostend,
Belgium; http://www.medcalc.org). P<0.05 was
considered to indicate a statistically significant difference.
Bland-Altman plots were used to evaluate the mean difference
between manual counts and macro-based automatic method of signal
counting in a calibration set of 22 slides. Cohen's kappa
coefficient was calculated to evaluate the strength of agreement
between the reference manual count and the macro-based automatic
method of signal counting in the first set of tumors, and between
the reference manual count and the semi-quantitative visual method
in the second set of tumors.
Results
Determination of BAC probes
thresholds
Manually counting red and green signals in 15
non-tumor tissues (normal epidermis and lymph nodes) hybridized
with the four BAC pairs resulted in a mean green/red signal ratio
of 1.0 (ranging from 0.9 to 1.1), with a standard deviation (SD) of
0.05. The threshold values were defined as the extreme (inferior
and superior) values ± 2 × SD. As a result, a green/red signal
ratio <0.8 or >1.2 was considered to reflect
intra-chromosomal imbalance.
Analyses of the first set of
TMA-included tumors with commercial and BAC probes
Within the first set of 62 TMA-included tumors using
the commercial FISH probe test, 45/47 (95.7%) melanomas presented
≥1 positive FISH criterion and 2/47 (4.3%) were FISH-negative.
Among the nevi, 2/15 (13.3%) tumors were FISH-positive and 13/15
(86.7%) were FISH-negative. The 2 FISH-positive nevi presented a
polysomy of chromosome 6 in 40 and 30% of tumor cells,
respectively, with gain of 11q13 signal in 41% (positive criterion)
and 28% (negative criterion) of tumor cells, respectively, thus
reflecting polyploid cells.
With the BAC probes, 47/47 (100.0%) melanomas
presented ≥1 chromosomal imbalance, and only 1 Spitz nevus
presented a chromosome 11 imbalance as a 11p15.5 HRAS
amplification, classically described in a subset of Spitz nevi
(16). The results of the FISH
analyses of the first set of tumors are summarized in Fig. 1.
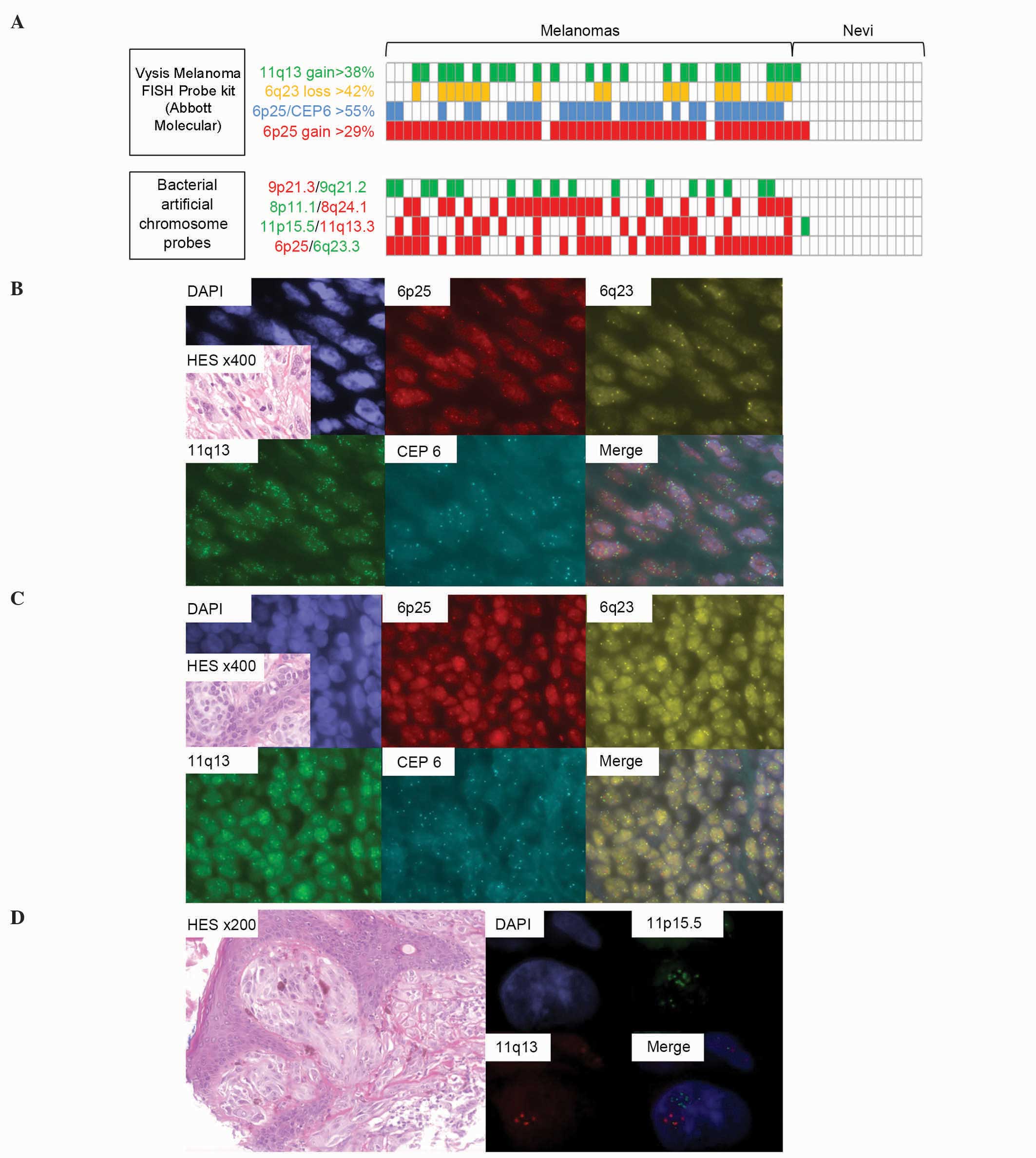 | Figure 1.Results of FISH analyses in the tissue
microarray-included tumors. (A) Summary of positive (colored cases)
and negative (white cases) FISH criteria of the 62 tumors analyzed
with commercial and BAC probes. Cases are represented vertically.
Using BAC probes, red cases indicated a predominance of red
signals, while green cases indicated a predominance of green
signals. (B) Example of a FISH-positive melanoma analyzed with the
commercial FISH probe, which exhibited a gain of 6p25, a loss of
6q23 and an amplification of 11q13 (split channels and merge; ×100
magnification for FISH; ×400 magnification for HES tumor
histological view). (C) Example of a FISH-negative nevus analyzed
with the commercial FISH probe, exhibiting no chromosome 6 or 11
gain or loss (split channels and merge; ×100 magnification for
FISH; ×400 magnification for HES tumor histological view). (D)
Focus on a Spitz nevus cell presenting polysomy of chromosome 11
and 11p15.5 (Harvey rat sarcoma viral oncogene homolog)
amplification with chromosome 11 BAC probes (split channels and
merge; ×100 magnification for FISH; ×200 magnification for HES
tumor histological view). FISH, fluorescence in situ
hybridization; BAC, bacterial artificial chromosome; HES,
hematoxylin and eosin; DAPI, 4′,6-diamidino-2-phenylindole; CEP6,
centromere 6. |
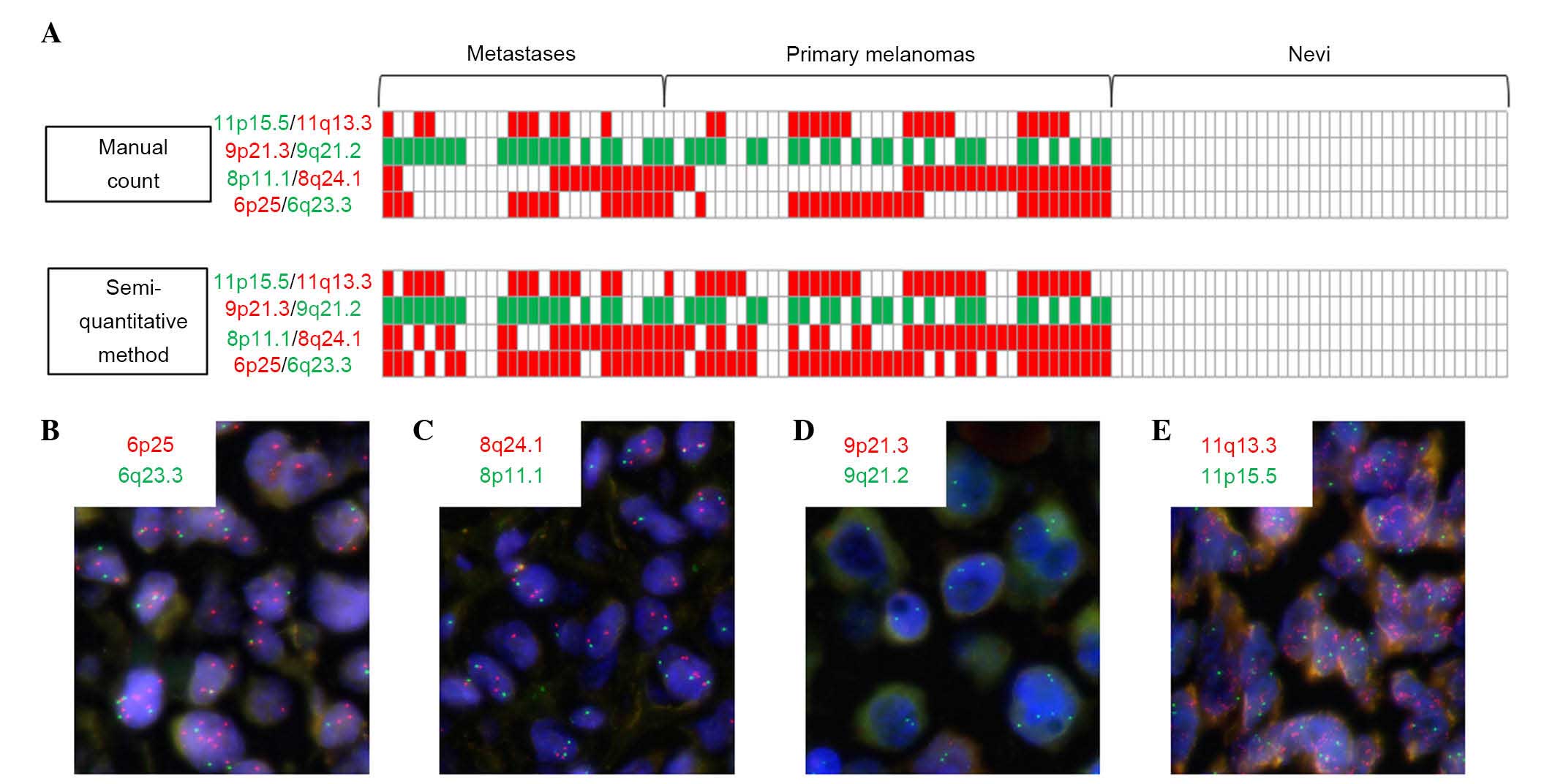
Analyses of the second set of tumors
with BAC probes on digital FISH images
The second set of tumors was analyzed with the four
BAC probes pairs by counting manually the green and red signals to
obtain green/red signal ratios. Of the 70 (7.1%) malignant lesions
(2/43 primary melanomas and 3/27 metastases), 5 did not present an
intra-chromosomal imbalance in chromosomes 6, 8, 9 or 11, whereas
≥1 intra-chromosomal imbalance was encountered in the other
malignant lesions (65/70, 92.9%). No intra-chromosomal imbalance
was observed in any of the 38 nevi. The strength of agreement
between the manual count, considered as reference, and the
automatic or semi-quantitative visual appreciation methods, was
subsequently studied.
A macro-based automatic method of signal counting
was developed on the basis of 22 FISH slides of good quality not
included in the present lesions set. The developed method exhibited
a good correlation in determining a green/red signal ratio in these
22 slides, with a mean difference between the manual count and the
automatic method of 0.000 [95% confidence interval (CI), −0.120 to
0.120]. Searching for intra-chromosomal imbalances in the second
set of tumors using this automatic method compared with the manual
count approach exhibited overall moderate strength of agreement
between the two methods (kappa-value, 0.515; 95% CI, 0.449–0.580).
Consideration of the quality of the digital images in the
interpretation of the results revealed that the agreement between
the manual count and the automatic method was better in
good-quality images than in images with moderate-to-poor quality
(Table III).
 | Table III.Strength of agreement between the
manual count and the alternative automatic count and
semi-quantitative visual methods. |
Table III.
Strength of agreement between the
manual count and the alternative automatic count and
semi-quantitative visual methods.
|
| Kappa-value (95%
CI) |
|---|
|
|
|
|---|
| Variable | Automatic
count | Semi-quantitative
visual method |
|---|
| BAC probes |
|
|
Chromosome 6 | 0.746
(0.627–0.866) | 0.726
(0.605–0.848) |
|
Chromosome 8 | 0.402
(0.277–0.527) | 0.751
(0.631–0.871) |
|
Chromosome 9 | 0.323
(0.189–0.458) | 1.000 |
|
Chromosome 11 | 0.471
(0.341–0.601) | 0.735
(0.609–0.861) |
| Image quality |
|
|
Good | 0.630
(0.536–0.723) | 0.846
(0.776–0.916) |
|
Moderate | 0.384
(0.277–0.492) | 0.846
(0.763–0.929) |
|
Poor | 0.480
(0.336–0.625) | 0.692
(0.557–0.828) |
Using the semi-quantitative visual method, the
strength of agreement was considered to be good-to-very good
(kappa-value, 0.816; 95% CI, 0.765–0.868). Contrary to the
automatic method, the quality of the images did not greatly
influence the strength of agreement with the semi-quantitative
visual method (Table III).
Differences in the results between the manual count and the
semi-quantitative visual method did not modify the global
positivity/negativity of the BAC FISH assay (no result changed
between ‘no chromosomal aberration detected’ and ‘≥1 chromosomal
imbalance detected’), but certain variations consisted of
overestimation of gain of red signals with the semi-quantitative
method in cases of high polysomy encountered in melanoma samples
but not in nevi (Fig. 2). Thus, it
appears that the less numerous polysomic cells frequently
encountered in nevi did not cause error of signal
semi-quantification in the present study.
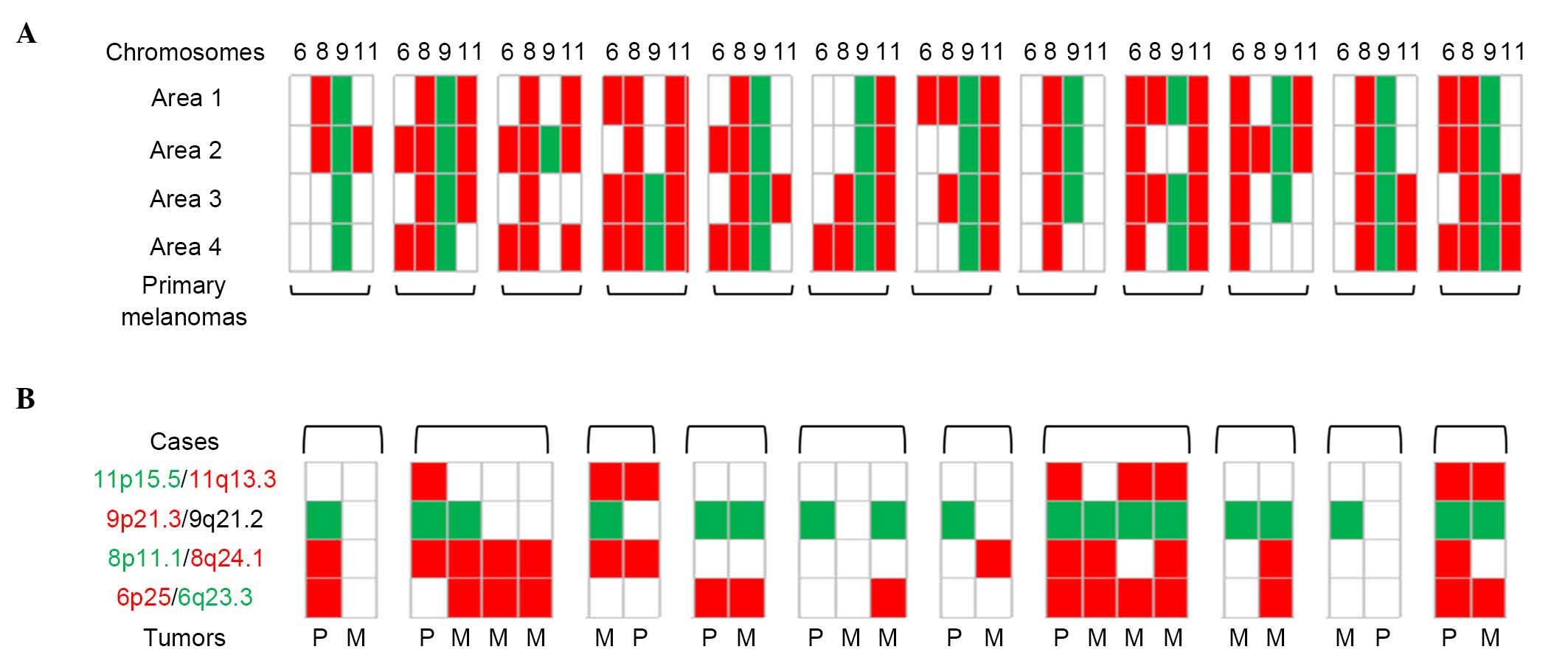
Intra- and inter-tumor
homogeneity/heterogeneity
Manual count of green/red signal ratio was used for
each of the chromosomes 6, 8, 9 and 11 to investigate tumor
heterogeneity within 12 primary melanomas considering four
different areas per tumor, and between paired tumor samples in 10
patients with metastatic melanomas. Intra-tumor heterogeneity was
constant within the 12 primary melanomas, as was inter-tumor
heterogeneity, which was present in 9 of 10 patients with ≥1
difference between two samples (Fig.
3).
Discussion
Previous CGH and CGH array analysis of numerous
melanocytic tumors revealed that melanomas exhibit multiple
chromosomal aberrations, whereas only a minority of benign nevi do,
particularly certain isolated aberrations in specific loci, such as
CDKN2A heterozygote deletion, HRAS gain, NRAS
gain or BAP1 loss (10–20). Those
results enabled the development of targeted FISH based on the most
recurrent unbalanced loci observed in melanomas (chromosomes 6 and
11) (21). Testing unambiguous nevi
demonstrated that nevi may present a mixture of diploid and
tetraploid cells, not revealed in CGH or CGH array studies
(26).
Intra-chromosomal imbalances, particularly when they
are multiple in a same lesion, appear to be more specific of a
malignant melanocytic tumor than polyploidy and whole-chromosome
aneuploidy (12,24,26,29).
However, the commercial FISH test considers both intra-chromosomal
imbalances and whole chromosome gain as malignancy criteria, which
leads to a number of false-positive results (26,29). In
the present study, 2 nevi were considered as ‘malignant’ lesions
using this commercial FISH test, in spite of definitively clinical
and histopathological benign features. Of these 2 lesions, 1 was a
tetraploid nevus that was not concluded as a genomic-unbalanced
lesion with the current eight-probe BAC FISH test, which focuses on
intra-chromosomal imbalances. The other lesion was concluded as a
specific, but benign, Spitz nevus, displaying an isolated
HRAS gain with the current test, whereas the commercial FISH
test concluded as a ‘malignant’ lesion due to tetraploidy appearing
as >2 signals for loci of chromosomes 6 and 11. Such an isolated
HRAS gain is well known as a specific signature of certain
Spitz nevi, but is not considered in the commercial FISH test
(16). This is unexpected, since the
aim of this ancillary cytogenetic analysis is to aid distinguishing
between benignity and malignancy in cases of difficult, ambiguous
melanocytic lesions that include numerous Spitzoid tumors.
Designing the most efficient FISH probe set to
accurately differentiate the majority of nevi from melanomas
remains difficult. Attempts were previously made to modify the
initial FISH test containing RREB1, MYB, CEP6 and
CCND1 probes by a more discriminatory test employing
RREB1, c-MYC, CDKN2A and CCND1 probes.
This latter test reached a sensitivity of 94% and a specificity of
98%, which is more efficient that the 75% sensitivity and 96%
specificity obtained with the former test (24). This improvement is due to a lesser
false-positive rate linked to tetraploidy and the fact that the new
test takes into account four different chromosomes instead of two.
However, FISH test algorithms remain complicated and exposed to
numerous technical artifacts. Visual selection of atypical cells
solely by the FISH reader is a well-known cause of excessive
positivity of the test; this ‘cherry-picking’ behavior can be
overcome using a systematic analysis of every tumor cells in a
given microscopic field (26).
Another limitation consists in the signal count per nucleus.
Artificial signal loss may be due to too-thin tissue sections,
whereas nuclei overlapping in too-thick tissue sections may lead to
false signal gains. The number of nuclei to be analyzed is also a
matter of debate. It is currently recommended to analyze only 30
nuclei, which may be unrepresentative of the whole tumor. However,
analyzing more nuclei, loci and probes, in addition to complex
diagnosis algorithms, markedly complicates the analysis and makes
it ‘time-consuming’. Such limitations indicate the requirement for
an easier interpretable and more automated test than the ones
currently available.
The present eight-probes/four-chromosomes test
presents numerous advantages but remains difficult to automate.
First, contrary to the CGH approach, FISH analysis enables
morphological confrontation, which may be very useful in case of
thin or very inflammatory lesions, where tumor cells are diluted in
non-tumor epidermal or inflammatory cells, or in case of suspicion
of melanoma developed on a pre-existing nevus. This morphological
approach also enables the selection of microscopic fields
containing a high proportion of apparently unbalanced cells. In
addition, working on digital images of these microscopic fields
avoids considering only spare atypical cells in signal counting.
Taking into account only the absolute number of signals per probe
in this given field also overcomes potential artificial losses or
gains of signals caused at the single nucleus level by tissue
section thickness. An attempt to computerized signal counting in
this digital image raises the possibility of rapid analysis of this
multiprobe/multichromosome FISH test. However, software-assisted
counting requires high-quality image files, and as a consequence,
FISH slides with strong signals without background are required.
Such a quality has not been reached in every tumor sample included
in the present study. This highlights a possible limitation of a
fully automated analysis in a routine workflow using samples from
different pathology laboratories with potentially different
fixation protocols and heterogeneous FISH slides quality. However,
besides this fully automated counting, the use of a cell counter
such as the built-in one included in ImageJ already avoids errors
in signal counting compared with direct microscopic observation
counting. A semi-quantitative visual method appears to be also
relevant in the present study, but it could be training- and
observer-dependent, thus requiring a learning curve with
confrontation of exact signal counts.
Due to these FISH limitations, CGH and CGH array may
be considered, which are also partially automated analyses that
explore the whole genome of a given tumor sample with an automated
fluorescence ratio-based analysis, providing an average profile of
chromosomal imbalances of the cells, tumoral or not, contained in
the sample used for DNA extraction (11,22,26).
However, morphological analysis is not permitted by these analyses.
Approximately 30% of cells presenting a given chromosomal imbalance
in the sample is considered as a rational threshold to allow the
detection of this imbalance using CGH-based analysis. Therefore,
limitations of CGH-based analyses are encountered in samples
containing a low ratio of tumor/non-tumor cells, such as
inflammatory, regressive or thin tumors. Cytogenetic intra-tumor
heterogeneity in melanomas could also impair the CGH-based
pangenomic analyses, but, to the best of our knowledge, limited
data are available in the literature (22). In the present study, this
heterogeneity was obvious within and between melanoma samples. In
this manner, we hypothesize that this tumor heterogeneity must be
taken into account in the interpretation of CGH and FISH
analyses.
To conclude, the present study highlighted that FISH
remains an efficient ancillary tool to argue for the malignancy or
benignity of a cutaneous melanocytic lesion. It also pointed out
limitations in FISH analysis associated with technical pitfalls and
tumor biological heterogeneity. Efficient automated image analysis
is difficult to calibrate in a FISH workflow, and visual
quantitative and semi-quantitative approaches remain more
efficient. Besides FISH analysis, CGH-based methods could be
technical alternatives, but caution must be observed in their
interpretation, taking into account the percentage of tumor cells
within the sample and a potential intra-tumor cytogenetic
heterogeneity.
Acknowledgements
The authors would like to thank the ‘Fighting
Melanoma’ association (Paris, France), ‘League Against Cancer’
(Brest, France) and ‘Omnium Group’ (Brest, France) for their
financial support. The authors would also like to acknowledge, for
their technical assistance and valuable advice, Professor Béatrice
Vergier, Professor Jean-Philippe Merlio, Dr Elodie Laharanne and Dr
Martina Prochazkova-Carlotti (EA2406, Histology and Molecular
Pathology of Tumours, University of Bordeaux, Bordeaux, France and
Department of Pathology, Centre Hospitalier Universitaire de
Bordeaux, Hôpital Haut-Lévêque, Pessac, France); Dr Arnaud De la
Fouchardière and Dr Daniel Pissaloux (Department of Biopathology,
Centre Léon Bérard, Lyon, France); Ms. Nadia Guéganic and Ms.
Corine Tous (Department of Cytogenetics and Reproduction, Brest,
France); and the pathologists of Brest, Lorient, Quimper,
Saint-Brieuc and the Brest Hospital Biobank.
References
|
1
|
Cochran AJ, Bailly C, Cook M, Crotty K,
McCarthy S, Mihm M, Mooi W and Sagebiel R: Recommendations for the
reporting of tissues removed as part of the surgical treatment of
cutaneous melanoma. The association of directors of anatomic and
surgical pathology. Am J Clin Pathol. 110:719–722. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Corona R, Mele A, Amini M, De Rosa G,
Coppola G, Piccardi P, Fucci M, Pasquini P and Faraggiana T:
Interobserver variability on the histopathologic diagnosis of
cutaneous melanoma and other pigmented skin lesions. J Clin Oncol.
14:1218–1223. 1996.PubMed/NCBI
|
|
3
|
Farmer ER, Gonin R and Hanna MP:
Discordance in the histopathologic diagnosis of melanoma and
melanocytic nevi between expert pathologists. Hum Pathol.
27:528–531. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jackson R: Malignant melanoma: A review of
75 malpractice cases. Int J Dermatol. 36:497–498. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kempf W, Haeffner AC, Mueller B, Panizzon
RG and Burg G: Experts and gold standards in dermatopathology:
Qualitative and quantitative analysis of the self-assessment slide
seminar at the 17th colloquium of the international society of
dermatopathology. Am J Dermatopathol. 20:478–482. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lodha S, Saggar S, Celebi JT and Silvers
DN: Discordance in the histopathologic diagnosis of difficult
melanocytic neoplasms in the clinical setting. J Cutan Pathol.
35:349–352. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piepkorn MW, Barnhill RL, CannonAlbright
LA, Elder DE, Goldgar DE, Lewis CM, Maize JC, Meyer LJ, Rabkin MS,
Sagebiel RW, et al: A multiobserver, population-based analysis of
histologic dysplasia in melanocytic nevi. J Am Acad Dermatol.
30:707–714. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Veenhuizen KC, De Wit PE, Mooi WJ,
Scheffer E, Verbeek AL and Ruiter DJ: Quality assessment by expert
opinion in melanoma pathology: Experience of the pathology panel of
the Dutch Melanoma working party. J Pathol. 182:266–272. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wakely SL, BaxendineJones JA, Gallagher
PJ, Mullee M and Pickering R: Aberrant diagnoses by individual
surgical pathologists. Am J Surg Pathol. 22:77–82. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balaban G, Herlyn M, Guerry D IV, Bartolo
R, Koprowski H, Clark WH and Nowell PC: Cytogenetics of human
malignant melanoma and premalignant lesions. Cancer Genet
Cytogenet. 11:429–439. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bastian BC, LeBoit PE, Hamm H, Bröcker EB
and Pinkel D: Chromosomal gains and losses in primary cutaneous
melanomas detected by comparative genomic hybridization. Cancer
Res. 58:2170–2175. 1998.PubMed/NCBI
|
|
12
|
Bastian BC, Olshen AB, LeBoit PE and
Pinkel D: Classifying melanocytic tumors based on DNA copy number
changes. Am J Pathol. 163:1765–1770. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cowan JM, Halaban R and Francke U:
Cytogenetic analysis of melanocytes from premalignant nevi and
melanomas. J Natl Cancer Inst. 80:1159–1164. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Limon J, Dal Cin P, Sait SN, Karakousis C
and Sandberg AA: Chromosome changes in metastatic human melanoma.
Cancer Genet Cytogenet. 30:201–211. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parmiter AH and Nowell PC: The
cytogenetics of human malignant melanoma and premalignant lesions.
Cancer Treat Res. 43:47–61. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bastian BC, LeBoit PE and Pinkel D:
Mutations and copy number increase of HRAS in Spitz nevi with
distinctive histopathological features. Am J Pathol. 157:967–972.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dubruc E, Balme B, Dijoud F, Disant F,
Thomas L, Wang Q, Pissaloux D and de la Fouchardiere A: Mutated and
amplified NRAS in a subset of cutaneous melanocytic lesions with
dermal spitzoid morphology: Report of two pediatric cases located
on the ear. J Cutan Pathol. 41:866–872. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park WS, Vortmeyer AO, Pack S, Duray PH,
Böni R, Guerami AA, Emmert-Buck MR, Liotta LA and Zhuang Z: Allelic
deletion at chromosome 9p21(p16) and 17p13(p53) in microdissected
sporadic dysplastic nevus. Hum Pathol. 29:127–130. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sini MC, Manca A, Cossu A, Budroni M,
Botti G, Ascierto PA, Cremona F, Muggiano A, D'Atri S, Casula M, et
al: Molecular alterations at chromosome 9p21 in melanocytic naevi
and melanoma. Br J Dermatol. 158:243–250. 2008.PubMed/NCBI
|
|
20
|
Wiesner T, Murali R, Fried I, Cerroni L,
Busam K, Kutzner H and Bastian BC: A distinct subset of atypical
Spitz tumors is characterized by BRAF mutation and loss of BAP1
expression. Am J Surg Pathol. 36:818–830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gerami P, Jewell SS, Morrison LE, Blondin
B, Schulz J, Ruffalo T, Matushek P IV, Legator M, Jacobson K,
Dalton SR, et al: Fluorescence in situ hybridization (FISH) as an
ancillary diagnostic tool in the diagnosis of melanoma. Am J Surg
Pathol. 33:1146–1156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Rao M, Fang Y, Hameed M, Viale A,
Busam K and Jhanwar SC: A genome-wide high-resolution array-CGH
analysis of cutaneous melanoma and comparison of array-CGH to FISH
in diagnostic evaluation. J Mol Diagn. 15:581–591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vergier B, ProchazkovaCarlotti M, de la
Fouchardière A, Cerroni L, Massi D, De Giorgi V, Bailly C,
Wesselmann U, Karlseladze A, Avril MF, et al: Fluorescence in situ
hybridization, a diagnostic aid in ambiguous melanocytic tumors:
European study of 113 cases. Mod Pathol. 24:613–623. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gerami P, Li G, Pouryazdanparast P,
Blondin B, Beilfuss B, Slenk C, Du J, Guitart J, Jewell S and
Pestova K: A highly specific and discriminatory FISH assay for
distinguishing between benign and malignant melanocytic neoplasms.
Am J Surg Pathol. 36:808–817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boone SL, Busam KJ, Marghoob AA, Fang Y,
Guitart J, Martini M and Gerami P: Two cases of multiple spitz
nevi: Correlating clinical, histologic, and fluorescence in situ
hybridization findings. Arch Dermatol. 147:227–231. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Busam KJ: Molecular pathology of
melanocytic tumors. Semin Diagn Pathol. 30:362–374. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gerami P and Zembowicz A: Update on
fluorescence in situ hybridization in melanoma: State of the art.
Arch Pathol Lab Med. 135:830–837. 2011.PubMed/NCBI
|
|
28
|
Isaac AK, Lertsburapa T, Pathria Mundi J,
Martini M, Guitart J and Gerami P: Polyploidy in spitz nevi: A not
uncommon karyotypic abnormality identifiable by fluorescence in
situ hybridization. Am J Dermatopathol. 32:144–148. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zembowicz A, Yang SE, Kafanas A and Lyle
SR: Correlation between histologic assessment and fluorescence in
situ hybridization using MelanoSITE in evaluation of histologically
ambiguous melanocytic lesions. Arch Pathol Lab Med. 136:1571–1579.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
DeBraekeleer E, DouetGuilbert N, Basinko
A, Morel F, Le Bris MJ, Férec C and De Braekeleer M: Using
bacterial artificial chromosomes in leukemia research: The
experience at the university cytogenetics laboratory in Brest,
France. J Biomed Biotechnol. 2011:3294712011.PubMed/NCBI
|