Introduction
Tumor neovascularization is a complex process that
plays a crucial role in the development of several types of cancer.
The mechanism of hematogenous metastasis requires newly formed
capillaries and overexpression of ≥1 positive regulators of
angiogenesis such as vascular endothelial growth factor (VEGF)
(1,2).
Previous studies reported the existence of another angiogenic
mitogen called endocrine gland-derived VEGF (EG-VEGF), which
selectively acts on the endothelium of endocrine gland cells
(3–5).
Both angiogenic proteins have been identified in a variety of
tissues, and are overexpressed in various cancers (6–12). Every
year, >1,000,000 women are diagnosed with breast cancer, which
is the first cause of mortality in females (13). In the USA, the incidence has been
estimated at 12.3%, and in Mexico, at 11.34%, according to the
National Cancer Institute of Mexico (13,14). Since
breast carcinoma may be asymptomatic and clinically undetectable,
numerous women are diagnosed with distant metastasis to the liver,
lungs, bones and brain, according to the American Cancer Society
(http://www.cancer.org), Centers for Disease
Control and Prevention (http://www.cdc.gov/cancer/breast/statistics) and
Cancer Research UK (http://www.cancerresearchuk.org/cancer-statistics/statistics)
(15–17). The expression of estrogen receptors
(ERs), progesterone receptors (PRs) and human epidermal growth
factor receptor 2 (HER-2/neu) are among the most prominent
predictive and prognostic factors in breast cancer. Chemotherapy is
the main treatment modality (17);
however, resistance to drugs is inherent in certain cases and
acquired during treatment in others (18). The resistance of malignant cells is
often the result of the overexpression of specific members of the
adenosine triphosphate-binding cassette family of transporters,
which actively export cytotoxic drugs out of the tumor cell, thus
preventing cell death (18). One of
the members of this family is breast cancer resistance protein
(BCRP) (19,20). The mechanisms of BCRP regulation
involve diverse factors such as hypoxia and steroid hormones
(21–23).
The messenger RNA (mRNA) and/or protein expression
of BCRP has been detected in numerous types of human cancer,
including pancreatic, gastric, renal, hepatocellular, endometrial
and colon carcinoma, as well as melanoma and leukemia (24). In addition, its overexpression has
been observed in placental choriocarcinoma (BeWo) (25) and human breast cancer (MCF-7) cell
lines (26). The aim of the present
study was to assess whether the expression of the angiogenic
factors VEGF, EG-VEGF and its receptor [prokineticin receptor-1
(PROKR1)] in breast samples of infiltrating canalicular carcinoma
(ICC) correlated with tumor staging and could be used as prognosis
factors.
Materials and methods
Reagents
TRIzol reagent was acquired from Ambion (Thermo
Fisher Scientific, Inc., Waltham, MA, USA), while Dulbecco's
modified Eagle's medium-high glucose (DMEM-HG) culture medium,
fetal bovine serum (FBS), oligonucleotides, molecular probes and
secondary antibodies were obtained from Invitrogen (Thermo Fisher
Scientific, Inc.). TaqMan® Reverse Transcription kit,
TaqMan® Universal PCR Master Mix and TaqMan®
probes were purchased from Applied Biosystems (Thermo Fisher
Scientific, Inc.). Capillaries were obtained from Roche Applied
Science (Pleasanton, CA, USA) and primary antibodies from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA) and Sigma Aldrich (St.
Louis, MO, USA). Paraformaldehyde, 4′-6-diamidino-2-phenylindole
dihydrochloride (DAPI), ProLong® Gold antifade reagent
and phosphate-buffered saline (PBS) were acquired from
Sigma-Aldrich.
Patients and tissue collection
Breast carcinomas from 50 patients treated at the
Department of Mammary Tumors, National Cancer Institute (Mexico
City, Mexico) were analyzed. Ethical approval for the present study
protocol was obtained from the Human Research Ethics Committee of
the National Cancer Institute. Written informed consent was
obtained from all subjects prior to tissue collection, and the
study was conducted in accordance with the guidelines stipulated in
the Declaration of Helsinki. Breast carcinoma (n=50) full-thickness
biopsies were obtained during diagnostic procedures between October
2008 and October 2010. A portion of each harvested tissue sample
was immediately frozen to −75°C in liquid N2 to await
RNA extraction. The remaining tissue was either fixed in 4%
paraformaldehyde and analyzed by immunohistochemistry (IHC), or
placed in DMEM-HG supplemented with 10% FBS, 250 mg/l penicillin,
50 mg/l streptomycin and 10−9 M estradiol
(E2) (Steraloids, Inc., Wilton, NH, USA), and
transported to the Department of Reproductive Biology, National
Institute of Medical Sciences and Nutrition Salvador Zubirán
(Mexico City, Mexcio) for in vitro culture. Patient
information was obtained from clinical charts, including age, size
of tumors, clinical presentation, family history and reproductive
factors.
Histology
For all patients, paraffin slides were used for
typing and grading the tumor. Histological grading was performed
according to the Scarff-Bloom-Richardson (SBR) histologic grading
system, which recommends the sum of individual scores for three
variables: i) Percentage of tubule differentiation; ii) degree of
nuclear pleomorphism; and iii) mitotic count within a defined field
area (27). From the total of
samples, 28 were classified as SBR grade 8, 9 as SBR grade 6 and 22
as SBR grade 7.
Primer design
Specific oligonucleotide primers for human VEGF,
EG-VEGF, PROKR1 and BCRP were designed based on published sequences
(Table I). To avoid false positives
due to the amplification of contaminating genomic DNA in the
complementary DNA (cDNA) preparation, all primers were designed to
anneal to exons separated by an intron. Hence, the primers
generated short amplicons (65–100 bp) that crossed an intron/exon
boundary within the PCR fragment. The VEGF, EG-VEGF, PROKR1 and
BCRP amplicons spanned the boundary of exons 7, 1, 2 and 16,
respectively. The primers for glyceraldehyde-3-phosphate
dehydrogenase (GAPDH), which served as control, amplified a region
between exons 2 and 3.
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| Gene | Sequence |
|---|
| VEGF |
|
|
(GenBank accession No.
NM_001025366) | Forward:
5′-GCAGCTTGAGTTAAACGAACG-3′ |
|
| Reverse:
5′-GGTTCCCGAAACCCTGAG-3′ |
| EG-VEGF |
|
|
(GenBank accession No.
NM_32414) | Forward:
5′-CCACGCGAGTCTCAATCA-3′ |
|
| Reverse:
5′-ACTGGACATCCCGCTCAC-3′ |
| PROKR1 |
|
|
(GenBank accession No.
NM_138964) | Forward:
5′-ACCTGCGCACTGTCTCTCTC-3′ |
|
| Reverse:
5′-CTCAGCGGATGGACAATAGC-3′ |
| BCRP |
|
|
(GenBank accession No.
NM_004827) | Forward:
5′-ATTTGGTAAAGCAGGGCATCC-3′ |
|
| Reverse:
5′-CAAGGCCACGTGATTCTT-3′ |
| GAPDH |
|
|
(GenBank accession No.
NM_002046) | Forward:
5′-AGCCACATCGCTGAGACAC-3′ |
|
| Reverse:
5′-GCCCAATACGACCAAATCC-3′ |
Total RNA isolation and qPCR
analysis
Total RNA was isolated from breast tissue using
TRIzol reagent according to the manufacturer's protocol. The
quantity and quality of RNA was determined by measuring the optical
density (OD) at 260 nm. The OD260/OD280 ratio of all RNA samples
was determined to be between 1.7 and 2.0, indicating that the
samples were exceptionally pure. RNA integrity was examined using
1.5% agarose gel electrophoresis with ethidium bromide staining
(data not shown). Single-strand cDNA was synthesized from 3.0 µg
purified total RNA using TaqMan® Reverse Transcription
kit in a reaction volume of 22 µl. RT-qPCR was performed using
TaqMan® Universal PCR Master Mix and in a
LightCycler® 2.0 instrument (Roche Diagnostics GmbH,
Mannheim, Germany). Each reaction mixture included 10 µl 2X
TaqMan® Universal PCR Master Mix, 5.2 µl sterile EMD
Millipore water (Billerica, MA, USA), 0.1 µl forward primer (20
nM), 0.1 µl reverse primer (20 nM), 0.1 µl TaqMan® probe
(10 nM) and 2.5 µl RT products. The PCR cycling conditions included
denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for
10 sec, annealing at 60°C for 30 sec, and extension at 72°C for 10
sec. The sizes of the resulting amplicons were 84, 88, 62, 94 and
66 bp, and the probes utilized were numbered 63, 22, 45, 12 and 60,
respectively, in the Universal ProbeLibrary (Roche Diagnostics
GmbH). All studies were performed at least in duplicate.
Quantification of relative mRNA levels was conducted by determining
the threshold cycle (Cq), which is defined as the cycle at which
the fluorescence emission intensity of the 6-carboxyfluorescein
reporter exceeds the standard deviation of the mean baseline
emission intensity for cycles 3 to 10 by a factor of 10 (25). Normalization of the cDNA load was
performed against the housekeeping gene GAPDH according to the
following formula: Cq (VEGF, EG-VEGF, PROKR1 or BCRP) - Cq (GAPDH)
= ∆Cq.
IHC
Serial sections (5-µm thick) were prepared from
paraffin-embedded tissues, and the sections were deparaffinized in
xylene and then rehydrated through decreasing concentrations of
ethanol. Antigen retrieval was performed by treating the sections
for 10 min in a 0.01 M citrate buffer, and endogenous peroxidase
activity was quenched with 10% (vol/vol)
H2O2/methanol at room temperature. To prevent
the nonspecific binding of antibodies, the sections were
preincubated with protein blocking buffer diluted in PBS/bovine
serum albumin (BSA; 1%; Sigma-Aldrich) for 1 h prior to overnight
incubation at 4°C with the corresponding primary antibody.
Anti-EG-VEGF (A-12; sc-30343; dilution, 1:300), anti-PROKR1
(HPA029396; dilution, 1:300), anti-HER-2/neu (C-18; sc-284;
dilution, 1:100) and anti-cytokeratin-7 (5F282; sc-70936; dilution,
1:100) were used. The slides were washed three times for 5 min in
PBS. Antibody binding was visualized with anti-goat-immunoglobulin
G (IgG)-rhodamine (sc-3945), anti-rabbit-IgG-Alexa
Fluor® 532 (A11009), anti-rabbit-IgG-Alexa
Fluor® 647 (A31573) and anti-mouse-fluorescein
isothiocyanate (sc-2010) secondary antibodies at 1:200 dilution at
4°C for 2 h. Subsequently, the sections were washed and mounted
with ProLong® Gold antifade reagent prior to be
visualized and photographed using a confocal laser scanning
microscope (TCS SP5; Leica Microsystems, Inc., Buffalo Grove, IL,
USA). In each case, negative controls without the primary antibody
were included (data not shown).
EG-VEGF, HER-2/neu and cytokeratin-7
in cell culture
A total of 10 random samples of breast cancer of
various degrees were obtained in order to study cultured cells on
glass chamber slides (Nalge Nunc International; Thermo Fisher
Scientific, Inc.). The cells were maintained in monolayer culture
in DMEM-HG supplemented with 10% FBS, 250 mg/l penicillin, 50 mg/l
streptomycin and 10−9 M E2. The cultures were
incubated at 37°C in a 5% CO2 humidified incubator, and
fresh medium was provided every other day. The cells were washed
twice with PBS and fixed in 4% paraformaldehyde in PBS for 10 min,
followed by a PBS wash and subsequent treatment with 50 mM ammonium
chloride in PBS for 10 min to reduce the auto-fluorescence of
aldehyde groups during immunofluorescence microscopy. The cells
were then washed again with PBS and incubated in permeabilization
buffer (0.2% Triton X-100 in PBS) for 5 min. To prevent the
nonspecific binding of antibodies, the cells were incubated with
blocking buffer containing 10% BSA diluted in PBS for 30 min at
room temperature. Next, the slides were incubated overnight at 4°C
with antibodies against EG-VEGF (gland-derived endothelial cell
marker), HER-2/neu (oncoprotein) and cytokeratin-7 (epithelial
tumor marker) at the aforementioned dilutions.
Following three washes for 5 min each, the slides
were incubated with the corresponding secondary antibody at 1:200
dilution at 25°C for 2 h. The sections were then washed, the nuclei
were counterstained with DAPI, and coverslips were attached.
Digitized images of the same microscopic field were captured using
four specific band-pass filters. The wavelength of the emitted
light are shown in Table II. Images
were obtained using a TCS-SP5 confocal laser scanning microscope
with 20X and 40X objectives, 1.4 oil immersion lens, and identical
exposure times. Simultaneous evaluation of the negative control
(without primary antibody) confirmed the absence of nonspecific
immunofluorescent staining, cross-immunostaining or fluorescence
bleed-through. Representative photomicrographs were processed using
Adobe Photoshop (version CS6; Adobe Systems, Inc., San Jose, CA,
USA) without any further adjustment to maintain the veracity of the
findings.
 | Table II.Antibodies and spectral
characteristics of rhodamine, Alexa Fluor® 532, Alexa
Fluor® 647, FITC and DAPI dyes. |
Table II.
Antibodies and spectral
characteristics of rhodamine, Alexa Fluor® 532, Alexa
Fluor® 647, FITC and DAPI dyes.
| Primary
antibody/dye | Secondary dye | Color | Absa | Ema | Extinction
coefficientb |
|---|
| Anti-EG-VEGF | Rhodamine | Red | 550 | 600 |
91,000 |
| Anti-PROKR1 | Alexa
Fluor® 532 | Yellow | 532 |
553c |
81,000 |
| Anti-HER-2/neu | Alexa
Fluor® 647 | Pink | 650 |
665c | 239,000 |
|
Anti-cytokeratin-7 | FITC | Green | 494 | 518 | >70,000 |
| DAPI | Nucleus
counterstaining | Blue | 350 | 461 | – |
Results
Although breast cancer is a heterogeneous disease,
the present study was conducted exclusively with the ICC histologic
phenotype (17). In accordance with
SBR scoring, there were 22 samples (44%) of grade 6/7 and 28 (56%)
of grade 8/9. The patients had a mean age of 53.4 years (ranging
from 26 to 86 years), and no significant differences were noticed
when comparing this parameter with tumor size. A family history of
breast cancer was recorded for 4 patients, and a history of
relatives with cancer at other sites was recorded for 10 patients.
Clinical staging criteria were based on the tumor-node-metastasis
(TNM) system, which considers the size of the tumor (T), lymph
nodes (N) and metastases (M) (17).
The size of the majority of tumors (32 samples, 64%) was classified
as T2/T3, being >20 and ≤50 mm, while in 16 samples (32%), it
was graded as T4. There were 34 patients (68%) presenting
infiltrated ipsilateral lymph nodes.
Regarding metastasis, 17 patients (34%) were
positive and 33 (66%) negative, according to the computed
tomography scan. Additional data were obtained, including the
status of the receptors for steroid hormones and two oncogenic
markers (Table III). Molecular
studies conducted on the mRNA expression of EG-VEGF and PROKR1
exhibited variable results. Whereas low levels of EG-VEGF were
identified in 28 samples, this protein was undetectable in 22
samples. Although PROKR1 is required for EG-VEGF to exert its
function, the mRNA of this receptor was only detectable in 17
samples, 14 of which were positive for EG-VEGF. To confirm the
expression of both proteins, IHC studies were conducted in tumoral
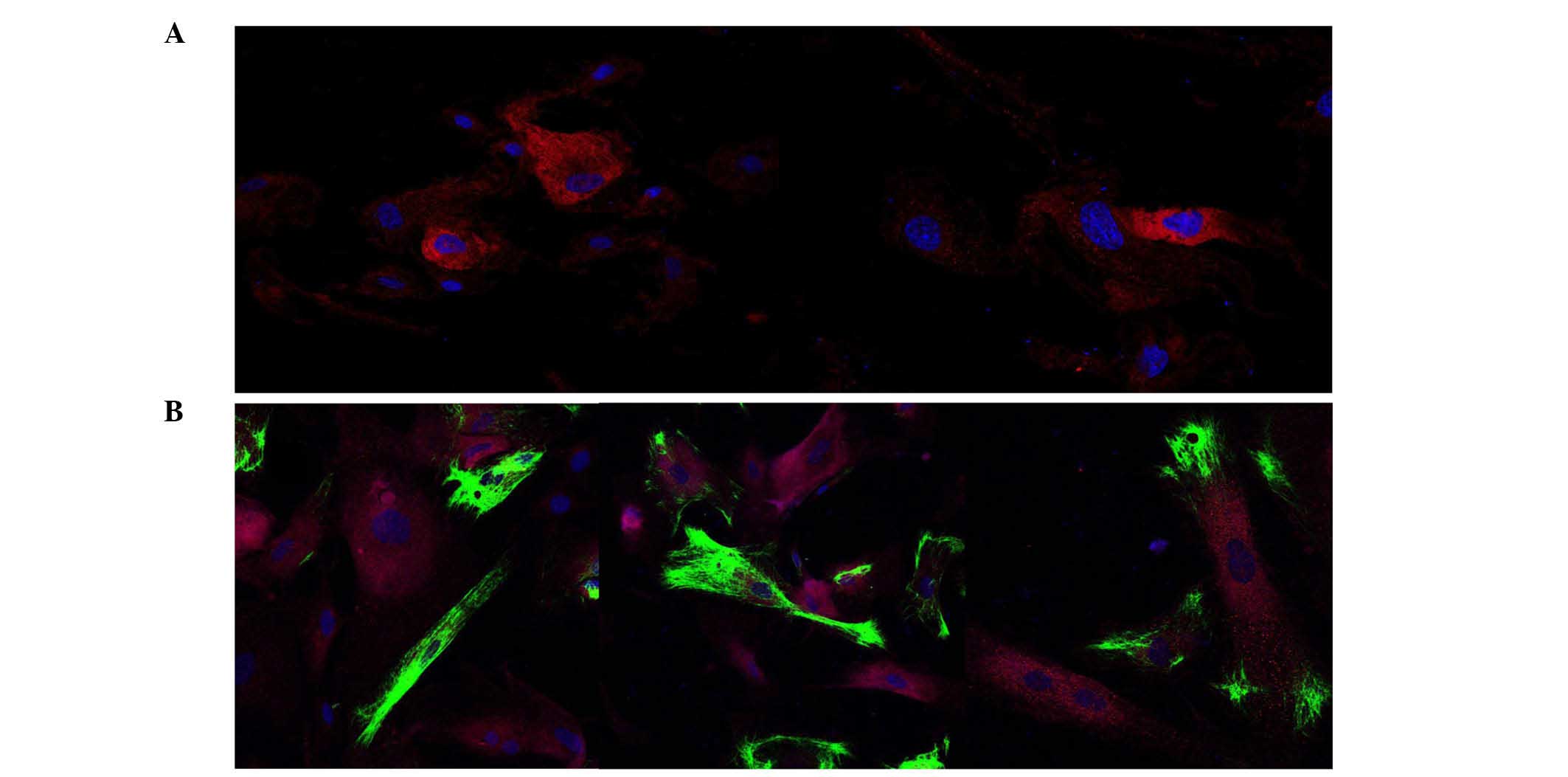
tissue and cell culture. EG-VEGF and PROKR1 were localized in the
cytoplasm (Fig. 1A and C,
respectively). Additionally, the expression of cytokeratin-7
(Fig. 1B) and HER-2/neu (Fig. 1D) was analyzed, since cytokeratin-7
expression is considered particularly useful for the diagnosis of
poorly differentiated tumors (29).
In cultures of several randomly selected samples that were grown to
80% confluence, positive immunostaining of EG-VEGF, HER-2/neu and
cytokeratin-7 was co-localized (Fig.
2).
 | Table III.Clinical and histological data. |
Table III.
Clinical and histological data.
|
Characteristics | No. of patients
(%) |
|---|
| Total no. of
patients | 50 (100) |
| Mean age, years
(range) | 53.4 (26–86) |
| Mean tumor size, mm
(range) | 20
(2–12) |
| Menopausal
status |
|
|
Pre | 7
(14) |
|
Post | 43 (86) |
| Histology, ICC | 50 (100) |
| TNM stage |
|
| Tumor
size |
|
|
pT1 | 2 |
|
pT2 | 20 |
|
pT3 | 12 |
|
pT4 | 16 |
| Nodal
status |
|
|
pN0 | 8 |
|
pN1 | 16 |
|
pN2 | 18 |
|
pN3 | 8 |
|
Metastatic status |
|
|
pM0 | 33 |
|
pM1 | 17 |
| Hormone receptor
status |
|
|
Positive ER | 6 |
|
Positive PR | 1 |
|
Positive ER and PR | 18 |
|
Positive HER-2/neu | 5 |
|
Negative ER, PR and
HER-2/neu | 11 |
|
Positive ER and HER-2/neu | 3 |
|
Positive PR and HER-2/neu | 2 |
|
Positive ER, PR and
HER-2/neu | 4 |
Discussion
In the normal human breast, two cell types have been
morphologically described, inner luminal cells (parenchyma) and
outer myoepithelial cells (stroma) (30). This anatomical distinction is
important for understanding the interactions between both cell
types during breast tumorigenesis. The majority of breast
malignancies (>95%) are derived from an epithelial lineage
(31). The epithelial-mesenchymal
interactions and the tissue-specific microenvironment modulate the
growth, progression and metastatic behavior of cancer cells
(31). The expression levels of
several biomarkers, including ERs, PRs and HER-2/neu, play a
critical role in the therapy and prognosis of breast tumors
(17).
Previous reports on human breast cancer cell lines,
patient tumor samples and clinical studies have all indicated that
progesterone is a risk factor for breast cancer, and that changes
in progesterone signaling pathways contribute to the early stage of
tumor progression (22,25). PR signaling stimulates epithelial cell
proliferation via an unknown mechanism in pre-neoplastic lesions
and mammary tumors (32). However,
primary tumors with negative PR expression have been associated
with a less differentiated, more invasive phenotype and a worse
prognosis than those expressing PR (32). The data from the current study differs
from the aforementioned previous evidence in the aggressiveness of
PR-negative tumors. Of 25 such tumors analyzed in the present
study, only 10 were poorly differentiated and 7 had an invasive
phenotype. In addition, of the 31 ER-positive tumors identified in
the present study, only 18 were moderately differentiated.
The HER-2/neu oncogene is amplified/overexpressed in
15–30% of breast cancers (17). This
overexpression/amplification of the HER-2/neu protein appears to be
of importance for the therapeutic benefit of anthracycline-based
treatments, and it is the target for trastuzumab
(Herceptin®), a humanized monoclonal antibody designed
as a therapy for metastatic breast cancer (33). The absence of ER, PR and HER-2/neu is
defined as triple negative breast cancer (TNBC), which is regarded
as an aggressive disease that affects young patients, and is
characterized by early relapse, particular visceral metastasis and
poor prognosis (34). In the current
study, 11 patients (22%) were TNBC, of which, 3 were young women
(<40 years of age), and 4 developed metastatic tumors. This
percentage is similar to that reported in previous studies
(35). However, other studies have
suggested that Hispanic women are more likely to present TNBC than
Caucasian women (36). In 2009,
Linderholm et al reported higher levels of VEGF in TNBC than
non-TNBC patients (37). The present
study obtained similar results, finding that all TNBC patients
exhibited slightly greater VEGF expression than non-TNBC patients.
Regarding the two angiogenic proteins assessed in the current
study, VEGF was expressed at a significantly higher level than
EG-VEGF. To the best of our knowledge, the present study is the
first to report the expression of EG-VEGF in mammary gland tumors.
A previous study demonstrated the expression of this protein in a
wide variety of human tissues, but did not include the mammary
gland (38).
Breast cancer is a heterogeneous disease that
presents different biological patterns and histologically diverse
subtypes (17). The development of
resistance to multiple chemotherapeutic drugs suggests the
involvement of BCRP during the treatment of numerous breast
carcinomas (18). Unexpectedly, the
expression of BCRP was detected in all tumors, independently of TNM
and the expression of steroid receptors. In 2011, Moitra et
al explained that the BCRP phenotype can be produced by an
extensive population of tumor cells, cancer stem cells, cells with
acquired resistance in chemotherapy and cells with induced genetic
changes (39). Large progress has
been made in recent years in countering BCRP-induced drug
resistance, and ~20 molecules and 6 steroids have been identified
that can inhibit BCRP activity (18,40–42). The
level of expression of BCRP and the treatment outcome in the
present series deserves further analysis.
The differential expression of the angiogenic
factors evaluated in the present study could be attributed to the
cancer molecular subtype, which is based on gene expression
profiles. Recent research has indicated that human breast cells can
exhibit extensive lineage plasticity (43), which may explain why marker profiles
have been difficult to associate with distinct tumors subtypes. In
2014, Santagata et al analyzed normal breast cells and
identified 11 cell subtypes in the luminal layer; in the case of
breast tumors, none of them exhibited a purely basal-like phenotype
(30).
The present IHC data from human breast cancer
biopsies indicate that only certain cells were positively stained
for EG-VEGF and PROKR1, while others exhibited abundant staining
for cytokeratin-7 and HER-2/neu.
Several antineoplastic therapies are aiming to block
the function of VEGF (44). However,
in the majority of cases, tumors produce a large number of other
angiogenic factors, indicating that angiogenesis is a complex
process involving multiple signaling pathways (45). Over the last two decades, researchers
around the world have developed new techniques involving drugs that
target VEGF, including aflibercept and metronomic chemotherapy
(46). Aflibercept binds to and
inhibits all isoforms of VEGF, and also binds to placental growth
factor. Metronomic chemotherapy blocks proliferating tumor cells
(47) and is important in breast
cancer metastasis (48). Bergers and
Hanahan (46) and Dempke and
Heinemann (44) reported the results
of preclinical studies indicating that certain mechanisms of tumor
adaptation and resistance are based on increased tolerance to
hypoxia, which leads to a decreased dependence on
neovascularization. The role of hormones in the regulation of VEGF
is controversial. Numerous studies on estrogen and progesterone
have demonstrated that both are able to increase VEGF mRNA and/or
protein expression (49,50). In contrast, other studies do not
support these findings (51–53).
The regulation of VEGF expression and function by
steroid hormones may act through distinct mechanisms in the various
cells types involved in breast cancer. Further studies are required
to elucidate the mechanism through which EG-VEGF expression is
reduced or absent relative to the expression of VEGF in steroid
hormone-dependent tumors. These factors could conceivably be used
as alternative targets to modulate angiogenesis. The development of
novel therapeutic drugs, anti-angiogenic molecules, hormonal agents
and biomarkers is important for a better understanding of the
molecular mechanisms involved in breast cancer. Successful
treatment of patients may depend on addressing the combination of
individual genotypes and alternative targets to modulate
angiogenesis and reduce drug resistance to chemotherapy.
Acknowledgements
The authors would like to thank the staff in the
operating room (Department of Mammary Tumors, National Cancer
Institute, Mexico City, Mexico) for their assistance in the
procurement of tissue samples, as well as Ms. Veronica Rodriguez of
the Histopathology Facility Faculty of Medicine and Dr Silvia Reyes
Maya of the Faculty of Medicine (both of the National Autonomous
University of Mexico) for her technical assistance with
immunofluorescence confocal microscopy.
References
|
1
|
Nishida N, Yano H, Nishida T, Kamura T and
Kojiro M: Angiogenesis in cancer. Vasc Health Risk Manag.
2:213–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schneider BP and Miller KD: Angiogenesis
of breast cancer. J Clin Oncol. 23:1782–1790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
LeCouter J, Lin R and Ferrara N: The role
of EG-VEGF in the regulation of angiogenesis in endocrine glands.
Cold Spring Harb Symp Quant Biol. 67:217–221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaser A, Winklmayr M, Lepperdinger G and
Kreil G: The AVIT protein family. Secreted cysteine-rich vertebrate
proteins with diverse functions. EMBO Rep. 4:469–473. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou QY and Meidan R: Biological function
of prokineticins. Results Probl Cell Differ. 46:181–199. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keramidas M, Faudot C, Cibiel A, Feige JJ
and Thomas M: Mitogenic functions of endocrine gland-derived
vascular endothelial growth factor and Bombina variegata 8 on
steroidogenic adrenocortical cells. J Endocrinol. 196:473–482.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kisliouk T, Levy N, Hurwitz A and Meidan
R: Presence and regulation of endocrine gland vascular endothelial
growth factor/prokineticin-1 and its receptors in ovarian cells. J
Clin Endocrinol Metab. 88:3700–3707. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fraser HM, Bell J, Wilson H, Taylor PD,
Morgan K, Anderson RA and Duncan WC: Localization and
quantification of cyclic changes in the expression of endocrine
gland vascular endothelial growth factor in the human corpus
luteum. J Clin Endocrinol Metab. 90:427–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morales A, Morimoto S, Díaz L, Robles G
and Díaz-Sánchez V: Endocrine gland-derived vascular endothelial
growth factor in rat pancreas: Genetic expression and testosterone
regulation. J Endocrinol. 197:309–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagano H, Goi T, Koneri K, Hirono Y,
Katayama K and Yamaguchi A: Endocrine gland-derived vascular
endothelial growth factor (EG-VEGF) expression in colorectal
cancer. J Surg Oncol. 96:605–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
PerrotApplanat M and Di Benedetto M:
Autocrine functions of VEGF in breast tumor cells: Adhesion,
survival, migration and invasion. Cell Adh Migr. 6:547–553. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kassim SK, ElSalahy EM, Fayed ST, Helal
SA, Helal T, Azzam Eel-D and Khalifa A: Vascular endothelial growth
factor and interleukin-8 are associated with poor prognosis in
epithelial ovarian cancer patients. Clin Biochem. 37:363–369. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DeSantis CE, Bray F, Ferlay J,
LortetTieulent J, Anderson BO and Jemal A: International variation
in female breast cancer incidence and mortality rates. Cancer
Epidemiol Biomarkers Prev. 24:1495–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Córdova JA, Hernández M, Ortiz ME, Lezana
MA, López-Gatell H and Alpuche CM: Epidemiological profile of
malignant tumors in Mexico. In: IEPSA. Mexico City. pp. 145–195.
2011;
|
|
15
|
Euhus D, Di Carlo PA and Khouri NF: Breast
cancer screening. Surg Clin North Am. 95:991–1011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kennecke H, Yerushalmi R, Woods R, Cheang
MC, Voduc D, Speers CH, Nielsen TO and Gelmon K: Metastatic
behavior of breast cancer subtypes. J Clin Oncol. 28:3271–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Chen Z, Su K and Zeng J:
Clinicopathological classification and traditional prognostic
indicators of breast cancer. Int J Clin Exp Pathol. 8:8500–8505.
2015.PubMed/NCBI
|
|
18
|
Mao Q and Unadkat JD: Role of the breast
cancer resistance protein (BCRP/ABCG2) in drug transport-an update.
AAPS J. 17:65–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Faneyte IF, Kristel PM, Maliepaard M,
Scheffer GL, Scheper RJ, Schellens JH and van de Vijver MJ:
Expression of the breast cancer resistance protein in breast
cancer. Clin Cancer Res. 8:1068–1074. 2002.PubMed/NCBI
|
|
20
|
Glavinas H, Krajcsi P, Cserepes J and
Sarkadi B: The role of ABC transporters in drug resistance,
metabolism and toxicity. Curr Drug Deliv. 1:27–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Javam M, Audette MC, Iqbal M, Bloise E,
Gibb W and Matthews SG: Effect of oxygen on multidrug resistance in
term human placenta. Placenta. 35:324–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vore M and Leggas M: Progesterone acts via
progesterone receptors A and B to regulate breast cancer resistance
protein expression. Mol Pharmacol. 73:613–615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Wang H, Wei L, Li G, Yu J, Gao Y,
Gao P, Zhang X, Wei F, Yin D and Zhou G: Transcriptional modulation
of BCRP gene to reverse multidrug resistance by toremifene in
breast adenocarcinoma cells. Breast Cancer Res Treat. 123:679–689.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Diestra JE, Scheffer GL, Catala I,
Maliepaard M, Schellens JH, Scheper RJ, Germà-Lluch JR and
Izquierdo MA: Frequent expression of the multi-drug
resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours
detected by the BXP-21 monoclonal antibody in paraffin-embedded
material. J Pathol. 198:213–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Lee EW, Zhou L, Leung PC, Ross DD,
Unadkat JD and Mao Q: Progesterone receptor (PR) isoforms PRA and
PRB differentially regulate expression of the breast cancer
resistance protein in human placental choriocarcinoma BeWo cells.
Mol Pharmacol. 73:845–854. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakagawa M, Schneider E, Dixon KH, Horton
J, Kelley K, Morrow C and Cowan KH: Reduced intracellular drug
accumulation in the absence of P-glycoprotein (mdr1) overexpression
in mitoxantrone-resistant human MCF-7 breast cancer cells. Cancer
Res. 52:6175–6181. 1992.PubMed/NCBI
|
|
27
|
Schumacher M, Schmoor C, Sauerbrei W,
Schauer A, Ummenhofer L, Gatzemeier W and Rauschecker H: The
prognostic effect of histological tumor grade in node-negative
breast cancer patients. Breast Cancer Res Treat. 25:235–245. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
PanchukVoloshina N and Haugland RP,
BishopStewart J, Bhalgat MK, Millard PJ, Mao F, Leung WY and
Haugland RP: Alexa dyes, a series of new fluorescent dyes that
yield exceptionally bright, photostable conjugates. J Histochem
Cytochem. 47:1179–1188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chu P, Wu E and Weiss LM: Cytokeratin 7
and cytokeratin 20 expression in epithelial neoplasms: A survey of
435 cases. Mod Pathol. 13:962–972. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Santagata S, Thakkar A, Ergonul A, Wang B,
Woo T, Hu R, Harrell JC, McNamara G, Schwede M, Culhane AC, et al:
Taxonomy of breast cancer based on normal cell phenotype predicts
outcome. J Clin Invest. 124:859–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Howlett AR and Bissell MJ: The influence
of tissue microenvironment (stroma and extracellular matrix) on the
development and function of mammary epithelium. Epithelial Cell
Biol. 2:79–89. 1993.PubMed/NCBI
|
|
32
|
Obr AE and Edwards DP: The biology of
progesterone receptor in the normal mammary gland and in breast
cancer. Mol Cell Endocrinol. 357:4–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shak S: Overview of the trastuzumab
(Herceptin) anti-HER2 monoclonal antibody clinical program in
HER2-overexpressing metastatic breast cancer. Herceptin
Multinational Investigator Study Group. Semin Oncol. 26(Suppl 12):
71–77. 1999.PubMed/NCBI
|
|
34
|
Schmadeka R, Harmon BE and Singh M:
Triple-negative breast carcinoma: Current and emerging concepts. Am
J Clin Pathol. 141:462–477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumors. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bauer KR, Brown M, Cress RD, Parise CA and
Caggiano V: Descriptive analysis of estrogen receptor
(ER)-negative, progesterone receptor (PR)-negative and
HER2-negative invasive breast cancer, the so-called triple-negative
phenotype: A population-based study from the California cancer
registry. Cancer. 109:1721–1728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Linderholm BK, Hellborg H, Johansson U,
Elmberger G, Skoog L, Lehtiö J and Lewensohn R: Significantly
higher levels of vascular endothelial growth factor (VEGF) and
shorter survival times for patients with primary operable
triple-negative breast cancer. Ann Oncol. 20:1639–1646. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
LeCouter J, Kowalski J, Foster J, Hass P,
Zhang Z, DillardTelm L, Frantz G, Rangell L, DeGuzman L, Keller GA,
et al: Identification of an angiogenic mitogen selective for
endocrine gland endothelium. Nature. 412:877–884. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moitra K, Lou H and Dean M: Multidrug
efflux pumps and cancer stem cells: Insights into multidrug
resistance and therapeutic development. Clin Pharmacol Ther.
89:491–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dankers AC, Sweep FC, Pertijs JC, Verweij
V, van den Heuvel JJ, Koenderink JB, Russel FG and Masereeuw R:
Localization of breast cancer resistance protein (Bcrp) in
endocrine organs and inhibition of its transport activity by
steroid hormones. Cell Tissue Res. 349:551–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ee PL, Kamalakaran S, Tonetti D, He X,
Ross DD and Beck WT: Identification of a novel estrogen response
element in the breast cancer resistance protein (ABCG2) gene.
Cancer Res. 64:1247–1251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Imai Y, Ishikawa E, Asada S and Sugimoto
Y: Estrogen-mediated post transcriptional down-regulation of breast
cancer resistance protein/ABCG2. Cancer Res. 65:596–604.
2005.PubMed/NCBI
|
|
43
|
Roy S, Gascard P, Dumont N, Zhao J, Pan D,
Petrie S, Margeta M and Tlsty TD: Rare somatic cells from human
breast tissue exhibit extensive lineage plasticity. Proc Natl Acad
Sci USA. 110:4598–4603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dempke WC and Heinemann V: Resistance to
EGF-R (erbB-1) and VEGF-R modulating agents. Eur J Cancer.
45:1117–1128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mercurio AM, Lipscomb EA and Bachelder RE:
Non-angiogenic functions of VEGF in breast cancer. J Mammary Gland
Biol Neoplasia. 10:283–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bergers G and Hanahan D: Modes of
resistance to anti-angiogenic therapy. Nat Rev Cancer. 8:592–603.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chan A: Antiangiogenic therapy for
metastatic breast cancer: current status and future directions.
Drugs. 69:167–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
BanysPaluchowski M, Schütz F, Ruckhäberle
E, Krawczyk N and Fehm T: Metronomic chemotherapy for metastatic
breast cancer - a systematic review of the literature. Geburtshilfe
Frauenheilkd. 76:525–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ruohola JK, Valve EM, Karkkainen MJ,
Joukov V, Alitalo K and Härkönen PL: Vascular endothelial growth
factors are differentially regulated by steroid hormones and
antiestrogens in breast cancer cells. Mol Cell Endocrinol.
149:29–40. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Takei H, Lee ES and Jordan VC: In vitro
regulation of vascular endothelial growth factor by estrogens and
antiestrogens in estrogen-receptor positive breast cancer. Breast
Cancer. 9:39–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bogin L and Degani H: Hormonal regulation
of VEGF in orthotopic MCF7 human breast cancer. Cancer Res.
62:1948–1951. 2002.PubMed/NCBI
|
|
52
|
Mirkin S, Wong BC and Archer DF: Effect of
17 beta-estradiol, progesterone, synthetic progestins, tibolone and
tibolone metabolites on vascular endothelial growth factor mRNA in
breast cancer cells. Fertil Steril. 84:485–491. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mirkin S, Wong BC and Archer DF: Effects
of 17beta-estradiol, progesterone, synthetic progestins, tibolone
and raloxifene on vascular endothelial growth factor and
Thrombospondin-1 messenger RNA in breast cancer cells. Int J
Gynecol Cancer. 16(Suppl 2): S560–S563. 2006. View Article : Google Scholar
|