Introduction
Wilms tumor (WT) is the most common pediatric renal
malignancy, affecting ~7 in 100,000 children (1). Therapy is efficacious with excellent
cure rates, yielding a >90% 5-year overall survival rate
(2). However, the survival times of
patients with high-risk histology nephroblastoma (i.e. blastemal
predominant subtype following chemotherapy or diffuse anaplasia) or
metastatic disease remain limited. Despite significant progress in
developing risk predictors for disease recurrence, current
molecular and epigenetic markers do not allow the reliable
identification and preemptive treatment of all high-risk patients.
Those patients who develop recurrent disease continue to suffer a
50% mortality rate, even after salvage therapy (2). Thus, an improved understanding of WT
tumorigenesis may indeed be useful in identifying new therapeutic
targets, and also more reliable prognostic markers.
Current models of Wilms tumorigenesis stress the
pivotal role of canonical Wnt signaling aberrations in tumor stem
cell development and tumor survival before the eighth week of
gestation (3,4). These ontogenetic models propose a
‘two-hit’ hypothesis wherein disrupted Wnt signaling is the
necessary adjunct to genetic (WT1, CTNNB1,
WTX) (4) and epigenetic [loss
of imprinting (LOI) at chromosome 11p] (2,5,6) aberrations of the tumor stem cell. In
brief, extracellular Wnt ligands trigger a signaling cascade that
culminates in the translocation of cytoplasmic β-catenin into the
nucleus. There, β-catenin induces target genes that cause the cell
to form pre-tubular aggregates (4).
Notably, β-catenin degradation is important for further epithelial
differentiation of the cell into nephron tissue. Should β-catenin
be stabilized in the nucleus, pre-tubular aggregate cells escape
apoptosis and undergo proliferation, while cellular epithelial
transition is fully blocked (4).
Thus, the stabilization of β-catenin in the nucleus is theorized to
be essential in the formation of nephrogenic blastemal rests and
presumed WT stem cells. It is thought to be involved in the
tumorigenesis of all WT, including those without mutations
(4). Alterations in Wnt signaling
have been described in all five subtypes (S1-S5) of a proposed new
ontogenic model of Wilms tumorigenesis, which groups tumors by gene
expression, mutation analysis, methylation analysis and canonical
Wnt activation (3).
Forkhead box (FOX) proteins are DNA-binding factors
that regulate transcription and DNA repair. Their role in
embryogenesis in addition to tumorigenesis has received great
attention (7–9). Notably, FOXM1 has been reported to be a
critical downstream component of Wnt signaling in glioma cells,
where it enhances and stabilizes β-catenin in the nucleus, forming
a transcription/activation complex with β-catenin (10). Previous work suggests that FOXM1 is
essential for maintaining an undifferentiated tumor state, thus
promoting tumorigenesis (8,10). Indeed, spontaneous differentiation of
neuroblastoma cells upon FOXM1 ablation has been
demonstrated (10). Furthermore,
FOXM1 has been implicated in cell migration, invasion, angiogenesis
and metastasis by upregulating vascular endothelial growth factor
and molecules of cell-cycle progression, such as cyclin B1, aurora
kinase B, polo-like kinase 1 and c-Myc (7,8). FOXM1 was
demonstrated to be substantially elevated in numerous types of
human tumor, where it seems to protect cells from senescence and
reactive oxygen species (8,9).
Given the outstanding importance attributed to the
stabilization of β-catenin in the nucleus for Wilms tumorigenesis
(3,4),
as well as the recent discovery that FOXM1 is a downstream
component of Wnt signaling (10), we
hypothesized that FOXM1 is critical in Wilms tumorigenesis and may
affect patient outcome. To test this hypothesis, FOXM1
expression in WT was measured and its impact on outcome was
assessed in the present study.
Materials and methods
Study population
A total of 46 WT specimens were investigated,
following cryopreservation in liquid nitrogen, from patients
undergoing surgical tumor resection at the Department of Pediatric
Surgery of the Dr. von Hauner Children's Hospital (Munich,
Germany). Only one sample per tumor was analyzed, and analysis
included all tumor samples available to us. Likewise, for bilateral
cases, one sample taken from the kidney with the larger tumor mass
was analyzed per patient. The median age at the time of surgery was
37.6 months (range, 0 months to 17 years), with a female:male
gender ratio of 1:1.9. All patients were treated according to the
International Society of Pediatric Oncology (SIOP) protocol
(11) and underwent chemotherapy
prior to surgery. Stage 1 disease was identified in 16 patients
(34.8%). A further 16 patients (34.8%) were found to have bilateral
WT. The control group (n=11) consisted of renal tissue from the
healthy part of the resected specimen following tumor nephrectomy.
There were 3 controls taken from patients with stage 1 disease, and
another 4 from patients suffering bilateral WT. The median age of
the control group was 36.9 months (range, 2–62 months) with a
female:male ratio of 1:1.8 (Table I).
Histological classification of each sample was performed by a
trained pathologist prior to nucleic acid isolation. The study was
approved by the ethics committee of the Ludwig Maximilian
University of Munich. Written consent was obtained from all
parents.
 | Table I.Overview of demographic data. |
Table I.
Overview of demographic data.
| Variable | Tumor tissue | Normal tissue |
|---|
| Number of samples
tested | 46 | 11 |
| Lost to follow-up, n
(%) | 3 (6.5) | 2 (18.2) |
| FOXM1 expression |
|
|
|
Median | 1.21 | 0.12 |
| SD | 1.53 | 0.14 |
| 95%
CI | 1.26–2.18 | 0.09–0.26 |
| Gender |
|
|
| Female,
n (%) | 16 (34.8) | 4 (36.4) |
| Male, n
(%) | 30 (65.2) | 7 (63.6) |
|
Female:male | 1:1.9 | 1:1.8 |
| Age at
operation |
|
|
|
Median | 37.6 months | 36.9 months |
|
Range | 0–17 years | 2–62 months |
| Relapse, n (%) | 5
(11.6) | 1 (11.1) |
| Mortality from
disease, n (%) | 4 (9.3) | 1 (11.1) |
| Histology, n
(%) | n=43 |
|
|
Blastemal | 15 (34.9) | n.a. |
| Diffuse
and focal anaplastic | 2 (4.7) | n.a. |
|
Epithelial | 7
(16.3) | n.a. |
|
Stromal | 2 (4.7) | n.a. |
|
Other | 17 (39.5) | n.a. |
| SIOP stage, n
(%) | n=43 | n=9 |
| 1 | 16 (37.2) | 3
(33.3) |
| 2 | 3 (7.0) | 0 (0.0) |
| 3 | 6
(14.0) | 2
(22.2) |
| 4 | 3 (7.0) | 0 (0.0) |
| 5 | 15 (34.9) | 4
(44.4) |
| Doxorubicin
administered, n (%) | 9
(20.9) | 3
(33.3) |
Reverse transcription
(RT)-quantitative polymerase chain reaction (qPCR)
TRI Reagent® (Sigma-Aldrich, Munich,
Germany) was used for the isolation of total RNA from native
samples according to the supplier's recommendations. Total RNA was
depleted of DNA and subsequently purified using DNase and an RNeasy
Mini Kit, respectively (Qiagen GmbH, Hilden, Germany). RT of total
RNA was performed using random hexamers (Roche Diagnostics,
Penzberg, Germany) and SuperScript II reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Carlsbad, CA, USA).
Subsequently, cDNA was analyzed by qPCR on a Mastercycler
RealPlex2 cycler (Eppendorf, Hamburg, Germany) using
iTaq™ Universal SYBR® Green Supermix (Bio-Rad
Laboratories GmbH, Munich, Germany) and the following primer pairs
(5′-3′ orientation): FOXM1 forward, CTCCCGCAGCATCAAGCAA, and
reverse GCCAGGACGCTGATGGTCTC; TATA box-binding protein (TBP)
forward, GCCCGAAACGCCGAATAT, and reverse, CCGTGGTTCGTGGCTCTCT. In
brief, samples were heated to 95°C for 2 min, and subsequently
processed over 40 cycles at 95, 55 and 68°C for 15, 15 and 20 sec,
respectively. TBP was used as a housekeeping gene to
standardize the amount of sample RNA. Relative quantization of gene
expression was performed using a previously described mathematical
model (12).
Methylation status of the insulin-like
growth factor 2 (IGF2)/H19 locus
Determination of methylation status was performed
using a previously described qPCR-based method (5,6). In brief,
genomic DNA was extracted from native tumor samples using standard
procedures. DNA was subsequently digested with either RsaI,
RsaI+HpaII or MspI for 4 h at 37°C. Restricted
DNA was then amplified using the following primer pairs (5′-3′
orientation): H19DMR forward, GGCCCTAGTGTGAAACCCTTCTCG, and
reverse, CAGGCGGTGAGACCGAAGGA; KvDMR forward, CCCGCTGGGCCAATCT, and
reverse, GAGTCTGGTTTTGATGCCACC. Subsequently, the generated
template DNA was analyzed by qPCR on a Mastercycler
RealPlex2 cycler as described (5). The amount of amplifiable template
remaining after RsaI+HpaII digestion was compared to
that remaining after single RsaI digest, allowing the
percentage of methylation to be estimated. An
RsaI+MspI digest was used as a control for complete
digestion. The percentage of methylated (undigested) DNA was
calculated by dividing the amount of DNA amplified from the
RsaI+HpaII digested sample by that obtained from the
control RsaI digest.
Data analysis
Data analysis was performed using SPSS software
version 21 (IBM SPSS, Armonk, NY, USA). Tests included the
one-sample Kolmogorov-Smirnov test of normal distribution, the
Kruskal-Wallis test and the Mann-Whitney test.
Results
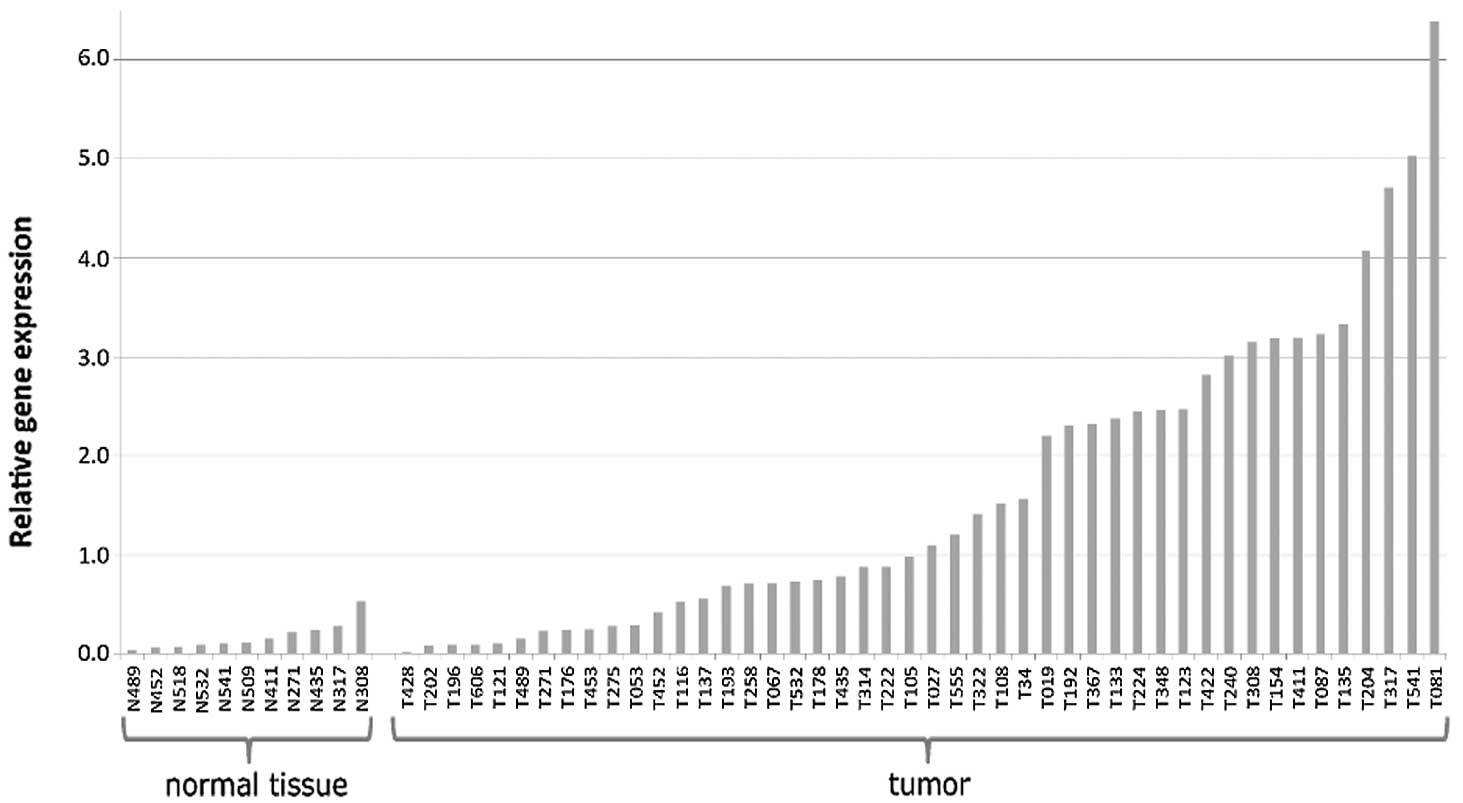
A total of 46 histopathologically confirmed WT
specimens as well as 11 controls of healthy kidney-tissue were
analyzed for FOXM1 expression (Fig. 1). Gene expression did not follow
normal distribution in either group (P<0.0001; one-sample
Kolmogorov-Smirnov test), and was present at highly elevated
FOXM1 levels in the tumors. Overall, a 10-fold elevation in
median FOXM1 expression was detected in the tumor samples as
compared with the controls (1.21 vs. 0.12; P<0.001; Mann-Whitney
U test) (Fig. 1; Table I).
Subsequently, the expression level of tumor cases
was assessed with regard to clinicopathological features, which was
based only on 43 tumor cases due to the loss of follow-up in 3
patients. FOXM1 expression varied greatly according to
histopathological subtype of tumor tissue (Fig. 2A). Blastemal and anaplastic tumors
exhibited the highest median values, with relative FOXM1
expression levels of 2.98 [standard deviation (SD), 1.71; 95%
confidence interval (CI), 1.36–3.35] and 2.34 (SD, 0.06; 95% CI,
1.84–2.84), respectively. Histopathological risk group according to
SIOP (11) were significantly
correlated (P<0.001, Kruskal-Wallis) with FOXM1
expression: Tumors of high-risk histology (blastemal and diffuse
anaplastic histology) exhibited significantly increased median
FOXM1 expression compared with tumors of intermediate risk
(regressive, epithelial, stromal, mixed or focal anaplastic
histology) (2.60 vs. 0.73; P=0.008, Mann-Whitney U) (Fig. 2B). All 4 mortalities occurred in
patients with high-risk histology tumors. Accordingly, a trend
towards higher median transcript levels was identified in those
tumor samples of patients with events (defined as relapse or
mortality from disease); in these, median FOXM1 expression
was 2.82 (SD, 1.33; 95% CI, 1.46–3.80), a >3-fold increase
compared with the median of 0.88 (SD 1.51; 95% CI, 1.49–2.08)
identified in tumors of good outcome (P=0.088; Mann-Whitney test)
(Fig. 2C).
There was a clear, albeit insignificant
(Kruskal-Wallis), trend for increased FOXM1 expression with
increasing tumor spread as indicated by SIOP stage (Fig. 2D). Tumors of stages 1, 3 and 5
exhibited significantly elevated median FOXM1 expression
levels in comparison to normal tissue (P=0.002, P=0.005 and
P=0.002, respectively; Mann-Whitney U; Fig. 2B), with stage 2 not tested due to
insufficient sample size. Stage 4 tumors, all of which were of
regressive pathology, proved an exception to this. Patients in this
group had received preoperative treatment with doxorubicin and the
median FOXM1 expression in this group (0.24; SD, 0.28; 95%
CI, 0.05–0.68) was insignificantly higher than in normal tissue
(0.12; SD, 0.14; 95% CI, 0.09–0.26).
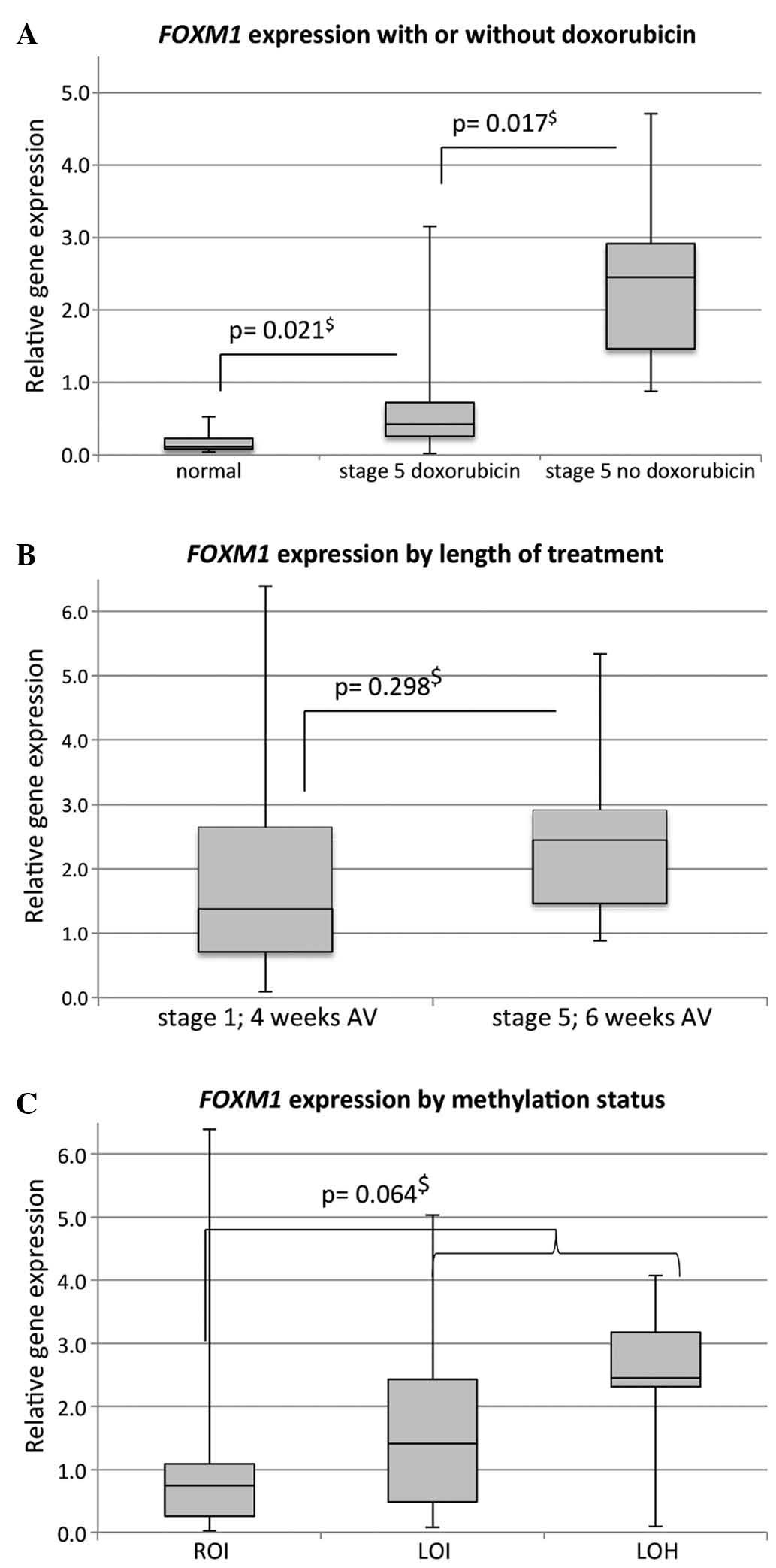
A significant associated between preoperative
therapy with doxorubicin and FOXM1 expression could be
observed (P<0.001, Kruskal-Wallis) (Fig. 3A). Significantly lower median
FOXM1 expression was identified in stage 5 patients who had
received 6 weeks of treatment with actinomycin D, vincristine and
doxorubicin when compared to stage 5 patients who had received 6
weeks of vincristine and actinomycin D only [0.42 (SD, 0.99; 95%
CI, 0.06–1.53) vs. 2.45 (SD, 1.19; 95% CI, 1.52–3.28)]. Notably,
prolongation of treatment alone did not have a significant impact
on median FOXM1 expression when comparing stage 1 tumors
that had received 4 weeks of vincristine and actinomycin D to those
stage 5 tumors that had received 6 weeks of treatment without
doxorubicin [1.39 (SD, 1.63; 95% CI, 1.12–2.72) vs. 2.45 (SD, 1.19;
95% CI, 1.52–3.28)] (Fig. 3B).
Statistical subgroup analysis regarding histopathology and outcome
was not conducted on those 9 patients that received doxorubicin
preoperatively, due to insufficient sample size.
Another known predictor of adverse outcome (5,6), namely
loss of heterozygosity (LOH) and LOI aberrations of the
IGF2/H19 locus, did not correlate with FOXM1
expression (P=0.110; Kruskal-Wallis), although a trend towards
higher FOXM1 expression in tumors with methylation
aberrations exists (P=0.064; Mann-Whitney U) (Fig. 3C).
All other clinical characteristics, including age at
surgery and gender, were not significantly associated with
FOXM1 expression.
Discussion
WT is considered a prime example of differentiation
failure in human neoplasia (4).
Nevertheless, oncogenesis in WT is currently not fully understood.
Despite the importance of genetic and epigenetic changes in Wilms
tumorigenesis (5,6), stabilization of β-catenin in the nucleus
is theorized to be the essential ‘first hit’ in a two-hit
hypothesis describing the formation of nephrogenic blastemal rests
and presumed tumor stem cells (3,4). Recently,
FOXM1 was shown to be a downstream component of Wnt signaling,
stabilizing β-catenin in the nucleus of glioma cells (10). Conversely, ablation of FOXM1 was
demonstrated to be therapeutic in neuroblastoma and glioma models
(8,10), suggesting that, in such tumors, FOXM1
may not only be a potential adjunct biomarker in determining
prognosis, but also a promising future target of chemotherapy.
To the best of our knowledge, the current study was
the first to demonstrate a 10-fold elevation of FOXM1
expression in WT cells as compared with normal kidney tissue. The
fact that no significant correlation was identified between
elevated FOXM1 expression and methylation status at the
differentially methylated region IGF2/H19 may conceivably
support an independent mechanism for such an elevation. Considering
the proposed role of FOXM1 as a downstream component of Wnt
signaling (10), these results
support the ontogenetic models proposed by Pode-Shakked and Dekel
(4) and Gadd et al (3), respectively.
Despite recent advances in genomic and epigenetic
analysis, tumor histology is still recognized as the most powerful
prognostic factor determining survival in pretreated WT (13). Undifferentiated tumor state, as
observed in diffuse anaplastic and blastemal WT, is associated with
decreased survival and relapse (13).
Previous studies assumed that, by stabilizing β-catenin in the
nucleus, undifferentiated tumor state is maintained in WT (3,4); work by
Zhang et al (10) on glioma
furthermore suggested that this may be a consequence of elevated
FOXM1 expression. Thus, we hypothesized that FOXM1
expression would be highest in those tumors of undifferentiated
histology and thus adverse clinical outcome. In line with this
hypothesis, significantly elevated FOXM1 expression levels
were observed in tumors with blastemal and diffuse, as well as
focal anaplastic, histopathology. Likewise, a trend towards
decreased survival and increased incidence of relapse was revealed
in those tumors of highest FOXM1 expression, possibly
reflecting the association of FOXM1 and histopathological
subtype.
In agreement with previous data showing inhibition
of FOXM1 by doxorubicin (14),
it was demonstrated in vivo that FOXM1 expression was
low in tumors pretreated with doxorubicin (P=0.009; Mann-Whitney
U). While the precise mechanism of doxorubicin action on
FOXM1 is currently not understood, its proposed mechanism of
action is via FOXO (14).
Besides regulating cell proliferation, differentiation, DNA damage
repair and apoptosis, FOXO is a known inhibitor of
FOXM1 expression (14).
Indeed, a diverse spectrum of anticancer drugs, including
paclitaxel, lapatinib, gefitinib, imatinib and cisplatin, have been
shown to derive their anticancer properties from acting on the
FOXO/FOXM1 axis (14).
As a hypothesis-generating study, this work has some
limitations that merit discussion, not the least of which is the
comparatively small number of samples analyzed and the concomitant
unusual distribution of gender. While this may limit the extent to
which definite conclusions may be drawn from the work herein
presented, it is certainly a motivation for future studies
including a larger number of cases and ideally also samples of
tumors resected by primary nephrectomy. This study has demonstrated
that FOXM1 expression is suppressed by chemotherapy. While
samples harvested at primary nephrectomy were unavailable for the
present study under the current SIOP protocol, the requirement for
assessing non-pretreated tissue for FOXM1 expression is one
further argument favoring the extensive inter-institutional
collaboration necessary in advancing the understanding of Wilms
tumorigenesis.
In conclusion, the present data demonstrated
significantly elevated FOXM1 expression levels in WT of
high-risk histology. Based on two current models of WT ontogenesis
(3,4)
as well as a glioma model (10), this
may indeed reflect a causative association, where high FOXM1
expression determines undifferentiated tumor state by stabilizing
β-catenin in the nucleus. The present data on the association of
FOXM1 expression with prognosis may be interpreted as a
reflection of this. Notably, the current study was the first to
demonstrate in vivo that pretreatment with doxorubicin
significantly reduces FOXM1 expression independently of the
increase in treatment length. Whether this latter observation has
any future impact on improving outcome by targeting FOXM1 in
those patients of high-risk histology remains the subject of
studies to come.
Glossary
Abbreviations
Abbreviations:
|
WT
|
Wilms tumor
|
|
SD
|
standard deviation
|
|
CI
|
confidence interval
|
|
SIOP
|
International Society of Pediatric
Oncology
|
References
|
1
|
Davidoff AM: Wilms tumor. Adv Pediatr.
59:247–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dome JS, Fernandez CV, Mullen EA,
Kalapurakal JA, Geller JI, Huff V, Gratias EJ, Dix DB, Ehrlich PF,
Khanna G, et al: Children's oncology group's 2013 blueprint for
research: Renal tumors. Pediatr Blood Cancer. 60:994–1000. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gadd S, Huff V, Huang CC, Ruteshouser EC,
Dome JS, Grundy PE, Breslow N, Jennings L, Green DM, Beckwith JB
and Perlman EJ: Clinically relevant subsets identified by gene
expression patterns support a revised ontogenic model of Wilms
tumor: A children's oncology group study. Neoplasia. 14:742–756.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
PodeShakked N and Dekel B: Wilms tumor-a
renal stem cell malignancy? Pediatr Nephrol. 26:1535–1543. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hubertus J, Lacher M, Rottenkolber M,
Müller-Höcker J, Berger M, Stehr M, von Schweinitz D and Kappler R:
Altered expression of imprinted genes in Wilms tumors. Oncol Rep.
25:817–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hubertus J, Zitzmann F, Trippel F,
Müller-Höcker J, Stehr M, von Schweinitz D and Kappler R: Selective
methylation of CpGs at regulatory binding sites controls NNAT
expression in Wilms tumors. PLoS One. 8:e676052013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katoh M, Igarashi M, Fukuda H, Nakagama H
and Katoh M: Cancer genetics and genomics of human FOX family
genes. Cancer Lett. 328:198–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Halasi M and Gartel AL: Targeting FOXM1 in
cancer. Biochem Pharmacol. 85:644–652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong A and Huang S: FoxM1 and
Wnt/β-catenin signaling in glioma stem cells. Cancer Res.
72:5658–5662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang N, Wei P, Gong A, Chiu WT, Lee HT,
Colman H, Huang H, Xue J, Liu M, Wang Y, et al: FoxM1 promotes
β-catenin nuclear localization and controls Wnt target-gene
expression and glioma tumorigenesis. Cancer Cell. 20:427–442. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
deKraker J, Graf N, van Tinteren H, Pein
F, Sandstedt B, Godzinski J and Tournade MF: SIOP: Reduction of
postoperative chemotherapy in children with stage I
intermediate-risk and anaplastic Wilms' tumour (SIOP 93-01 trial):
A randomised controlled trial. Lancet. 364:1229–1235. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vokuhl C, Vogelgesang W, Leuschner I,
Furtwängler R, Graf N, Gessler M, Dörner E and Pietsch T: 1q gain
is a frequent finding in preoperatively treated Wilms tumors, but
of limited prognostic value for risk stratification in the
SIOP2001/GPOH trial. Genes Chromosomes Cancer. 53:960–962. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilson MS, Brosens JJ, Schwenen HD and Lam
EW: FOXO and FOXM1 in cancer: The FOXO-FOXM1 axis shapes the
outcome of cancer chemotherapy. Curr Drug Targets. 12:1256–1266.
2011. View Article : Google Scholar : PubMed/NCBI
|