Introduction
Monoclonal gammopathies are characterized by the
presence of a serum monoclonal component (MC) produced by clonal
plasma cells. The MC can be an intact immunoglobulin alone or in
combination with a free light chain (FLC), either κ or λ (1,2).
Precise and accurate MC quantification is one of the
key parameters required to discriminate between monoclonal
gammopathy of undetermined significance (MGUS), multiple myeloma
(MM) and smoldering MM (SMM) (1,2). The
monitoring of MC levels provides indications of the response to
therapy and, with the advent of immunomodulatory drugs, MC
quantification has also been identified as a quick method to assess
the depth of response, providing prominent prognostic information
(3,4).
Traditionally, quantification and characterization
of the MC relies on serum protein electrophoresis (sPEP) and serum
immunofixation (sIFE), respectively. A lack of standardization,
limited sensitivity and subjectivity, to a certain extent, are the
main limitations of these techniques (5,6).
The introduction of Freelite® (The
Binding Site Group Ltd., Birmingham, UK) for the automatic
nephelometric/turbidimetric measurement of serum (s)FLC in 2001
(7) radically changed the diagnostic
procedure in clinical practice, and FLC is now an established tool
for hematological diagnostics (8–10).
More recently, a new assay was introduced:
Hevylite® (The Binding Site Group Ltd.) for heavy/light
chain (HLC) quantification. Hevylite targets the junctional
epitopes, allowing the measurement of couples of immunoglobulin
(Ig)‘κ/Ig’λ (IgGκ, IgGλ, IgAκ, IgAλ, IgMκ and IgMλ), thus,
providing novel insight into the quantification and
characterization of MC (11).
FLC and HLC assays have routinely been performed at
San Gennaro Hospital (Naples 1 Local Health Center, Naples, Italy)
for the follow-up of MM patients. The current study demonstrates
the clinical utility of these assays, providing 3 examples of their
combined use and displaying the advantages with respect to
traditional tools.
Patients and methods
Patient selection and frequency of
monitoring with FLC and HLC
Between January 2012 and March 2014, 300 samples
were collected from 90 patients (49 MM, 6 SMM and 35 MGUS) treated
in the Complex Operative Unit (U.O.C.) of Hematology of San Gennaro
Hospital (Naples 1 Local Health Center). The mean age was 69.14
years, and the 49 MM patients were split into 21 IgGκ MM, 16 IgGλ
MM, 6 IgAκ MM, 3 IgAλ MM and 3 light-chain MM (LCMM).
FLC and HLC were repeated every 3 months in MM
patients under treatment, to assess their response to therapy. SMM
patients were monitored every 6 months, whereas MGUS patients were
monitored every 6–12 months, depending on their risk assessment.
MGUS and SMM were re-assessed by the clinicians every 4 months.
Every month, a clinical evaluation was performed on
the MM patients under chemotherapy; during follow-up they were
monitored every 3 months.
This study was approved by the Ethical Committee of
San Gennaro Hospital and written informed consent was obtained from
all patients.
Treatment options
The MM patients received different treatment
protocols. In total, 7 patients were eligible to receive autologous
stem cell transplantation (ASCT); 3 received a bortezomib,
thalidomide and dexamethasone regimen and the remaining 4 received
a bortezomib, doxorubicin and dexamethasone (PAD) regimen. Among
the remaining 42 patients, 16 received a melphalan, prednisone and
bortezomib (MPV) regimen and 3 an MP regimen, while the patients
who experienced relapse received lenalidomide and dexamethasone
(Rd; 19 patients) or bortezomib and dexamethasone (VD; 4 patients)
therapy. In detail, the therapeutic regimens were as follows: i)
1.3 mg/m2 bortezomib (Velcade®) on days 1, 4,
8 and 11, 50 mg/day thalidomide on days 1–14, 100 mg/day
thalidomide on days 15–28, and 40 mg dexamethasone on days 1–4 and
8–11 (4 cycles every 28 days); ii) PAD regimen: 1.3
mg/m2 bortezomib on days 1, 4, 8 and 11, 30
mg/m2 liposomal doxorubicin (Caelix®) on day
1, and 20 mg dexamethasone on days 1–2, 4–5, 8–9 and 11–12 (6
cycles every 28 days); iii) MPV regimen: 1.3 mg/m2
bortezomib on days 1, 8, 15 and 22, 9 mg/m2 melphalan on
days 1–4, and 60 mg/m2 prednisone on days 1–4 (9 cycles
every 28 days); iv) MP regimen: 9 mg/m2 melphalan on
days 1–4 and 60 mg/m2 prednisone on days 1–4 (6 cycles
every 28 days); v) Rd regimen: 25 mg lenalidomide on days 1–21, and
20 mg dexamethasone on days 1–4, 9–12 and 17–20 (every 28 days
until to progression); and vi) VD regimen: 1.3 mg/m2
bortezomib on days 1, 4, 8 and 11, and 40 mg dexamethasone on days
1, 4, 8 and 11 (6 cycles every 28 days).
Laboratory tests
Serum samples were analyzed with the standard
diagnostic work up for MC (12–14), and
stored at −20°C. sPEP with 0.8% agarose gel was used for separating
β1/β2 bands, and black staining was analyzed by scanning
densitometry (Hydrasys 2; Sebia, Lisses, France) to quantify the CM
in % and g/dl. sIFE and urine IFE on agarose gel with acid violet
staining (Sebia) were employed for confirmation of clonality and
subsequent typing.
Total IgA, IgG and IgM (Roche Diagnostics, Basel,
Switzerland) were measured using serum immunifixation, as well as
azotemia and creatinemia for the assessment of renal function.
Freelite and Hevylite (The Binding Site Group Ltd.)
assays were used for FLC determination and independent HLC isotype
quantification, respectively. The Hevylite assay relies on the
targeting of junctional epitopes between the heavy and light chains
of intact Igs. The associated measurements were performed on
turbidimetric platform SPAplus® (The Binding Site Group
Ltd.).
Measurements of these parameters were used to derive
IgGκ/IgGλ, which were compared with reference ranges. A HLC ratio
(HLCr) outside of the reference range was considered to be
indicative of a clonal process.
Patients were categorized using FLC ratio (FLCr)
prognostic values, which were broadly identified using receiver
operator characteristic analysis with final cut-offs being
identified by trial and error.
Results
MGUS, MM and SMM frequency
The study population consisted of 49 MM patients (37
IgG, 9 IgA and 3 LCMM), 35 MGUS and 6 SMM. Patients were selected
from the population treated at the U.O.C. of Hematology of San
Gennaro Hospital. This accounts for the lower percentage of MGUS
with respect to MM, as low-risk MGUS patients are usually referred
to a general practitioner.
In the study population, the majority of MC cases
were IgGκ, followed by IgGλ at 30% and IgAκ at 11%. Only a small
percentage (5%) of LCMM was found (Fig.
1).
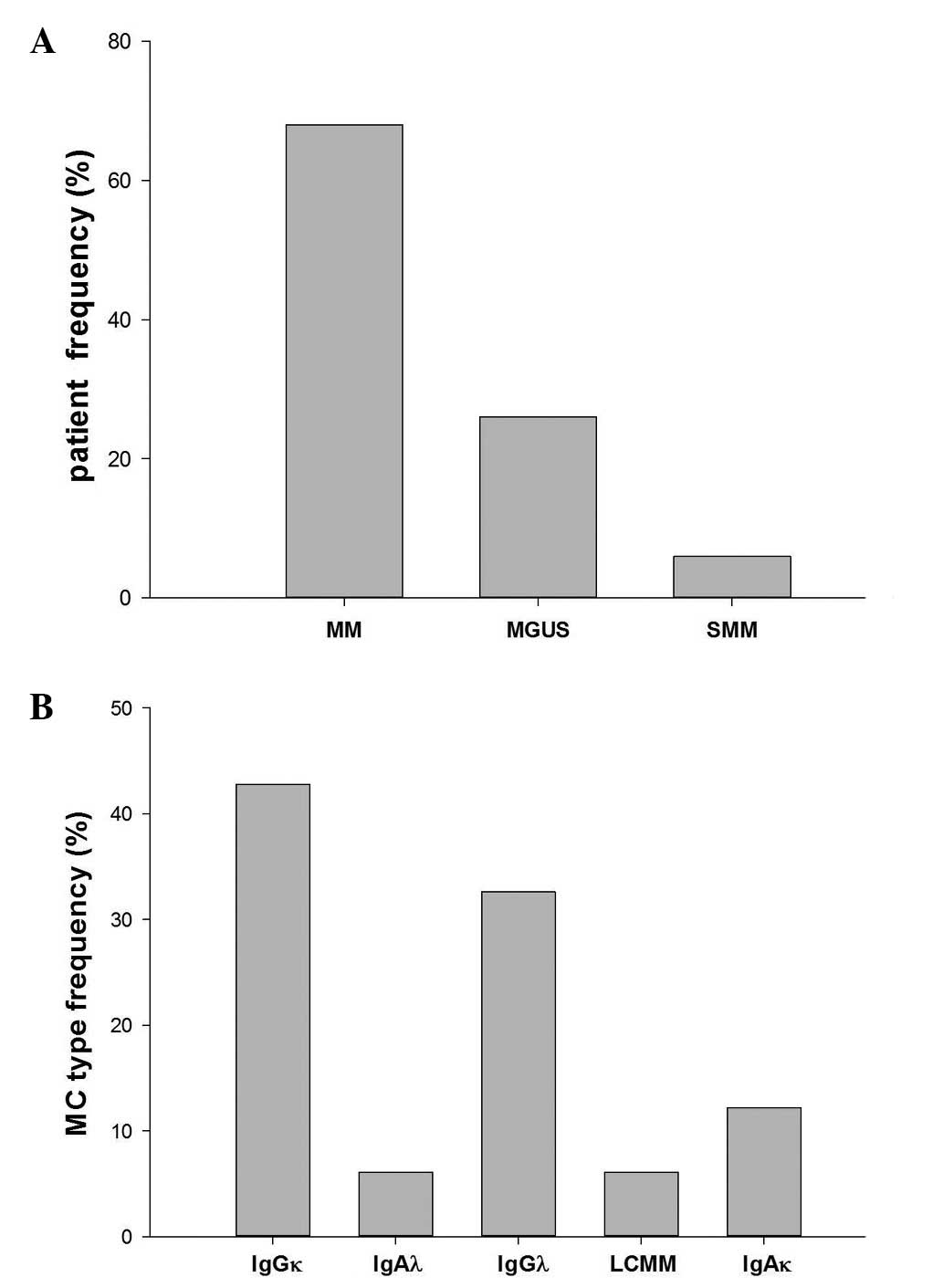 | Figure 1.MGUS, MM and SMM patient frequency and
MC type distribution in the study population. (A) Percentage of
patients with MGUS, MM and SMM, accounting for 6, 26 and 68, of the
population, respectively. (B) MC type frequency in the population,
consisting of 42.8% IgGκ, 6.1% IgAλ, 32.6% IgGλ, 6.1% LCMM and
12.2% IgAκ MM patients. MM, multiple myeloma; SMM, smoldering MM;
MGUS, monoclonal gammopathy of undetermined significance; Ig,
immunoglobulin; MC, monoclonal component. |
FLC abnormality was present in 82% of all patients
(MM, SMM and MGUS) when considering the first sample received,
which was regarded as the diagnostic sample (Fig. 2).
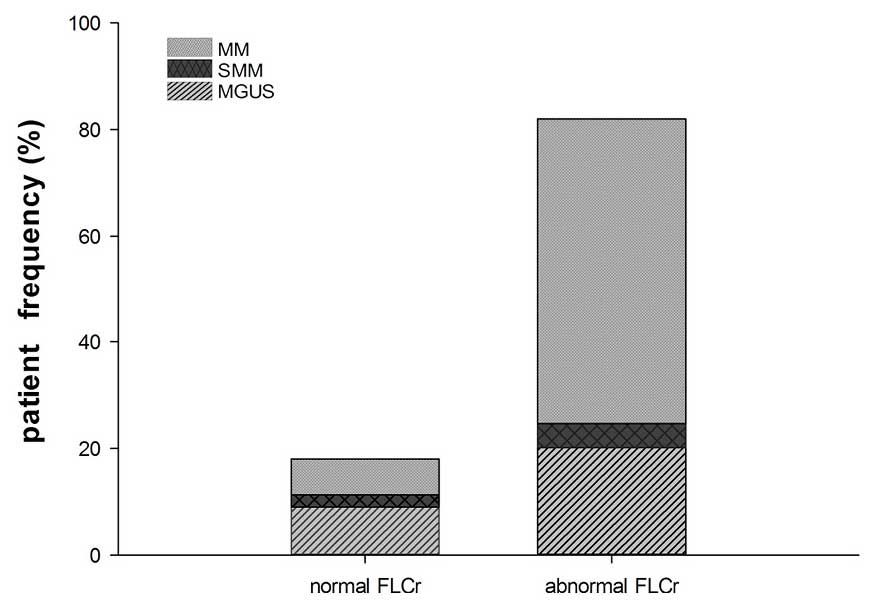 | Figure 2.Abnormality of FLCr in the overall
population, and MGUS, SMM and MM frequency. FLC abnormality was
present in 82% of all patients; among these samples, 69.9% were MM
patients, 24.6% were MGUS patients and 5.5% were SMM patients. With
regard to the remaining 18% of patients without FLCr abnormality,
37.5% were MM patients, 50% were MGUS patients and 12.5% were SMM
patients. MM, multiple myeloma; SMM, smoldering MM; MGUS,
monoclonal gammopathy of undetermined significance; FLC, free light
chain; FLCr, FLC ratio. |
In total, 69.9% of the samples were MM patients,
24.6% were MGUS patients and 5.5% were SMM patients; however, care
must be taken when assessing these results due to the limited
number of SMM patients (6).
Notably, ~70% of the intact immunoglobulin MM (IIMM)
samples had an abnormal FLC (15),
thus supporting the use of FLCr as an additional marker to diagnose
and monitor IIMM.
FLCr abnormality: Distribution across
MGUS, SMM and MM patients
The FLCr is an important indicator of FLC pair
suppression, with prominent prognostic meaning in MM patients at
diagnosis and in the course of treatment (9); a high degree of abnormality correlates
with reduced overall survival and progression-free survival times
(16). In the present study
population, ratio abnormality was compared in the three categories
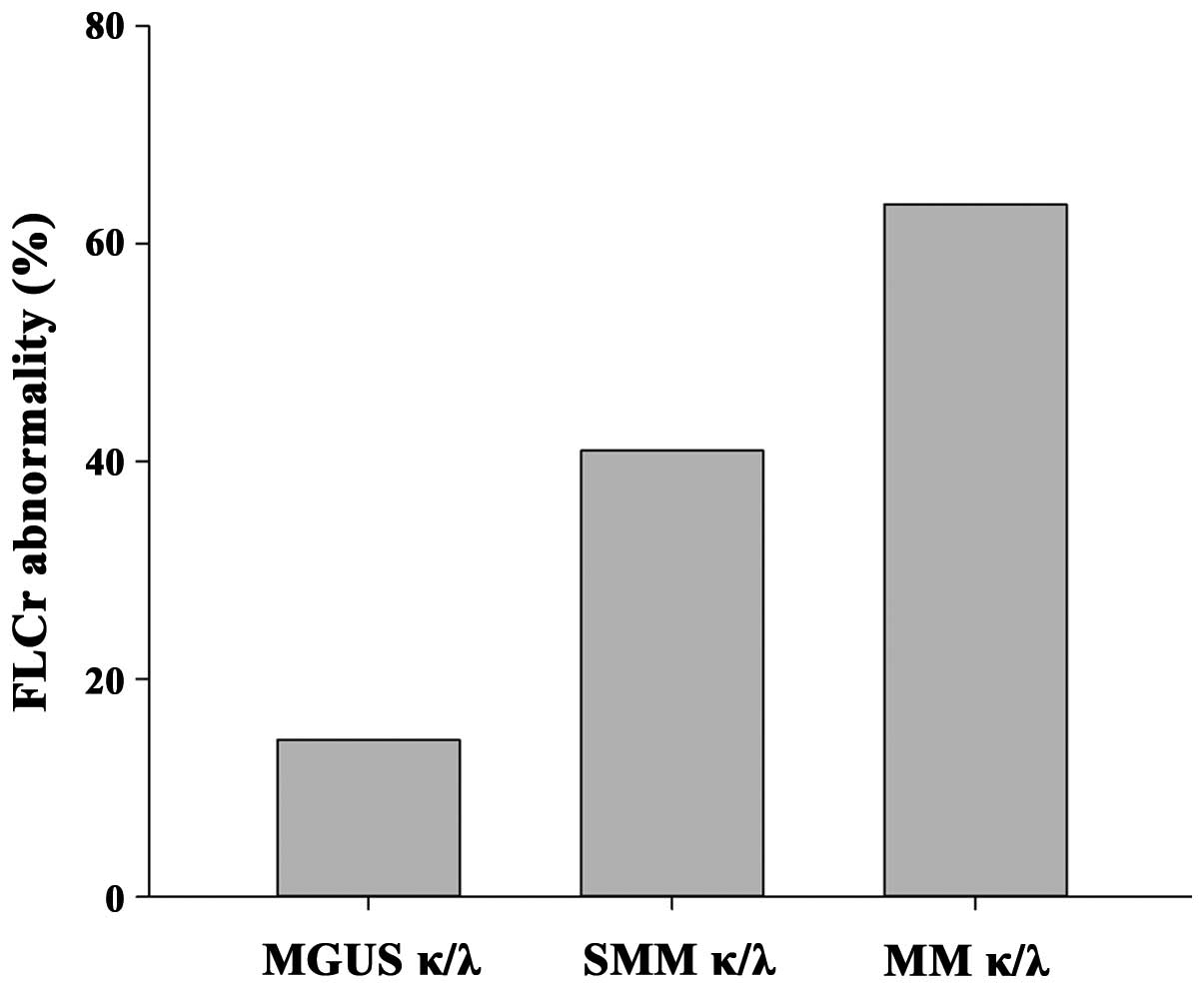
of MGUS, SMM and MM (Fig. 3), to
assess the level of pair suppression. The majority of the patients
had an IgGκ MC; therefore, this type of patient was focused
upon.
The mean value of the IgGκ/IgGλ ratios was obtained,
and a rising trend of the FLCr level was found in the three
categories, suggesting that the severity of the FLC imbalance could
be associated with overt disease.
Selected clinical cases
Case 1: Evolution of an IgGλ MC patient to double
MC and then LCMM (
Fig. 4)
A 60-year-old man was diagnosed with IgGλ MM in
March 2006 and received VAC (2 mg/m2 vincristine on day
1, 30 mg/m2 liposomal doxorubicin on day 2, 100 mg
cyclofosfamide on days 1–4; 6 cycles every 28 days) at the
Hematology Unit of S. Giovanni Bosco Hospital (Naples, Italy). In
February 2009, the patient commenced therapy with the Rd regimen at
the U.O.C. of Clinical Pathology of San Gennaro Hospital, and was
routinely monitored with sPEP, sIFE and FLC.
From March 2012, HLC was introduced at the U.O.C. of
Clinical Pathology of San Gennaro Hospital and was used together
with FLC.
In October 2012 the patient had a small IgGλ MC (4.7
g/l), quantified by sPEP; FLCλ (620 mg/l) was significantly above
the normal range (NR) of 5.7–26.3 mg/l and the FLCr was highly
abnormal at 0.01 (NR, 0.26–1.65). IgGλ HLC (4.26 g/l) was within
the NR (1.91–6.74 g/l), but the IgGκ (1.82 g/l) was below NR
(3.84–12.07 g/l), thus the IgG HLCr abnormality was consistent with
sIFE. This suggested the presence of persistent monoclonality even
with relatively low MC levels, aiding in the assessment of a small
MC together with the response to therapy.
In February 2013, a secondary small IgGλ MC was
identified through sIFE and sPEP, and FLCλ increased had to 1,049
mg/l. By contrast, IgGλ HLC levels were decreased with respect to
the previous time point (3.23 g/l), while IgGκ HLC (1.88 g/l)
remained below the NR. HLC pair suppression assisted in identifying
monoclonality even in the presence of small electrophoretic peaks
that were hardly quantifiable with sPEP.
In April 2013, therapy was suspended due to a
vertebral collapse requiring kyphoplasty, as well as severe anemia
requiring blood transfusions.
When the patient returned in October 2013, although
HLCr was restored to within the NR, in agreement with the sIFE, the
IgGκ and IgGλ levels were below the NR. As MC disappeared in
response to therapy, with normalization of Hevylite values and
ratio, Freelite never normalized and emerged a secondary clone
producing free light chains [light chain escape (LCE)]. FLCλ
reached 3,297 mg/l and the patient was diagnosed with LCMM.
Treatment with the PAD regimen was then commenced and the patient
was eligible for ASCT. Double ASCT was performed in another center
prior to returning to the San Gennaro Hospital in March 2014. A
marked reduction in FLCλ to 230 mg/l was observed, which was
abnormal but significantly lower with respect to the previous
values.
For this patient, the combined monitoring of FLC and
HLC supplied more detailed information compared with sPEP and sIFE.
At the most recent follow-up in June 2016, the patient had
commenced therapy with pomalidonmide (4 mg daily for 21 days, every
28 days) and exhibited stable disease.
Case 2: FLC and HLC persistent abnormality
correctly identifies monoclonality of MCs in the presence of
secondary clones and predicts clinical relapse (
Fig. 5)
A 78-year-old woman was diagnosed with IgAλ and FLCλ
MM in October 2012. The IgAλ MC, estimated by sPEP, was 22.4 g/l,
while FLCλ was 258.6 mg/l. The patient underwent 5 cycles of
therapy with MP. In November 2012, a partial response was obtained
and the sPEP did not show any monoclonal peak. Hevylite IgAκ and
IgAλ were within the NR; importantly, the HLCr was also normal at
this time point, suggesting that patient had responded to therapy.
However, FLCλ (48 mg/l) and FLCr (0.01) remained above the NR, thus
indicating the presence of residual disease. The shorter FLC
half-time compared with IgA meant that an earlier FLC response was
expected with respect to intact Igs. Therefore, this could be
suggestive of clonal heterogeneity with coexisting FLC and HLC
clones, and possibly a diverse sensitivity to the chemotherapy
agent.
The patient was constantly monitored and, in June
2013, the sPEP profile suggested the reappearance of an MC visible
in the γ zone, confirmed by sIFE as IgAλ. Densitometry measurement
of the MC indicated a 5.8 g/l peak, whereas IgAλ HLC was 11.5 g/l
with HLC pair suppression of IgAκ and an altered HLC A ratio. The
Freelite ratio did not normalize and in September 2013, the patient
experienced a clinical relapse, revealed by the MC increasing to
11.8 g/l and an IgAλ HLC level amounting to 15.3 g/l. Relapse was
confirmed during follow-up. The patient received 6 cycles of the VD
regimen, but monitoring with sPEP was not reliable, as the
polyclonal Igs in the γ zone interfered with an accurate IgA
quantification, resulting in an underestimation of the MC (19.60
g/l by sPEP vs. 33.5 g/l IgAλ HLC). Being unable to quantify the MC
by sPEP, HLC A was chosen in combination with Freelite to monitor
the patient. In February 2014, a reduction in FLCλ and IgAλ HLC was
observed. However, FLCr and HLCr were highly abnormal, suggesting a
significant degree of pair suppression at the expense of the
uninvolved FLCκ and IgAκ HLC. Despite an apparent reduction in the
MC upon densitometric analysis (10.6 g/l), the severe degree of
pair suppression, revealed by the ratios, suggested that there was
no specific tumor killing by chemotherapy. This was confirmed in
May 2014 through the observation of an increase of all biochemical
disease markers: IgAκ. 0.0010 g/l (NR 0.57–2.08 g/l); IgAλ, 23.79
g/l (NR 0.44–2.04 g/l); Ratio, 0.0004; FLCκ, 1.22 mg/l (NR 3.3–19.4
mg/l); FLCλ, 298.57 mg/l (NR 5.71–26.3 mg/l); Ratio, 0.004. No
further treatment was planned and the patient succumbed in July
2014.
Case 3: Clonal heterogeneity in a patient with
IgAκ MM and a secondary MC (
Fig.
6)
In August 2012, a 77-year-old woman was diagnosed
with IgAκ MM accompanied by diffuse osteolytic lesions at the
U.O.C. of Hematology of San Gennaro Hospital. Treatment commenced
with 2 cycles of PAD, followed by 4 cycles of VD, with 8 monthly
injections of zoledronate (4 mg) to prevent skeletal fractures.
The monoclonal IgAκ was visible in the β2 zone of
the sPEP migration pattern, a frequent occurrence with IgA MCs,
thus hindering identification and accurate measurement of the
monoclonal peak. The patient also exhibited elevated FLCκ (27
mg/l).
In June 2013, the patient suffered from severe
anemia and infiltration of the mandibular bone by clonal plasma
cells, prompting a change of therapy and the beginning of treatment
with the Rd regimen as salvage treatment.
In September 2013, the IgAκ (9.08 g/l; NR 0.57–2.0
g/l) and FLCκ (378 mg/l; NR 3.3–19.4 mg/l) levels remained stable
and, thus, above the NR. This suggested that no response to therapy
occured. At the same time, a secondary MC was identified in the γ
zone of the migration pattern, and sIFE results were suggestive of
IgGκ and FLCλ.
However, when sIFE was compared with FLC and HLC, no
abnormality was observed in the λ chains and Hevylite ratio G
(0.76) clearly indicated the presence of an IgGλ.
This case is a clear example of how sIFE
interpretation can be challenging even for a skilled operator when
more than one MC is present, with FLC and intact Igs.
Hevylite and Freelite assisted in clarifying the
isotypes and allowed for subsequent monitoring.
Notably, the IgGκ and IgGλ levels were below the NR,
possibly suggesting a condition of systemic immunoparesis. IgAκ was
increased (9.08 g/l) above the NR, whereas the IgAλ uninvolved
chain was suppressed (0.017 g/l), with a highly abnormal HLC A
ratio of 534.
The patient continued to receive lenalidomide and
dexamethasone. At the following time point in November 2013, there
was an improvement in the biochemical parameters of IgGκ and IgGλ,
the HLCr became closer to the NR, and there was a reduction in the
difference between FLCκ and FLCλ (dFLC) and HLC A.
In February 2014, the patient returned to hospital
and the secondary MC IgG was no longer visible upon sIFE, whereas
the IgAκ HLC (9.79 g/l) and FLCκ (58.43 mg/l) levels were increased
and highly above the NR. IgAκ HLC (20.35 g/l) and FLCκ (141.45
mg/l) steadily increased over the next months despite treatment
with the Rd regimen, until the patient succumbed in October
2014.
Discussion
Accurate quantification of the MC is fundamental for
forming a differential diagnosis and to assess the response to
therapy (17), as well as to define
immune system reconstitution in patients undergoing ASCT (18). Traditional routine tests include sPEP
and sIFE, which occasionally lack sensitivity and specificity. On
the other side, the current advances in molecular biology and
cytogenetics have led to a major breakthrough in the development of
newer and highly resolutive technologies, although they are not
easily accessible for all patients, thus confirming the requirement
for quick, reliable and standardized serum markers (19).
Within this scenario, serum immunoglobulin FLC
measurement with polyclonal antisera represents an important
diagnostic tool for monitoring patients (8).
Unlike total light chain assays, Freelite allows
separate measurement of sFLC κ and λ, thus allowing the
determination of the κ/λ ratio (FLCr), which serves as a reliable
marker of monoclonality that is particularly relevant for a
diagnosis (10). On the other hand,
concerning the monitoring, it is also important to consider the
dFLC and the individual concentrations of involved and uninvolved
FLC. The degree of the Freelite ratio abnormality has been
associated with disease progression. When considering IgGκ
patients, which were the most abundant in the present study
population, in agreement with previous reports (20), a trend of greater FLC abnormality in
MGUS, SMM and MM IgG patients was observed.
FLC is a reliable marker that can be used together
with traditional tests to diagnose and monitor all monoclonal
gammopathies (21). In the present
study, it was observed that the FLCr was abnormal in 82% of all
patients, including SMM and MGUS patients, supporting the use of
FLC in LCMM and non-secretory MM monitoring (22,23).
However, the clinical utility is not restricted to
these patients. FLCs are also fundamental for IIMM: In 80% of IIMM,
FLC determination is required. As shown in the first case, FLCs
were independent markers of disease and allowed identification of
LCE (24).
Hevylite has been used since 2012 in San Gennaro
Hospital and the current study presents 3 exemplar cases where the
introduction of Hevylite, together with Freelite, provided
additional information with respect to standard techniques.
Similarly to FLC, HLCrs are powerful and sensitive markers of
monoclonality, providing novel insight into the balance between the
involved and uninvolved Igs (25,26).
Clinical case 1 is a perfect example of how FLC and
HLC can be reliable markers of disease, particularly for small MCs.
Double MCs can be difficult to evaluate since the migration
patterns could prevent accurate quantifications (27). At the same time, total Igs cannot
distinguish between the MC and the polyclonal background (28). Hevylite, in this case, allowed the
quantification of the IgGλ MC and confirmed the disappearance of
the IgG component.
The combined use of FLC and HLC is emerging as a
novel strategy to detect MC and to enable better monitoring of
patients, allowing the depth of response to treatment as well as
the minimal residual disease (MRD) to be assessed; it can also be
used to detect relapse in MM patients (29). Clinical case 2 demonstrated Freelite
sensitivity for residual disease, in spite of clinical remission
and low MC levels assessed by sPEP. In this case, the MC was not
accurately quantifiable with sPEP, but only Hevylite allowed
precise MC measurement and also identified its reappearance,
anticipating the clinical relapse. FLCλ and FLCr did not normalize,
thus suggesting persistence of the disease, and Hevylite G also
became abnormal several months prior to clinical relapse.
A noteworthy point of discussion within the clinical
community is the requirement for earlier indicators of relapse and
reliable markers of MRD that could support the intention to treat
(30). In clinical case 2, the
persistent FLCr abnormality and the early identification of relapse
by HLCr, with respect to clinical symptoms, can be taken as an
example of how a careful and evidence-based selection of novel
markers could lead to a change in the paradigm of clinical practice
(31).
The scientific evidence in support of HLC shows that
it has greater sensitivity, providing quantitative results also in
the absence of an MC detectable by sPEP; in addition, it can
clarify dubious sIFE patterns (32,33).
The third case presented in the current study is a
clear example of the advantages of Hevylite and Freelite over sIFE;
this case also highlights the usefulness of these assays for
laboratory specialists and clinicians. With regard to their use in
the laboratory, these techniques could change the usual way of
monitoring patients, possibly replacing traditional tests and
providing more rapid, quantitative and qualitative results all in
one. The potential clinical use of Hevylite and Freelite could be
even wider and have a strong impact on prognosis (34,35).
As the attention focused on these novel biomarkers
increases, the most pressing issue will be their application in the
improvement of patient management.
Potentially, HLC could become part of a panel of
parameters required to support the intention to treat prior to the
observation of a severe clonal expansion accompanied by the
aggravation of symptoms. All these parameters move in the same
direction as the recently updated International Myeloma Working
Group criteria for MM diagnosis, introducing the concept of
‘myeloma defining events’, which are expected to become as
important as CRAB (calcium elevated, renal failure, anemia, bone
lesions) symptoms (10,36). At the same time, the possibility to
closely monitor the response to therapy, or the lack thereof, could
provide indications to support a change of treatment or an
adjustment in the frequency of monitoring.
Certainly, more evidence will be required prior to
the implementation of such a paradigmatic change. However, in our
experience, the benefits of Freelite and Hevylite monitoring in
patients with monoclonal gammopathies are already visible. In this
regard, the present clinical cases, clearly demonstrating the
positive advantages of HLC and FLC assessment with respect to
traditional tests, could provide valid support in illustrating the
beneficial impact of these assays for the clinical and laboratory
management of MM patients.
References
|
1
|
Durie BG, Kyle RA, Belch A, Bensinger W,
Blade J, Boccadoro M, Child JA, Comenzo R, Djulbegovic B, Fantl D,
et al: Scientific Advisors of the International Myeloma Foundation:
Myeloma management guidelines: A consensus report from the
Scientific Advisors of the International Myeloma Foundation.
Hematol J. 4:379–398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kyle RA, Durie BG, Rajkumar SV, Landgren
O, Blade J, Merlini G, Kröger N, Einsele H, Vesole DH, Dimopoulos
M, et al: International Myeloma Working Group: Monoclonal
gammopathy of undetermined significance (MGUS) and smoldering
(asymptomatic) multiple myeloma: IMWG consensus perspectives risk
factors for progression and guidelines for monitoring and
management. Leukemia. 24:1121–1127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aritaka N, Ichikawa K, Nakamura H, Yasuda
H, Ogura K, Matsumoto T, Komatsu N and Hirano T: Attainment of a
stringent complete response in multiple myeloma with thalidomide
monotherapy. Intern Med. 51:2781–2783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moustafa M Alhaj, Rajkumar SV, Dispenzieri
A, Gertz MA, Lacy MQ, Buadi FK, Hwa YL, Dingli D, Kapoor P, Hayman
SR, et al: Utility of serum free light chain measurements in
multiple myeloma patients not achieving complete response to
therapy. Leukemia. 29:2033–2038. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katzmann JA, Kyle RA, Benson J, Larson DR,
Snyder MR, Lust JA, Rajkumar SV and Dispenzieri A: Screening panels
for detection of monoclonal gammopathies. Clin Chem. 55:1517–1522.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murray DL, Ryu E, Snyder MR and Katzmann
JA: Quantitation of serum monoclonal proteins: Relationship between
agarose gel electrophoresis and immunonephelometry. Clin Chem.
55:1523–1529. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bradwell AR, Carr-Smith HD, Mead GP, Tang
LX, Showell PJ, Drayson MT and Drew R: Highly sensitive, automated
immunoassay for immunoglobulin free light chains in serum and
urine. Clin Chem. 47:673–680. 2001.PubMed/NCBI
|
|
8
|
Dispenzieri A, Kyle R, Merlini G, Miguel
JS, Ludwig H, Hajek R, Palumbo A, Jagannath S, Blade J, Lonial S,
et al: International Myeloma Working Group: International Myeloma
Working Group guidelines for serum-free light chain analysis in
multiple myeloma and related disorders. Leukemia. 23:215–224. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rajkumar SV, Harousseau JL, Durie B,
Anderson KC, Dimopoulos M, Kyle R, Blade J, Richardson P, Orlowski
R, Siegel D, et al: International Myeloma Workshop Consensus Panel
1: Consensus recommendations for the uniform reporting of clinical
trials: Report of the International Myeloma Workshop Consensus
Panel I. Blood. 117:4691–4695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rajkumar SV, Dimopoulos MA, Palumbo A,
Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E,
Richardson P..et al: International Myeloma Working Group updated
criteria for the diagnosis of multiple myeloma. Lancet Oncol.
15:e538–e548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bradwell AR, Harding SJ, Fourrier NJ,
Wallis GLF, Drayson MT, Carr-Smith HD and Mead GP: Assessment of
monoclonal gammopathies by nephelometric measurement of individual
immunoglobulin kappa/lambda ratios. Clin Chem. 55:1646–1655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guidelines Working Group of UK Myeloma
Forum, . British Committee for Standards in Haematology, British
Society for Haematology: Guidelines on the diagnosis and management
of AL amyloidosis. Br J Haematol. 125:681–700. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bird J, Behrens J, Westin J, Turesson I,
Drayson M, Beetham R, D'Sa S, Soutar R, Waage A, Gulbrandsen N, et
al: Haemato-oncology Task Force of the British Committee for
Standards in Haematology, UK Myeloma Forum and Nordic Myeloma Study
Group: UK Myeloma Forum (UKMF) and Nordic Myeloma Study Group
(NMSG): Guidelines for the investigation of newly detected
M-proteins and the management of monoclonal gammopathy of
undetermined significance (MGUS). Br J Haematol. 147:22–42. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dimopoulos MA, Kyle R, Fermand JP,
Rajkumar SV, Miguel J San, Chanan-Khan A, Ludwig H, Joshua D, Mehta
J, Gertz M, et al: International Myeloma Workshop Consensus Panel
3: Consensus recommendations for standard investigative workup:
Report of the International Myeloma Workshop Consensus Panel 3.
Blood. 117:4701–4705. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mead GP, Carr-Smith HD, Drayson MT, Morgan
GJ, Child JA and Bradwell AR: Serum free light chains for
monitoring multiple myeloma. Br J Haematol. 126:348–354. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siegel D, Bilotti E and van Hoeven KH:
Serum free light chain analysis for diagnosis, monitoring, and
prognosis of monoclonal gammopathies. Lab Med. 40:363–366. 2009.
View Article : Google Scholar
|
|
17
|
Smith A, Wisloff F and Samson D: UK
Myeloma Forum; Nordic Myeloma Study Group; British Committee for
Standards in Haematology: Guidelines on the diagnosis and
management of multiple myeloma 2005. Br J Haematol. 132:410–451.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Donato LJ, Zeldenrust SR, Murray DL and
Katzmann JA: A 71-year-old woman with multiple myeloma status after
stem cell transplantation. Clin Chem. 57:1645–1648. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fulton RB and Fernando SL: Serum free
light chain assay reduces the need for serum and urine
immunofixation electrophoresis in the evaluation of monoclonal
gammopathy. Ann Clin Biochem. 46:407–412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vernocchi A, Gelsumini S and Piazza E:
Prevalence of the monoclonal gammopathy of undetermined
significance (MGUS) in out-patients >50 years old: An indication
to carry out electrophoresis of serum protein? Biochim Clin.
38:154–155. 2014.
|
|
21
|
Gertz MA: Utility of the immunoglobulin
free light chain assay for plasma cell disorders 2015. Leuk
Lymphoma. 56:2757–2758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bradwell AR, Carr-Smith HD, Mead GP,
Harvey TC and Drayson MT: Serum test for assessment of patients
with Bence Jones myeloma. Lancet. 361:489–491. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Drayson M, Tang LX, Drew R, Mead GP,
Carr-Smith H and Bradwell AR: Serum free light-chain measurements
for identifying and monitoring patients with nonsecretory multiple
myeloma. Blood. 97:2900–2902. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brioli A, Giles H, Pawlyn C, Campbell JP,
Kaiser MF, Melchor L, Jackson GH, Gregory WM, Owen RG, Child JA, et
al: Serum free immunoglobulin light chain evaluation as a marker of
impact from intraclonal heterogeneity on myeloma outcome. Blood.
123:3414–3419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Katzmann JA and Rajkumar SV: A window into
immunoglobulin quantitation and plasma cell disease: Antigen
epitopes defined by the junction of immunoglobulin heavy and light
chains. Leukemia. 27:1–2. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ludwig H, Milosavljevic D, Zojer N, Faint
JM, Bradwell AR, Hübl W and Harding SJ: Immunoglobulin heavy/light
chain ratios improve paraprotein detection and monitoring, identify
residual disease and correlate with survival in multiple myeloma
patients. Leukemia. 27:213–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lakomy D, Lemaire-Ewing S, Denimal D,
Bastie JN, Lafon I and Caillot D: Evaluation of the new Hevylite™
IgA assay for the diagnosis and follow-up of monoclonal
gammopathies. Ann Biol Clin (Paris). 71:157–163. 2013.(In French).
PubMed/NCBI
|
|
28
|
Keren DF: Heavy/light-chain analysis of
monoclonal gammopathies. Clin Chem. 55:1606–1608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Astolfi M, Omedè P, Redoglia V, Oddolo D,
Ferrero MP and Ciaiolo C: Simultaneous evaluation of serum Hevylite
and Freelite assays for relapse prediction in multiple myeloma.
Biochim Clin. 37:383–388. 2013.
|
|
30
|
Bhutani M, Landgren O and Usmani SZ:
Multiple myeloma: Is it time for biomarker-driven therapy? Am Soc
Clin Oncol Educ Book. 35:e493–e503. 2015. View Article : Google Scholar
|
|
31
|
Landgren O and Morgan GJ: Biologic
frontiers in multiple myeloma: From biomarker identification to
clinical practice. Clin Cancer Res. 20:804–813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paolini L, Di Noto G, Maffina F,
Martellosio G, Radeghieri A, Luigi C and Ricotta D: Comparison of
Hevylite™ IgA and IgG assay with conventional techniques for the
diagnosis and follow-up of plasma cell dyscrasia. Ann Clin Biochem.
52:337–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Katzmann JA, Willrich MA, Kohlhagen MC,
Kyle RA, Murray DL, Snyder MR, Rajkumar SV and Dispenzieri A:
Monitoring IgA multiple myeloma: Immunoglobulin heavy/light chain
assays. Clin Chem. 61:360–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bradwell A, Harding S, Fourrier N, Mathiot
C, Attal M, Moreau P, Harousseau JL and Avet-Loiseau H: Prognostic
utility of intact immunoglobulin Ig'κ/Ig'λ ratios in multiple
myeloma patients. Leukemia. 27:202–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koulieris E, Panayiotidis P, Harding SJ,
Kafasi N, Maltezas D, Bartzis V, Tzenou T, Dimou M, Georgiou G,
Mirbahai L, et al: Ratio of involved/uninvolved immunoglobulin
quantification by Hevylite™ assay: Clinical and prognostic impact
in multiple myeloma. Exp Hematol Oncol. 1:92012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rajkumar SV, Landgren O and Mateos MV:
Smoldering multiple myeloma. Blood. 125:3069–3075. 2015. View Article : Google Scholar : PubMed/NCBI
|