Introduction
It was previously demonstrated that in solid tumors
only a minority of cancer cells have the capacity to proliferate
extensively and form new tumors. The so-called cancer stem cells
are defined by common properties: the capability of self-renewal
and the ability of differentiation (1,2). For
cancer to develop, a population of continuously proliferating cells
must arise (3). These have to be
transformed and survive by altering the pathway of cancer cell
differentiation and proliferation over the lifetime of the host.
This new implication of the cancer stem cell model has been
suggested to account for potential differences in drug sensitivity
and may identify individual risk of metastasis. An improved
understanding of cancer stem cells could improve our ability to
regulate target therapy (1).
In 2003, Al-Hajj et al suggested the ability
to distinguish tumorigenic (tumor-initiating) cells from
non-tumorigenic cancer cells based on the expression of cell
surface markers including cluster of differentiation (CD)24 and
CD44 (4). This group proposed that
CD44+/CD24−/low breast cancer cells were
capable of forming a small number of tumors in immunocompromised
mice. The cells gave rise to phenotypically diverse cancer cells,
which they may be similar to stem cells.
The CD44 protein is involved in multiple distinct
cellular functions, including proliferation, adhesion and migration
(5). It has been associated with stem
cells in normal and malignant breast tissues (6). CD24 protein is considered to be a
molecule having the functions of adhesion, development and
progression (7,8).
However, the molecular characteristics and clinical
significance of CD44 and CD24 are unclear. In this study, we
assessed the clinical implications of CD44 and CD24 as markers of
breast cancer stem cells by identifying their correlation with
clinicopathological factors of invasive breast cancer.
Materials and methods
Patients and clinicopathological
data
The present study was approved by the institutional
review board (IRB) of Konkuk University Hospital (Seoul, Korea; IRB
number KUH1210036), and patient's informed consent was waived.
A total of 262 patients with invasive breast cancer
underwent surgery at Hanyang University Medical Center, Seoul,
Korea, from 1989 to 1999. None of the patients had a history of
previous therapies with anticancer drugs or radiation therapy. All
patients received routine chemotherapy or endocrine therapy
following surgery. In the present study, we retrospectively
analyzed the clinical and pathological data of the patients.
The evaluation variables were age, tumor size,
axillary lymph node metastasis status, tumor stage, histological
grade, estrogen receptor (ER) status, progesterone receptor (PR)
status and human epidermal growth factor receptor 2 (HER2) status.
We also analyzed the survival time of patients. The median
follow-up time was 91.9 months.
The baseline characteristics of the patients are
summarized in Table I. Tumor size and
axillary nodal status were categorized according to the
tumor-node-metastasis (TNM) system criteria from the American Joint
Committee on Cancer classification. The tumor histological grade
was classified as 1, 2 or 3 according to the guidance of Elston and
Ellis (9).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | No. of patients
(%) |
|---|
| All patients | 262 |
| Age, years |
|
|
<50 | 156 (59.5) |
| ≥50 | 106 (40.5) |
| Tumor size, cm |
|
| ≤2 | 115 (43.9) |
|
>2–5 | 127 (48.5) |
|
>5 | 20
(7.6) |
| Lymph node
metastasis |
|
| Yes | 129 (49.2) |
| No | 133 (50.8) |
| Stage |
|
| I | 52
(19.8) |
| II | 150 (57.3) |
| III | 60
(22.9) |
| Histological
gradea |
|
| 1 | 25
(11.4) |
| 2 | 116 (52.7) |
| 3 | 79
(35.9) |
| Estrogen
receptorb |
|
|
Positive | 144 (55.6) |
|
Negative | 115 (44.4) |
| Progesterone
receptorb |
|
|
Positive | 142 (54.8) |
|
Negative | 117 (45.2) |
| HER2c |
|
|
Positive | 73
(28.5) |
|
Negative | 183 (71.5) |
Tissue microarray construction
For tissue microarray (TMA) construction,
hematoxylin and eosin-stained sections of each tumor were examined.
Representative areas of tumors were selected and marked on the
corresponding paraffin block. The TMAs were assembled using a
tissue-array instrument (AccuMac Arrayer, ISU ABXIS Co. Ltd, Seoul,
Korea) consisting of thin-walled stainless steel punches and
stylets used to empty and transfer the needle content. The selected
area in the corresponding paraffin block was punched out and
embedded in microarray blocks. Two 3-mm cores of the selected area
in the corresponding paraffin block from each case were
arrayed.
Immunohistochemical assessment of
hormone receptor and HER2
Table II lists the
primary antibodies against ER (Lab Vision/Neomarkers, Fremont, CA,
USA), PR (Lab Vision/Neomarkers), HER2 (Dako, Glostrup, Denmark),
CD44 (Lab Vision/Neomarkers) and CD24 (Lab Vision/Neomarkers),
their dilutions, and the pretreatment conditions. Bound secondary
antibiotics were visualized by standard avidin-biotin-peroxidase
techniques using diaminobenzidine as a chromogen. ER and PR status
were determined by immunohistochemistry using ASCO/CAP guidelines
(10). In general, tumors with >1%
positively stained tumor cells were classified as positive for ER
and PR.
 | Table II.Antibodies and antigen retrieval
techniques used. |
Table II.
Antibodies and antigen retrieval
techniques used.
| Antibody | Clone | Source | Antigen
retrieval | Dilution |
|---|
| ER | Sp1 | Lab
Vision/Neomarkers | Benchmark XT
protocol | 1:600 |
| PR | Sp2 | Lab
Vision/Neomarkers | Benchmark XT
protocol | 1:600 |
| HER2 | Polyclonal | Dako | Benchmark XT
protocol | 1:100 |
| CD44 | 156-3C11 | Lab
Vision/Neomarkers |
Citrate/autoclave | 1:300 |
| CD24 | SN3 | Lab
Vision/Neomarkers |
Citrate/autoclave | 1:100 |
HER2 status was also determined by
immunohistochemistry. HER2-positive tumors were defined as 3+ and
HER2-negative tumors were defined as 0 or 1+, using the American
Society of Clinical Oncology/College of American Pathologists
(ASCO/CAP) guidelines as the criteria for immunohistochemical
staining (11). For tumors with
equivocal immunoreactivity (2+), we performed silver in situ
hybridization (SISH) of the HER2 gene to determine an accurate HER2
status.
Silver in situ hybridization for
assessment of HER2 status
For the determination of HER2 status in 2+ cases of
HER2 immunohistochemistry, HER2 SISH was performed on an automated
Ventana Benchmark instrument (Roche Diagnostics GmbH, Mannheim,
Germany), according to the manufacturer's instructions for the
INFORM HER2 Dual ISH DNA Probe cocktail. The evaluation of HER2
gene amplification status was performed in a blind manner using the
updated 2013 ASCO/CAP guidelines (11). HER2/CEP17 SISH signals were selected
and 20 non-overlapping nuclei were analyzed. Amplification was
defined by first examining the HER2/CEP17 ratio followed by the
average HER2 copy number. A ratio of ≥2.0 indicated amplification
of the gene regardless of the average HER2 copy number. A ratio of
<2.0 with an average HER2 copy number between 4.0 and <6.0
was defined as equivocal. For the equivocal cases, signals from 20
further tumor nuclei were counted in a second target area and a new
ratio was calculated. A ratio of <2.0 with an average HER2 copy
number <4.0 was defined as having no amplification.
Immunohistochemical assessment of CD44
and CD24
Four-micrometer sections were made from the
formalin-fixed, paraffin-embedded blocks, which were then
deparaffinized in xylene and rehydrated in a graded series of
alcohol solutions. For antigen retrieval, paraffin tissue sections
were cooked with 10 mM sodium citrate buffer, pH 6.0, at a
sub-boiling temperature for 15 min and cooled for 20 min at room
temperature. The sections were washed twice with Tris-buffered
saline for 10 min.
The antibody incubations were carried out at room
temperature for 1 h. CD44 and CD24 were detected with standard
avidin-biotin-peroxidase techniques using diaminobenzidine as the
chromogen. Afterwards, the slides were briefly counterstained with
hematoxylin, dehydrated and mounted.
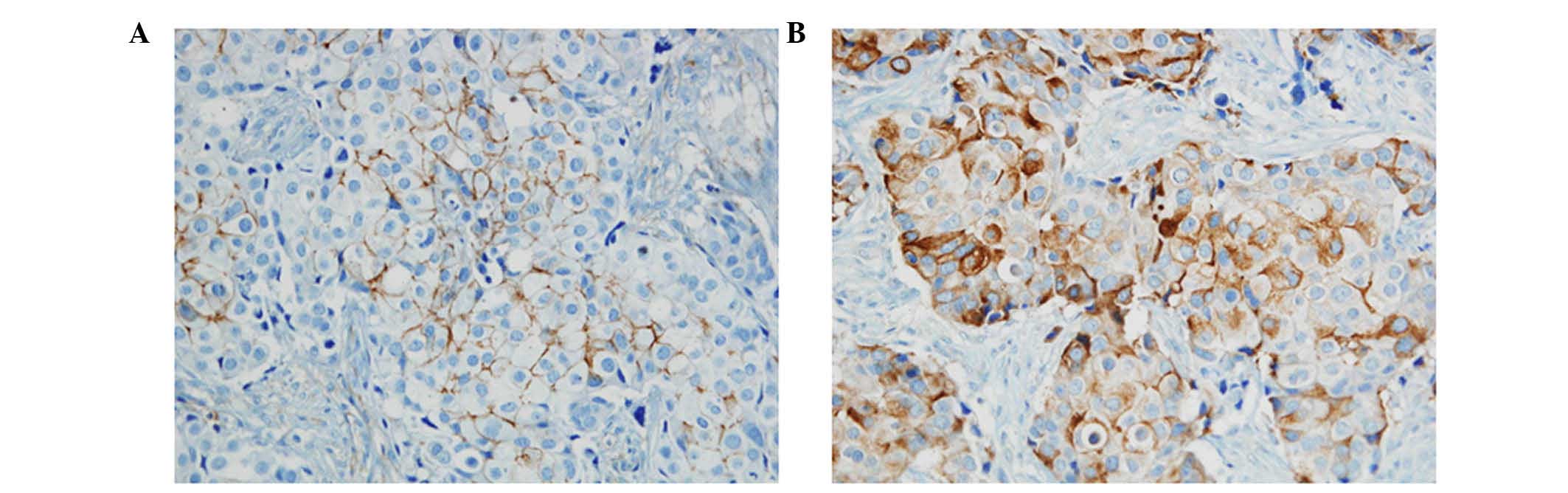
Immunostained samples were evaluated by two
pathologists. The expression of CD44 and CD24 was graded in terms
of the percentage of cytoplasmic membrane staining in each block as
well as the intensity of staining. However, there is no common
cut-off value on the patterns of staining. In this study, the grade
was designated according to the following criteria: 0, no staining;
1, staining in less than 50%; and 2, staining in more than 50% of
tumor cells. The grades were classified into two groups: (−), grade
0; (+), grade 1 and grade 2 (Fig.
1).
Statistical analysis
The Statistical Package for the Social Sciences (IBM
SPSS, Armonk, NY, USA) for Windows version 19.0 was used for all
the analyses. The Pearson's χ2 test was used to examine
statistically significant differences between the expression of
CD44 and CD24 and clinicopathological parameters. P<0.05 was
considered to indicate a statistically significant difference. The
overall survival was estimated using the Kaplan-Meier analysis.
Results
Clinicopathological
characteristics
All 262 cases had invasive breast cancer. The median
age of the patients was 47 years (range, 26–78). A total of 156
cases (59.5%) were younger than 50 years and 106 cases (40.5%) were
50 years or older. A total of 115 cases (43.9%) had a tumor size of
2 cm or less, 127 cases (48.5%) had tumors between 2 and 5 cm, and
20 cases (7.6%) had a tumor size greater than 5 cm. A total of 133
cases (50.8%) demonstrated negative axillary lymph node metastasis
and 129 cases (49.2%) demonstrated positive axillary lymph node
metastasis. A total of 52 cases (19.8%) were stage I, 150 cases
(57.3%) were stage II, and 60 cases (22.9%) were stage III
(Table I).
Immunohistochemistry
Among the 262 cases, only 259 were assessed for
expression of ER and PR. A total of 144 cases (55.6%) were
ER-positive and 115 cases (44.4%) were ER-negative. A total of 142
cases (54.8%) were PR-positive and 117 cases (45.2%) were
PR-negative. HER2 status was evaluated in 256 cases. A total of 73
cases (28.5%) demonstrated HER2 positivity and 183 cases (71.5%)
were HER2-negative (Table I). Among
the 262 cases, 259 cases were assessed for the expression of CD44
and CD24 protein. A total of 165 cases (63.7%) were CD44
protein-positive, whereas 94 cases (36.3%) were CD44
protein-negative. A total of 188 cases (71.7%) were CD24
protein-positive, whereas 74 cases (28.3%) were CD24
protein-negative (Table III).
 | Table III.Correlation between expression of
CD44, CD24 and clinicopathological variables. |
Table III.
Correlation between expression of
CD44, CD24 and clinicopathological variables.
|
| CD44 | CD24 |
|
|
|
|
|
Characteristics | Positive (%) | Negative (%) | P-value | Positive (%) | Negative (%) | P-value |
| All cases | 165 (63.7) | 94 (36.3) |
| 188 (71.7) | 74 (28.3) |
|
| Age, years |
|
| 0.276 |
|
| 0.057 |
|
<50 | 101 (61.2) | 51 (54.3) |
| 107 (56.9) | 49 (66.2) |
|
|
≥50 | 64 (38.8) | 43 (45.7) |
| 81 (43.1) | 25 (33.8) |
|
| Tumor size, cm |
|
| 0.056 |
|
| 0.854 |
| ≤2 | 79 (47.9) | 35 (36.6) |
| 84 (44.7) | 31 (41.9) |
|
|
>2 | 78 (47.3) | 49 (52.7) |
| 89 (47.3) | 38 (51.4) |
|
|
>5 | 8 (4.8) | 10 (10.7) |
| 15 (8.0) | 5 (6.7) |
|
| LN metastasis |
|
| 0.413 |
|
| 0.137 |
|
Yes | 86 (52.1) | 44 (46.8) |
| 98 (52.1) | 31 (41.9) |
|
| No | 79 (47.9) | 50 (53.2) |
| 90 (47.9) | 43 (58.1) |
|
| Stage |
|
| 0.393 |
|
| 0.746 |
| I | 36 (21.8) | 18 (18.0) |
| 37 (19.7) | 15 (20.3) |
|
| II | 90 (54.5) | 52 (57.0) |
| 107 (56.9) | 43 (58.1) |
|
|
III | 39 (23.7) | 24 (25.8) |
| 44 (23.4) | 16 (21.6) |
|
| Histological
gradea |
|
| 0.181 |
|
| 0.256 |
| 1 | 20 (14.7) | 5 (6.3) |
| 15 (9.7) | 10 (15.4) |
|
| 2 | 68 (50.0) | 43 (54.4) |
| 82 (52.9) | 34 (52.3) |
|
| 3 | 48 (35.3) | 31 (39.3) |
| 58 (37.4) | 21 (32.3) |
|
| Estrogen
receptorb |
|
| 0.857 |
|
| 0.661 |
|
Positive | 93 (56.7) | 51 (55.4) |
| 105 (56.5) | 39 (53.4) |
|
|
Negative | 72 (43.3) | 41 (44.6) |
| 81 (43.5) | 34 (46.6) |
|
| Progesterone
receptorb |
|
| 0.556 |
|
| 0.095 |
|
Positive | 90 (54.9) | 54 (58.7) |
| 108 (58.1) | 34 (46.6) |
|
|
Negative | 74 (45.1) | 38 (41.3) |
| 78 (41.9) | 39 (53.4) |
|
| HER2c |
|
| <0.001 |
|
| <0.001 |
|
Positive | 33 (20.5) | 39 (42.4) |
| 65 (35.3) | 8 (11.1) |
|
|
Negative | 128 (79.5) | 53 (57.6) |
| 119 (64.7) | 64 (88.9) |
|
Correlation between CD44 and CD24 and
clinicopathological variables
Table III shows the
correlations between the expression of CD44 and CD24 and
clinicopathological factors. HER2-negative status was associated
with positive CD44, and HER2-positive status was associated with
positive CD24 (P<0.001 and P<0.001, respectively). There was
no correlation between the expression of CD44 and CD24 and other
clinicopathological factors, including age, tumor size, axillary
lymph node metastasis status, stage, histological grade or hormonal
status.
The median follow-up time was 91.9 months. The
Kaplan-Meier method was used to identify the prognostic
significance of CD44 and CD24. There was no significant difference
between overall survival and the expression of CD44 or CD24
(P=0.437, P=0.976, respectively).
Discussion
Cancer stem cells have the common ability to
self-renew, differentiate, acquire drug resistance, survive and
migrate. Breast cancer stem cells are a small population of cells
which have classic features of cancer stem cells, and become
tumorigenic cells through the accumulation of mutations (12). The initial identification of breast
cancer stem cells was based on a combination of CD44 and CD24; in
particular, the CD44+/CD24−/low phenotype has
been reported to have stem cell properties (4).
In contrast, other studies have revealed that the
prevalence of CD44+/CD24−/low cells was not
significantly associated with breast tumor progression or patient
survival (13,14). Further studies demonstrated that the
CD44+/CD24−/low cells were transit
progenitors; however, they did not determine either the molecular
subtype or clinical parameters in breast cancer (15).
CD44 is a type I transmembrane glycoprotein receptor
that binds primarily to the extracellular glycosaminoglycan
hyaluronan. This protein is also known as a cellular adhesion
molecule and has been linked to diverse effects including cellular
adhesion, migration and invasion, which are significant in cancer
progression (16,17).
CD24 is a glycosylphosphatidylinositol-anchored
membrane protein that is also known as a cell surface molecule, and
represents small membrane microdomains endowed with cell adhesion
and cell signaling properties (18,19).
The aim of this study was to investigate the
clinical significance of CD44 and CD24 expression in
paraffin-embedded sections of breast cancer. We examined the
correlation between expression of the markers and
clinicopathological parameters.
According to Horiguchi et al, higher CD44
expression was significantly correlated with smaller tumor size,
negative axillary lymph node metastasis and lower stage (20). In this study, we did not observe any
correlation between CD44 expression and clinicopathological
factors, with the exception of HER2 status. Our data revealed that
the expression of CD44 was significantly correlated with
HER2-negative status (P<0.001). HER2-negative is part of the
basal phenotype. Bànkfalvi et al indicated that
myoepithelial cells expressed CD44 in normal breast epithelium, and
that this is implicated in the early stage of breast carcinogenesis
(21). Herrera-Gayol et al
observed that CD44 expression was involved in two of the three
steps of the invasive cascade and could not be confidently used as
a reliable prognostic indicator (22). Sanchez et al revealed a
deregulation in the CD44 expression pattern in malignant tumors,
but did not identify a correlation between this deregulation and
clinicopathological factors (23).
Conversely, Bànkfalvi et al observed that increased levels
of CD44 expression were correlated with poor prognosis and
metastatic involvement of the axillary lymph nodes in breast cancer
(21). Looi et al revealed
that CD44 played a role in the progression of breast cancer
(24).
According to Horiguchi et al, higher CD24
expression was significantly correlated with larger tumor size,
positive axillary lymph node metastasis and higher stage (20). In this study, we did not observe a
correlation between CD24 expression and clinicopathological
factors, with the exception of HER2 status. Our data revealed that
the expression of CD24 was significantly correlated with
HER2-positive status (P<0.001). The HER2-positive tumor is
generally considered an aggressive form of breast cancer. It has
been associated with rapid tumor growth through angiogenesis and
invasion in breast tumorigenesis. Honeth et al indicated
that HER2-positive groups highly expressed CD24 (25). Baumann et al revealed that CD
24 expression increased tumor cell metastasis in vivo,
proliferation and spreading, and induced cell motility and invasion
(26). Athanassiadou et al
revealed that CD24 expression was correlated with adverse
prognostic parameters, including increased stage, tumor grade 3,
positive lymph nodes and increased tumor size (27). Conversely, Schindelmann et al
observed that CD24 was significantly downregulated in invasive cell
lines, and this downregulation might be associated with a more
aggressive behavior of the tumor (28).
In conclusion, our results demonstrate that CD44
expression is significantly correlated with HER2-negative status in
invasive breast cancer cells. This finding suggests that CD44 is
correlated with tumorigenesis in HER2-negative breast cancer.
Conversely, CD24 expression is significantly correlated with
HER2-positive status. This finding suggests that CD24 is associated
with the aggressive phenotype of breast cancer cells. However,
there is no difference in clinical outcome and survival with
respect to CD44 and CD24 expression. Therefore, we may conclude
that CD44 and CD24 are markers associated with tumorigenesis in
breast cancer, but not sufficient factors to determine the
prognosis of invasive breast cancer.
Acknowledgements
This study was supported by grants from the Korea
Breast Cancer Foundation.
References
|
1
|
Al-Hajj M, Becker MW, Wicha M, Weissman I
and Clarke MF: Therapeutic implications of cancer stem cells. Curr
Opin Genet Dev. 14:43–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smalley M and Ashworth A: Stem cells and
breast cancer: a field in transit. Nat Rev Cancer. 3:832–844. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clarke MF and Fuller M: Stem cells and
cancer: two faces of eve. Cell. 124:1111–1115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ponta H, Sherman L and Herrlich PA: CD44:
from adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hebbard L, Steffen A, Zawadzki V, Fieber
C, Howells N, Moll J, Ponta H, Hofmann M and Sleeman J: CD44
expression and regulation during mammary gland development and
function. J Cell Sci. 113:2619–2630. 2000.PubMed/NCBI
|
|
7
|
Aigner S, Sthoeger ZM, Fogel M, Weber E,
Zarn J, Ruppert M, Zeller Y, Vestweber D, Stahel R, Sammar M and
Altevogt P: CD24, a mucin-type glycoprotein, is a ligand for
P-selectin on human tumor cells. Blood. 89:3385–3395.
1997.PubMed/NCBI
|
|
8
|
Kristiansen G, Winzer KJ, Mayordomo E,
Bellach J, Schlüns K, Denkert C, Dahl E, Pilarsky C, Altevogt P,
Guski H and Dietel M: CD24 expression is a new prognostic marker in
breast cancer. Clin Cancer Res. 9:4906–4913. 2003.PubMed/NCBI
|
|
9
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. Arch Pathol Lab Med. 134:907–922. 2010.PubMed/NCBI
|
|
11
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abraham BK, Fritz P, McClellan M,
Hauptvogel P, Athelogou M and Brauch H: Prevalence of
CD44+/CD24-/low cells in breast cancer may not be associated with
clinical outcome but may favor distant metastasis. Clin Cancer Res.
11:1154–1159. 2005.PubMed/NCBI
|
|
14
|
Mylona E, Giannopoulou I, Fasomytakis E,
Nomikos A, Magkou C, Bakarakos P and Nakopoulou L: The
clinicopathologic and prognostic significance of CD44+/CD24(−/low)
and CD44−/CD24+ tumor cells in invasive
breast carcinomas. Hum Pathol. 39:1096–1102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lü X, Xu K, Lü H, Yin Y, Ma C, Liu Y, Li H
and Suo Z: CD44(+)/CD24(−) cells are transit progenitors and do not
determine the molecular subtypes and clinical parameters in breast
carcinomas. Ultrastruct Pathol. 35:72–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iida N and Bourguignon LY: New CD44 splice
variants associated with human breast cancers. J Cell Physiol.
162:127–133. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Naor D, Sionov RV and Ish-Shalom D: CD44:
Structure, function, and association with the malignant process.
Adv Cancer Res. 71:241–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Henniker AJ: CD24. J Biol Regul Homeost
Agents. 15:182–184. 2001.PubMed/NCBI
|
|
19
|
Fogel M, Friederichs J, Zeller Y, Husar M,
Smirnov A, Roitman L, Altevogt P and Sthoeger ZM: CD24 is a marker
for human breast carcinoma. Cancer Lett. 143:87–94. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Horiguchi K, Toi M, Horiguchi S, Sugimoto
M, Naito Y, Hayashi Y, Ueno T, Ohno S, Funata N, Kuroi K, et al:
Predictive value of CD24 and CD44 for neoadjuvant chemotherapy
response and prognosis in primary breast cancer patients. J Med
Dent Sci. 57:165–175. 2010.PubMed/NCBI
|
|
21
|
Bànkfalvi A, Terpe HJ, Breukelmann D, Bier
B, Rempe D, Pschadka G, Krech R and Böcker W: Gains and losses of
CD44 expression during breast carcinogenesis and tumour
progression. Histopathology. 33:107–116. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herrera-Gayol A and Jothy S: Adhesion
proteins in the biology of breast cancer: contribution of CD44. Exp
Mol Pathol. 66:149–156. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lockhart M Sanchez, Hajos SE, Basilio FM,
Mongini C and Alvarez E: Splice variant expression of CD44 in
patients with breast and ovarian cancer. Oncol Rep. 8:145–151.
2001.PubMed/NCBI
|
|
24
|
Looi LM, Cheah PL, Zhao W, Ng MH and Yip
CH: CD44 expression and axillary lymph node metastasis in
infiltrating ductal carcinoma of the breast. Malays J Pathol.
28:83–86. 2006.PubMed/NCBI
|
|
25
|
Honeth G, Bendahl PO, Ringnér M, Saal LH,
Gruvberger-Saal SK, Lövgren K, Grabau D, Fernö M, Borg A and
Hegardt C: The CD44+/CD24- phenotype is enriched in basal-like
breast tumors. Breast Cancer Res. 10:R532008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baumann P, Cremers N, Kroese F, Orend G,
Chiquet-Ehrismann R, Uede T, Yagita H and Sleeman JP: CD24
expression causes the acquisition of multiple cellular properties
associated with tumor growth and metastasis. Cancer Res.
65:10783–10793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Athanassiadou P, Grapsa D, Gonidi M,
Athanassiadou AM, Tsipis A and Patsouris E: CD24 expression has a
prognostic impact in breast carcinoma. Pathol Res Pract.
205:524–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schindelmann S, Windisch J, Grundmann R,
Kreienberg R, Zeillinger R and Deissler H: Expression profiling of
mammary carcinoma cell lines: Correlation of in vitro invasiveness
with expression of CD24. Tumour Biol. 23:139–145. 2002. View Article : Google Scholar : PubMed/NCBI
|