Introduction
Solitary fibrous tumor (SFT), also known as
hemangiopericytoma, is a fibroblastic mesenchymal tumor that was
first described by Klemperer and Rabin in 1992 (1). SFTs are rare entities accounting for
<2% of all soft tissue sarcomas (2). Although SFTs are commonly found in the
pleura, according to the literature, 50–70% of these tumors are
extrapleural and can manifest in the head and neck, abdomen and
pelvis (3). This condition is rare
and only limited cases of SFT have been reported. Due to the rarity
of this tumor, preoperative diagnosis is challenging and no
consensus has been reached on standard treatment. The present study
summarizes the diagnosis, treatment and prognosis of 47 cases of
SFTs from the literature, in order to determine the
clinicopathological profile of SFT. To the best of our knowledge,
the present retrospective study on SFT is the largest reported to
date.
Patients and methods
Patients
Patient files from the Department of Pathology of
the Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing
University Medical School, (Nanjing, China) from the period between
January 2002 and September 2014 were retrospectively reviewed, and
47 cases of SFT were identified, including 39 inpatients and 8
outpatients. The present study was approved by the Institutional
Review Board of Nanjing Drum Tower Hospital. Informed consent for
participation in the study was obtained from all patients. All
patients had been diagnosed with SFT by a pathological examination.
Patient information, including age and gender, details of the
lesion, clinical symptoms at the time of diagnosis, auxiliary and
pathological examination findings, treatment and follow-up, was
recorded.
Auxiliary examination types and
findings
The findings of different types of auxiliary
examination, including computed tomography (CT), magnetic resonance
imaging (MRI) scans and color Doppler ultrasound, were reviewed. A
histopathological examination of paraffin-embedded surgical
specimens was performed to confirm the diagnosis using standard
hematoxylin and eosin staining and immunohistochemical techniques.
Immunohistochemical staining was performed using a Dako EnVision
System (Dako, Glostrup, Denmark), according to the manufacturer's
instructions. The primary antibodies included rabbit polyclonal
antibodies against vimentin (sc-5565; 1:200), cluster of
differentiation (CD)34 (sc-9095; 1:100), actin (sc-7210; 1:200),
desmin (sc-14026; 1:200), cytokeratin (CK; sc-134493; 1:100), S100
(sc-7849-R; 1:100), B-cell lymphoma 2 (bcl-2; sc-783; 1:200), CD99
(sc-241355; 1:200), CD117 (sc-3936; 1:200), epithelial membrane
antigen (EMA; sc-6826; 1:200) and smooth muscle actin (SMA;
sc-92040; 1:200; all Santa Cruz Biotechnology Inc., Dallas, TX,
USA). 3,3′-diaminobenzidine was used as the chromogen.
Results
Baseline characteristics
Out of the 47 screened patients, full clinical
characteristics were collected from 37 (18 men and 19 women; age
range, 13–72 years; mean age, 44.1 years) and are summarized in
Table I. The maximum diameter of the
tumors was 1.5–25 cm, with a mean diameter of 8.8 cm. The patients'
symptoms were various and non-specific, and included pain, cough,
inhibited defecation and frequent micturition. In total, 23 of the
37 patients (62.2%) had no symptoms at all, and the tumor was
discovered incidentally during physical examination. Common lesion
locations included the lungs, pleura, chest wall and pelvic cavity,
while certain rare sites, including the thyroid, groin, eyebrow
bow, bladder, kidney, eye, mediastinum and sigmoid mesocolon, were
discovered.
 | Table I.Clinical data of 37 patients with
solitary fibrous tumor. |
Table I.
Clinical data of 37 patients with
solitary fibrous tumor.
| Case
no./gender/agea | Symptom | Location | Treatment | Margin status | Diameter | Recurrence | Metastasis | Follow-up,
months |
|---|
| 1/F/48 | No | Chest wall | SE | Negative | 3.0 | No | No | NED, 130 |
| 2/M/35 | No | Lung | SE | Negative | 6.0 | NA | NA | NA |
| 3/F/48 | No | Thoracic cavity | SE | Negative | 21.0 | NA | NA | NA |
| 4/M/49 | Pharyngeal
dryness | Pharynx side
clearance | SE | Negative | 4.0 | NA | NA | NA |
| 5/F/41 | Pain | Infratemporal
fossa | SE | Negative | 5.7 | NA | NA | NA |
| 6/F/44 | No | Diaphragm | SE | Negative | 8.0 | NA | NA | NA |
| 7/F/53 | Abdominal pain | Mesentery of small
intestine | SE | Negative | 7.5 | NA | NA | NA |
| 8/M/55 | Chest pain | Posterior superior
iliac spine | SE | Negative | NA | No | No | NED, 40 |
| 9/F/44 | No | Kidney | SE | Negative | 11.0 | NA | NA | NA |
| 10/M/47 | No | Forearm | SE | Negative | 10.0 | NA | NA | NA |
| 11/F/62 | No | Chest wall | SE | Negative | 8.0 | No | No | NED, 68 |
| 12/F/13 | Knees ache | Knee | SE | Negative | 4.0 | NA | NA | NA |
| 13/M/23 | No | Nasal cavity | SE | Negative | 5.0 | No | No | NED, 65 |
| 14/M/62 | No | Pelvic cavity | SE | Negative | 9.0 | NA | NA | NA |
| 15/F/51 | Neck Pain | Cervical
vertebra | SE | Negative | 2.0 | No | No | NED, 57 |
| 16/M/53 | No | Lung | SE | Negative |
3.0 | No | No | NED, 55 |
| 17/M/49 | Abdominal
distension | Abdomen | SE | Negative | 18.0 | NA | NA | NA |
| 18/M/47 | No | Thoracic
cavity | SE | Negative |
8.0 | No | No | NED, 50 |
| 19/M/53 | No | Hip joint | SE | Negative | 10.0 | No | No | NED, 45 |
| 20/M/55 | Frequency of
urination | Pelvic cavity | SE | Negative | 14.0 | No | No | NED, 44 |
| 21/F/13 | Cough | Lung | SE | Negative |
5.0 | No | No | NED, 39 |
| 22/M/27 | Pain | Hip | SE | Negative | 16.0 | NA | NA | NA |
| 23/F/48 | Headache,
tinnitus | Temporal lobe | SE | Negative |
6.0 | No | No | NED, 35 |
| 24/F/39 | Chest pain | Mediastinum | SE | Negative | 11.0 | No | No | NED, 33 |
| 25/M/61 | No | Gastrocolic
ligament | SE | Negative |
5.0 | No | No | NED, 33 |
| 26/F/58 | No | Kidney | SE | Negative |
8.0 | Yes | No | NED, 15 after 2nd
surgery |
| 27/F/40 | No | Bladder | SE | Negative |
5.0 | No | No | NED, 30 |
| 28/F/64 | No | Lung | SE | Negative | NA | No | No | NED, 18 |
| 29/M/49 | No | Pleura | SE | Negative |
3.5 | No | No | NED, 18 |
| 30/M/60 | No | Testis | SE | Negative |
7.0 | No | No | NED, 18 |
| 31/F/22 | No | Inguinal
ligament | SE | Negative |
7.0 | No | No | NED, 14 |
| 32/M/21 | No | Diaphragm | SE | Negative | 24.0 | No | No | NED, 12 |
| 33/M/72 | No | Lung | SE | Negative |
6.0 | No | No | NED, 11 |
| 34/F/16 | No | Chest wall | SE | Negative |
8.5 | No | No | NED, 10 |
| 35/F/34 | Abdominal pain | Stomach | SE | Negative | 11.0 | No | No | NED, 9 |
| 36/M/24 | Constipation | Pelvic cavity | SE | Negative | NA | No | No | NED, 10 |
| 37/F/50 | No | Sigmoid
mesocolon | SE | Negative | 25.0 | No | No | NED, 5 |
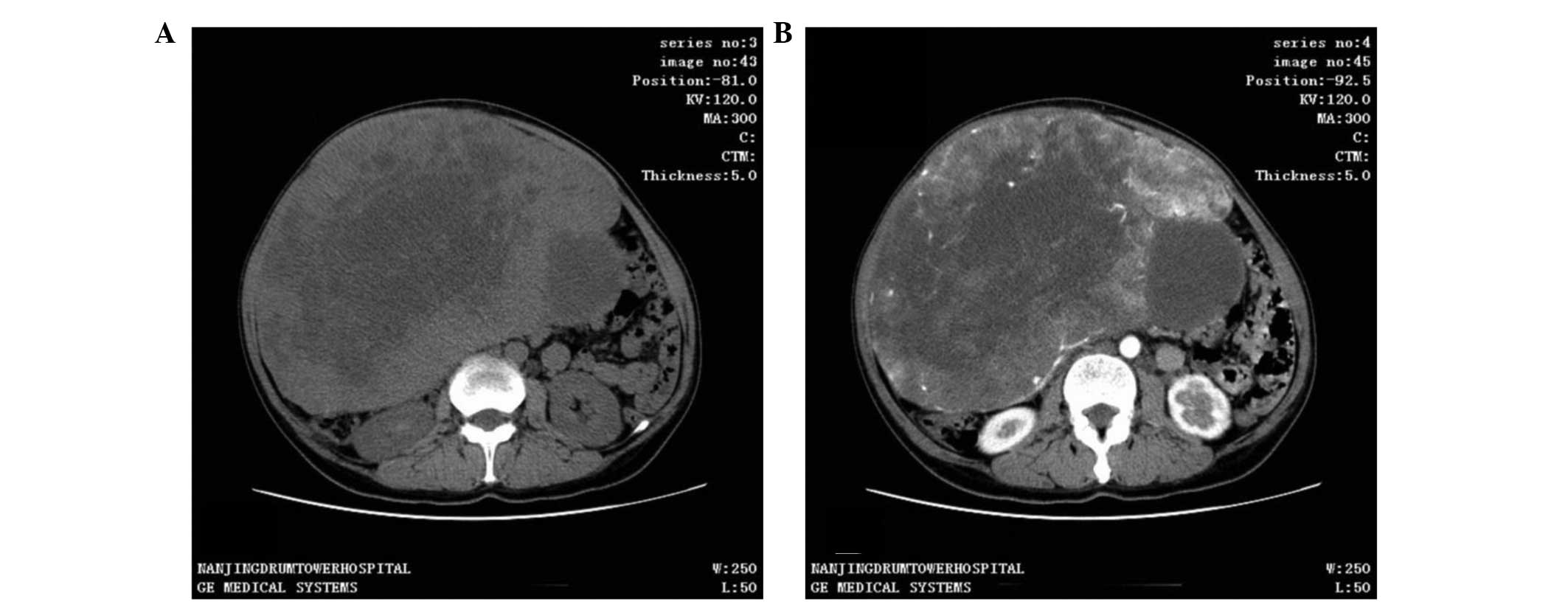
CT scan was the most frequent diagnostic imaging
technique used. CT images captured following iodinated contrast
administration from 30 of the 37 cases showed SFTs as well-defined,
cystic or solid mass, and enhanced (Fig.
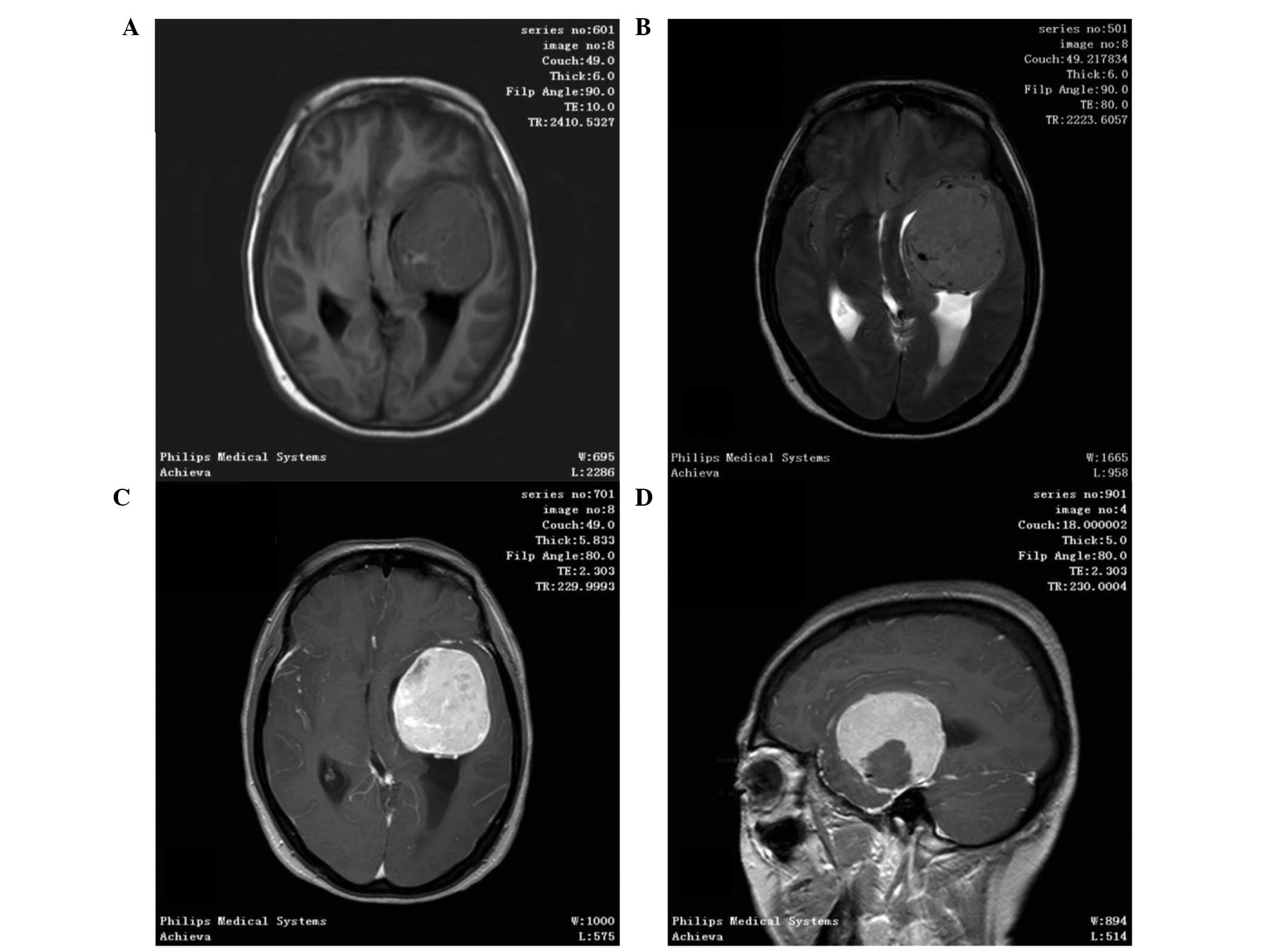
1). In total, 11 out of 37 patients underwent an MRI scan, in
which SFTs were shown as lobulated, heterogeneous soft tissue with
short T1-weighted (T1WI) and a flake long T2-weighted imaging
(T2WI) signal. Contrast-enhanced MRI scans demonstrated
heterogeneous contrast enhancement (Fig.
2). Color Doppler ultrasound was performed in 14 out of 37
patients and found the tumors to be hypoechoic, clear, irregular
masses. All patients underwent successful surgical resection with
no serious complications, and no postoperative mortality was
recorded. No patient underwent other adjuvant therapy.
Pathological findings
All patients were definitively diagnosed with SFT by
postoperative pathological and immunohistochemical examination.
Macroscopically, most tumors appeared as solid, well-encapsulated,
smooth to firm soft tissue masses, with a gray-white to red-brown
color on the cut surface. Microscopically, the tumors were shown to
be comprised of spindle or short spindle cells and various
quantities of vascular tissue. In certain regions, the cells were
arranged in short, ill-defined fascicles, whereas in others they
were arranged irregularly.
Immunohistochemical findings
Immunohistochemistry showed the following: The
positive rates of vimentin, CD34, CD99, bcl-2, actin, desmin, CK,
S100, CD117, EMA and SMA were 13/13 (100.0%), 40/46 (87.0%), 25/31
(80.6%), 30/38 (79.0%), 6/21 (28.6%), 2/35 (5.7%), 0/18 (0%), 6/40
(15%), 2/18 (11.1%), 3/13 (23.1%) and 7/15 (46.7%), respectively.
The immunohistochemical indexes were selected according to the
location of primary lesion, as different tissues have different
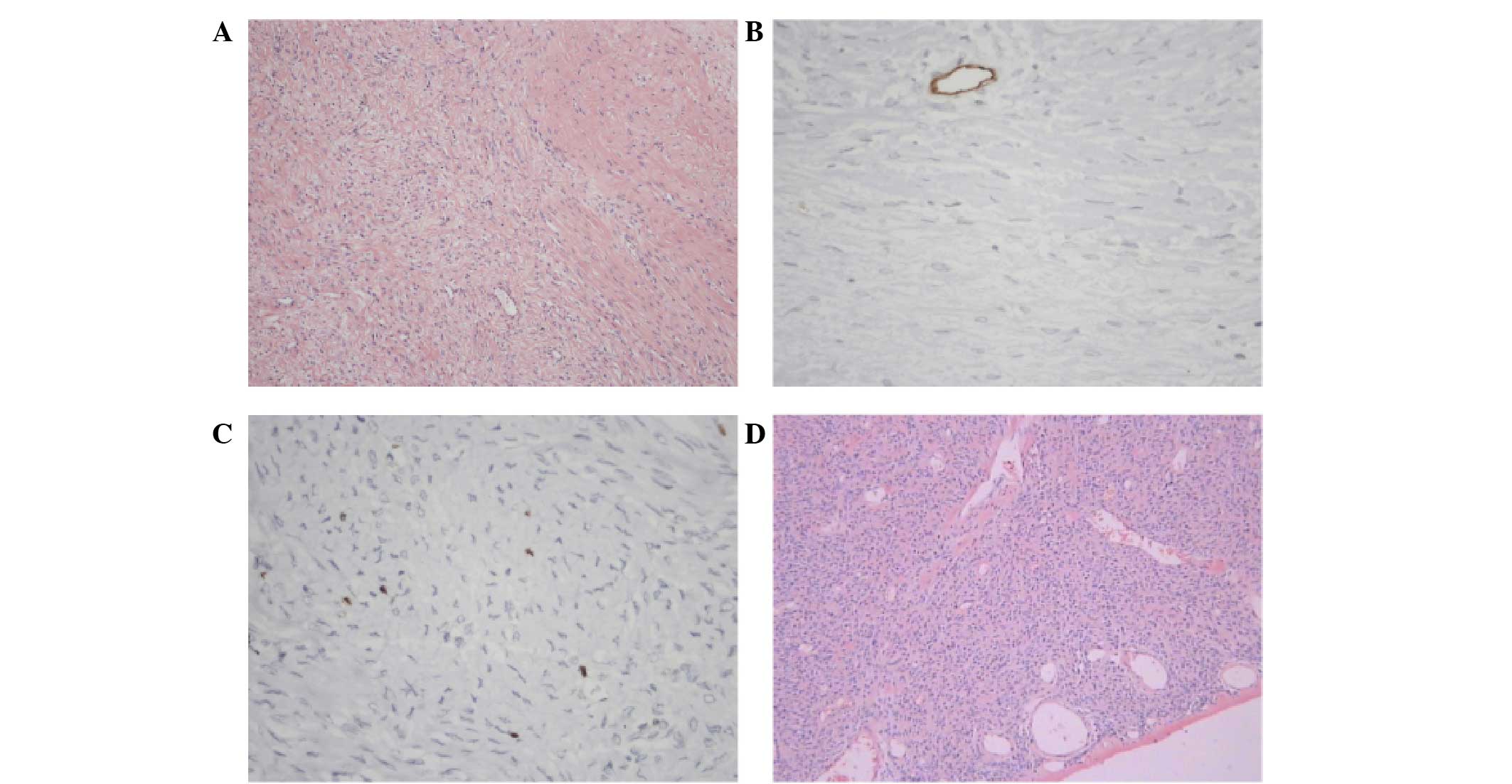
specific markers. The representative pathological and
immunohistochemical images of a patient that presented with SFT in
the diaphragm are shown in Fig. 3.
The immunohistochemical results for this patient were as follows:
CD34−, CD99−, neurofilament−,
bc1-2+, desmin−, actin−,
S100−, Ki67+ 1% and
β-catenin+.
Follow-up information
A total of 25 patients received complete follow-up
lasting 5–130 months (median follow-up period, 35.2 months), during
which they underwent a color Doppler ultrasound or CT scan every
6–12 months. At the time of writing, all patients were alive and
healthy. Only 1 patient (4%) presented with recurrent SFT 15 months
after the first surgery, and had a disease-free survival following
the second operation. The status of each patient at the last
follow-up is summarized in Table I.
The follow-up data from 22 patients of the total 47 (12 of the 37)
were either lost or could not be obtained.
Discussion
SFT was first described as a rare spindle cell tumor
that arises from the visceral pleura (4); however, over time, SFT was identified in
various other locations outside of the thoracic cavity, such as the
meninges (5,6), orbit (7),
nasal cavity (8), salivary gland
(9), parapharyngeal space (10) and paranasal sinuses (11). The present study reviewed numerous
cases of SFT manifesting in locations outside the thoracic cavity,
such as the thyroid, groin, bladder, sigmoid mesocolon and
posterior superior iliac spine, locations that had not been
previously reported. According to the literature (12), SFT is usually encountered in
middle-aged people with an equal distribution between genders;
however, it has also been reported in young patients (13). Consistent with these findings, the
gender ratio in the present study was 0.95 (18 men and 19 women)
and the mean age was 44.1 years. The symptoms varied depending on
the lesions; the majority of patients (23/37) presented with
physical symptoms. The diameter of SFTs is usually large (commonly
>8cm) (14). In the present study,
50% patients had a tumor measuring >8 cm.
SFTs do not express any tumor markers, and
diagnostic techniques include CT, MRI scans and color Doppler
ultrasound, despite the fact that their results may not be specific
(9). These imaging tests often
provide the first clue to the identification of the tumors,
depiction of local extent and invasion of adjacent structures,
which is useful in guiding surgery. On CT scan, SFT appears as a
well-defined, heterogeneous or homogeneous isodense mass, and shows
moderate to marked enhancement following contrast administration
(15,16). On color Doppler ultrasound, it has the
appearance of a hypoecho, clear, and irregular mass (17). On MRI, it has been shown to exhibit
intermediate signal intensity on T1WI images, and enhancement on
T2WI images, which was in accordance with the findings of the
present study (18,19). None of these methods, however, could
achieve accurate diagnosis; the final diagnosis has been reported
to rely on surgical resection and pathological examination
(20,21).
In the present study, all patients underwent
surgical resection. Macroscopically, the majority of SFTs appear as
rounded, encapsulated masses of homogenous density, with a
yellow/brown-to-white whorled appearance of the cut surface
(22). Microscopically, SFTs are
shown to be comprised of spindle or short spindle cells and varying
quantities of vascular tissue (9).
The micro and macroscopic results of the present study were in
accordance with the findings of previous studies.
Immunohistochemically, SFT shows immunoreactivity with vimentin and
CD34, with the largest part of tumor also displaying positive
results for bcl-2 and CD99; however, the specimens do not express
EMA or S-100 proteins (23). The
immunohistochemical analysis results of the current study validated
the previous findings.
The primary treatment of SFT is surgical resection
with negative margins (24). All
patients from the present study underwent complete resection
without adjuvant treatments, such as radiotherapy or chemotherapy.
Tumors that cannot be completely excised or that show malignant
histological features may respond to radiation and/or chemotherapy.
Xue et al (25) described a
case of non-recurrent malignant SFT of the nasal and paranasal
areas, and found that the combination of cytoreductive surgery with
intensity-modulated radiation and stereotactic body radiation
therapies have a good result. Studies have also demonstrated that
preoperative embolization may be employed prior to surgical
resection for highly vascular tumors (26,27).
Immunotherapy, for example with interferon, may also be effective
(28).
A previous study showed that the prognosis of
patients with SFT is favorable (29).
Patients that underwent complete tumor resection showed 100%
survival at a mean follow-up period of 1.9 years (29). In the present study, all 25 patients
that received a complete follow-up were alive at the time of
writing. The longest follow-up period among these patients was 130
months. The recurrence rate of SFT was 4%.
In conclusion, SFT is a rare systemic disease with
no particular clinical manifestations. In the cases reviewed in the
present study, CT and MRI scans and color Doppler ultrasound were
important for the diagnosis of SFT; however, the final diagnosis
relied on pathological and immunohistochemical examinations.
Surgery was the primary treatment for SFT, and the prognosis
following complete tumor resection was favorable.
References
|
1
|
Klemperer P and Coleman BR: Primary
neoplasms of the pleura. A report of five cases. Am J Ind Med.
22:1–31. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gold JS, Antonescu CR, Hajdu C, Ferrone
CR, Hussain M, Lewis JJ, Brennan MF and Coit DG: Clinicopathologic
correlates of solitary fibrous tumors. Cancer. 94:1057–1068. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
vanHoudt WJ, Westerveld CM, Vrijenhoek JE,
van Gorp J, van Coevorden F, Verhoef C and van Dalen T: Prognosis
of solitary fibrous tumors: A multicenter study. Ann Surg Oncol.
20:4090–4095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
England DM, Hochholzer L and McCarthy MJ:
Localized benign and malignant fibrous tumors of the pleura. A
clinicopathologic review of 223 cases. Am J Surg Pathol.
13:640–658. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ogawa K, Tada T, Takahashi S, Sugiyama N,
Inaguma S, Takahashi SS and Shirai T: Malignant solitary fibrous
tumor of the meninges. Virchows Arch. 444:459–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saceda-Gutiérrez JM, Isla-Guerrero AJ,
Pérez-López C, Ortega-Martínez R, de la Riva A Gómez,
Gandia-González ML, Gutiérrez-Molina M and Rey-Herranz JA: Solitary
fibrous tumors of the meninges: Report of three cases and
literature review. Neurocirugia (Astur). 18:496–504. 2007.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adeleye AO, Ogun OA and Ogun GO: Orbital
solitary fibrous tumor. Another rare case from Africa. Int
Ophthalmol. 30:315–318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kessler A, Lapinsky J, Berenholz L,
Sarfaty S and Segal S: Solitary fibrous tumor of the nasal cavity.
Otolaryngol Head Neck Surg. 121:826–828. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Regan EM, Vanguri V, Allen CM, Eversole
LR, Wright JM and Woo SB: Solitary fibrous tumor of the oral
cavity: Clinicopathologic and immunohistochemical study of 21
cases. Head Neck Pathol. 3:106–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeong AK, Lee HK, Kim SY and Cho KJ:
Solitary fibrous tumor of the parapharyngeal space: MR imaging
findings. AJNR Am J Neuroradiol. 23:473–475. 2002.PubMed/NCBI
|
|
11
|
Kim TA, Brunberg JA, Pearson JP and Ross
DA: Solitary fibrous tumor of the paranasal sinuses: CT and MR
appearance. AJNR Am J Neuroradiol. 17:1767–1772. 1996.PubMed/NCBI
|
|
12
|
Cho KJ, Ro JY, Choi J, Choi SH, Nam SY and
Kim SY: Mesenchymal neoplasms of the major salivary glands:
Clinicopathological features of 18 cases. Eur Arch
Otorhinolaryngol. 265(Suppl 1): S47–S56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mathew GA, Ashish G, Tyagi AK,
Chandrashekharan R and Paul RR: Solitary Fibrous Tumor of Nasal
Cavity: A Case Report. Iran J Otorhinolaryngol. 27:307–312.
2015.PubMed/NCBI
|
|
14
|
Huang SC, Li CF, Kao YC, Chuang IC, Tai
HC, Tsai JW, Yu SC, Huang HY, Lan J, Yen SL, et al: The
clinicopathological significance of NAB2-STAT6 gene fusions in 52
cases of intrathoracic solitary fibrous tumors. Cancer Med.
5:159–168. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chis O and Albu S: Giant solitary fibrous
tumor of the parotid gland. Case Rep Med.
2014:9507122014.PubMed/NCBI
|
|
16
|
Li XM, Reng J, Zhou P, Cao Y, Cheng ZZ,
Xiao Y and Xu GH: Solitary fibrous tumors in abdomen and pelvis:
Imaging characteristics and radiologic-pathologic correlation.
World J Gastroenterol. 20:5066–5073. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park SB, Park YS, Kim JK, Kim MH, Oh YT,
Kim KA and Cho KS: Solitary fibrous tumor of the genitourinary
tract. AJR Am J Roentgenol. 196:W132–W137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bauer JL, Miklos AZ and Thompson LD:
Parotid gland solitary fibrous tumor: A case report and
clinicopathologic review of 22 cases from the literature. Head Neck
Pathol. 6:21–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Messa-Botero OA, Romero-Rojas AE, Olaya SI
Chinchilla, Díaz-Pérez JA and Tapias-Vargas LF: Primary malignant
solitary fibrous tumor/hemangiopericytoma of the parotid gland.
Acta Otorrinolaringol Esp. 62:242–245. 2011.(In Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hunt I, Ewanowich C, Reid A, Stewart K,
Bédard EL and Valji A: Managing a solitary fibrous tumour of the
diaphragm from above and below. ANZ J Surg. 80:370–371. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kita Y: Pleural solitary fibrous tumor
from diaphragm, being suspected of liver invasion; report of a
case. Kyobu Geka. 65:338–340. 2012.(In Japanese). PubMed/NCBI
|
|
22
|
Wang H, Chen P, Zhao W, Shi L, Gu X and Xu
Q: Clinicopathological findings in a case series of abdominopelvic
solitary fibrous tumors. Oncol Lett. 7:1067–1072. 2014.PubMed/NCBI
|
|
23
|
Zhong Q and Yuan S: Total resection of a
solitary fibrous tumor of the sellar diaphragm: A case report.
Oncol Lett. 5:1783–1786. 2013.PubMed/NCBI
|
|
24
|
Gengler C and Guillou L: Solitary fibrous
tumour and haemangiopericytoma: Evolution of a concept.
Histopathology. 48:63–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xue Y, Chai G, Xiao F, Wang N, Mu Y, Wang
Y and Shi M: Post-operative radiotherapy for the treatment of
malignant solitary fibrous tumor of the nasal and paranasal area.
Jpn J Clin Oncol. 44:926–931. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zerón-Medina J, Rodríguez-Covarrubias F,
García-Mora A, Guerrero-Hernandez M, Chablé-Montero F,
Albores-Saavedra J and Medina-Franco H: Solitary fibrous tumor of
the pelvis treated with preoperative embolization and pelvic
exenteration. Am Surg. 77:112–113. 2011.PubMed/NCBI
|
|
27
|
Botchu R, Khan AN, Adair W and Elabassy M:
Solitary fibrous tumor made resectable after successful
endovascular embolization. J Gastrointest Cancer. 42:287–291. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cuello J and Brugés R: Malignant solitary
fibrous tumor of the kidney: Report of the first case managed with
interferon. Case Rep Oncol Med. 2013:5649802013.PubMed/NCBI
|
|
29
|
Manglik N, Patil S and Reed MF: Solitary
fibrous tumour of the parotid gland. Pathology. 40:89–91. 2008.
View Article : Google Scholar : PubMed/NCBI
|