Introduction
Schofield introduced the concept of the
hematopoietic stem cell (HSC) niche in 1978 (1). The niche contains the endosteal niche
and the vascular niche. The endosteal niche is a prominent
compartment of the HSC niche and it assists HSCs to maintain the
quiescent state under normal conditions. The pathological niche of
leukemia models promotes leukemia stem cell (LSC) proliferation,
survival and protects LSCs from the killing effects of
chemotherapeutic drugs. The osteoblast niche becomes the region for
the settlement and proliferation of certain leukemia cells,
particularly the stem cells (2).
Despite the uncontrolled growth and survival features, the majority
of primary acute myeloid leukemia (AML) cells rely on the
microenvironment, which may be a targetable weakness (3).
Future studies should assess the effect of an
abnormal niche on the biological characteristics of leukemia cells
and investigate the mechanism of interaction between the bone
marrow microenvironment and leukemia cells. These studies may
promote the treatment of leukemia. The conventional in vitro
two-dimensional (2D) co-culture system does not reproduce the in
vivo microenvironment (4).
However, cells maintained in spatial distribution can mimic the
complex, cellular organization of the bone marrow microenvironment.
The 3D culture system has been used compellingly in recent years
(5).
Osteopontin (Opn) is a negatively charged
phosphorylated glycoprotein that is secreted by osteoblasts. The
overexpression of Opn is a feature of hematologically malignant
tumors. Opn is the key factor that induces HSCs and leukemia cells
to stay in the endosteal niche (6)
and maintain the G0 phase. Integrin αvβ3 is a membrane receptor
protein that can recognize a key structure of Opn, namely the RGD
sequence. The combination of the two proteins can play a vital role
in tumor cell adhesion, migration, invasion and tumor angiogenesis
(7). If the domain structure of αvβ3
has defects or mutations, it will lead to loss of promoting
adhesion function. Due to its high expression in tumor cells and
its low expression in normal cells, integrin αvβ3 may be an ideal
target for tumor therapy. There is no evidence that
cyclo(Arg-Gly-Asp-d-Phe-Val) [c(RGDfV)], which blocks αvβ3, can
mobilize leukemia cells to the peripheral blood, and it is not
clear whether c(RGDfV) can increase the sensitivity of the leukemia
cells to chemotherapy drugs after disrupting the
microenvironment.
The present study mainly clarified the following: i)
How a 3D culture system compared with the common 2D culture system;
ii) the effect of c(RGDfV) on the adhesion function of the
endosteal niche; iii) the effects of c(RGDfV) on leukemia cell
apoptosis and the cell cycle; and vi) the changes in leukemia cell
sensitivity to cytarabine (Ara-C) following the administration of
the drug.
Materials and methods
Materials and reagents
The monoclonal mouse anti-human αvβ3-fluorescein
isothiocyanate (FITC; catalog no. 336403), cluster of
differentiation (CD)34-FITC (catalog no. 343603), CD45-phycoerythin
(PE; catalog no. 368509), CD90-FITC (catalog no. 328107) and
CD105-PE (catalog no. 323205) antibodies were obtained from
BioLegend Inc., (San Diego, CA, USA). Test size products are
transitioning from 20 to 5 µl per test. The suggested use of this
reagent for flow cytometry is per million cells in 100 µl staining
volume. c(RGDfV) was provided by GL Biochem (Shanghai) Ltd.
(Shanghai, China). Human mesenchymal stem cell (MSC) osteogenic
differentiation medium was provided by Cyagen Biosciences, Inc.
(Santa Clara, CA, USA), and contained basal medium, ascorbate,
b-glycerosphophate and dexamethasone; these components were also
purchased individually from Sigma-Aldrich (St. Louis, MO, USA).
Dulbecco's modified Eagle's medium (DMEM)/F12 medium, RPMI 1640
medium, trypsin and fetal bovine serum (FBS) were purchased from GE
Healthcare Life Sciences (Hyclone; Logan, UT, USA). A human Opn
enzyme-linked immunosorbent assay (ELISA) kit was purchased from
R&D Systems (Minneapolis, MN, USA). The Transwell Insert system
for the migration assay was purchased from BD Biosciences (Bergen,
NJ, USA) The Cell Cycle Analysis kit and BCECF-AM were provided by
Beyotime Institute of Biotechnology (Haimen, China). The Annexin
V-FITC apoptosis detection kit was obtained from BD Pharmingen (San
Diego, CA, USA).
Patient cells
In total, 10 AML (M5) samples from the bone marrow
of AML patients at the time of preliminary diagnosis were acquired
after obtaining written informed consent. Patients were enrolled
between March and October 2013 from the Department of Hematology of
Xinqiao Hospital, The Third Military Medical University (Chonqing,
China). Ethical approval for this study was provided by the Medical
Ethics Committee of Xinqiao Hospital (The Third Military Medical
University). For the culture of osteoblasts, bone marrow aspirates
underwent Ficoll-Paque gradient centrifugation (Tianjin Hao Yang
Biological Products Technology Co., Ltd., Tiajin, China) at 400 × g
for 20 min. After two passages, the human MSC osteogenic
differentiation medium was used as previously described (8). FLT3-internal tandem duplication
(ITD)-positive leukemia MV4-11 cells were obtained from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in complete RPMI 1640 medium at 37°C in a humidified environment
with 5% CO2 and the cells began with passage every 2
days.
Isolation and culture of human
MSCs
Samples of ~5 ml of heparinized bone marrow were
collected from the AML patients at the preliminary diagnosis after
obtaining written informed consent. The mononuclear cell fraction
was isolated by density gradient centrifugation on Ficoll-Hypaque
(density, 1.077 g/cm3) and cultured in DMEM/F12
supplemented with 10% FBS. After two passages, the cells were
identified and used for osteodifferentiation.
Biomimetic osteoblast niche
For the 3-dimensional (3D) scaffolds, MSCs that had
been passaged twice were digested with 0.25% trypsin and
re-suspended in medium. A total of 6.5×104 MSCs in 60 µl
osteoinductive culture medium containing 10−8 mol/l
dexamethasone, 10 mmol/l β-glycerophosphate and 50 µg/ml retinoic
acid was dripped into the wells of 24-well plates. The cell-seeded
scaffolds were put into the incubator for 3 h prior to adding
another 940 µl of fresh medium. Additionally, 1.2×104
MSCs were cultured without scaffolds in the 24-well plates to
accord with the same cell seeding density (number of
cells/cm2). The medium was changed twice every week. The
cells were identified after 3 weeks. Alizarin red staining and
alkaline phosphatase (ALP) staining were conducted for the
identification of osteoblasts. Positive staining results
demonstrated that the cells were cultured successfully.
Electron microscopy
The cells were cultured and fixed in 2.5%
glutaraldehyde in a 4°C refrigerator overnight, and then the
samples were dehydrated in a graded series of ethanol, air-dried
and gold sputtered. The cells were analyzed using a KYKY-EM3200
scanning electron microscope (KYKY Technology Development, Inc.,
Beijing, China).
Measurement of Opn and ALP levels in
osteoblast supernatant
Supernatant was collected on the 7th, 14th and 21st
days following the culture of the cells, and they were analyzed
using a human OPN ELISA kit, according to the manufacturer's
instructions. Various known concentrations of standard solution (50
µl), to generate the standard curve, or 40 µl samples plus 10 µl
sample diluent were added into each hole. Next, 50 µl horseradish
peroxidase-labeled detection antibody was added into the sample
standard bore holes for 60 min. The liquid was then discarded, and
50 µl each of substrate A and B (visualization reagents from the
ELISA kit) were mixed and incubated for 15 min in the dark.
Finally, stop solution was added into each hole and the optical
density value was measured using a microplate reader. Besides, the
supernatant was collected when cells were cultured at the 7th, 14th
and 21st days, and 1 ml of 2% Triton X-100 was added to the cells
for long-term refrigeration. Next, the lysate (100 µl) was added to
a 96-well plate, then use the ALP kit to test the activity of
ALP.
Group of experiments
Once the osteoblasts had been cultured successfully,
they were cultured in RPMI 1640 medium for 2 days. MV4–11 cells
(1.8×105 cells/ml in the 2D culture system and
1×106 cells/ml in the 3D culture system) were
co-cultured with leukemia osteoblasts in RPMI 1640 medium. The
experiments used two groups: The experimental group received
c(RGDfV) (35 nmol/ml) and the control group received an equal
volume of phosphate-buffered saline (PBS) only.
Adhesion test by fluorometric
assay
MV4–11 cells (1.8×105 cells/ml in the 2D
culture system and 1×106 cells/ml in the 3D culture system) were
prelabeled with BCECF-AM (3 µM), mixed with c(RGDfV) and
co-cultured with osteoblasts for 4 h at 37°C. Non-adherent cells
were washed away using PBS three times, and images of the adherent
cells were captured prior to fluorescence intensity being
measured.
Flow cytometric analyses of αvβ3
expression
To analyze the expression of αvβ3 surface markers on
the leukemia cells prior to and after co-culture with osteoblasts,
the cells were stained with a mouse anti-human monoclonal αvβ3-FITC
antibody. The expression of the surface antigens was analyzed using
a flow cytometer.
Transwell migration assay
Osteoblasts were suspended in 24-well plates. When
the osteoblasts reached 80% confluence, untreated or
c(RGDfV)-treated MV4–11 cells were seeded in the upper chamber of
the Transwell inserts. After a 4-h incubation, the stromal layer
containing cells that had migrated was carefully washed twice with
PBS and counted.
Detection of in vitro apoptosis and
cell cycle analysis
The leukemia cells were seeded alone, or with
osteoblasts in the 2D or 3D culture system. The cells were then
treated with 35 nmol/ml c(RGDfV) for 24 h. Next, Ara-C (0.02, 0.2
and 2 µg/ml) was applied for 24 h. The cells (1×106)
were then washed. A double staining method with Annexin
V-FITC/propdium iodide (PI) was used for the detection of in
vitro apoptosis. Annexin V-FITC and propidium iodide (PI) were
added, and the cells were incubated for 15 min at 4°C. The cells
were washed and analyzed for apoptosis with the use of flow
cytometry. PI staining was also used to detect the levels of DNA in
order to assess cell cycle distribution. The cells
(1×106) were gathered and washed with PBS, and were
fixed with 75% ethanol prior to the addition of 50 µg/ml PI. The
cell cycle distribution was analyzed by flow cytometry.
Statistical analysis
Data were analyzed using SPSS software version 17.0
(Chicago, IL, USA). Student's t-test was used for comparisons
between two groups and an analysis of variance was used for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell culture, osteogenic
differentiation of MSCs and the different growth conditions in the
2D and 3D culture systems
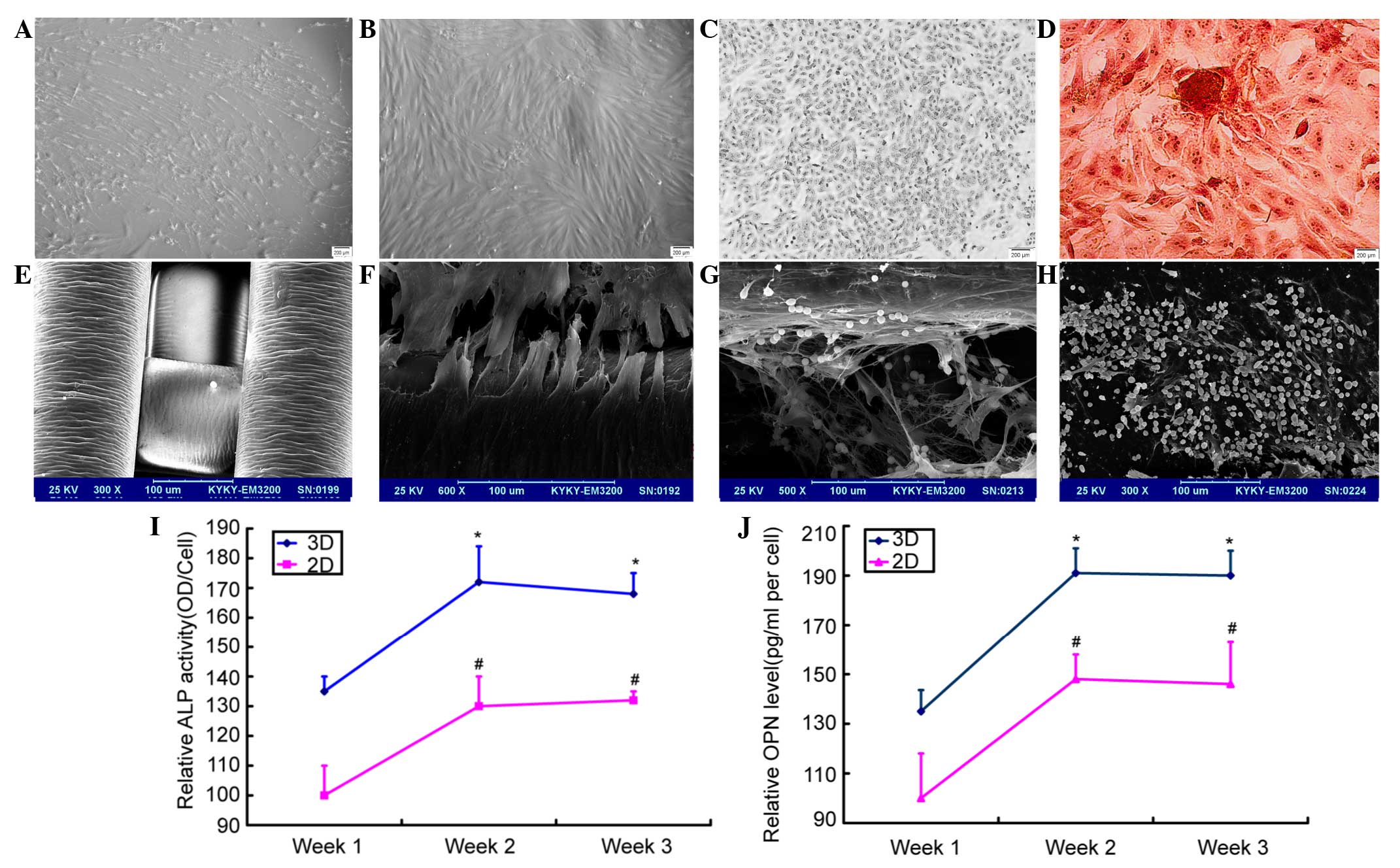
MSCs were obtained from the bone marrow of leukemia
patients and cultured (Fig. 1A). The
cells were identified successfully as CD90−− and CD105-positive,
but CD34− and CD45-negative. Next, the MSCs underwent ossification
induction and identification (Fig.
1B-D) In the PS scaffolds, interconnected networks of pores
were observed upon scanning microscopy. The cells gradually became
spindled after they were seeded on the scaffolds. The cells adhered
on the inner surfaces of the scaffolds, and the compatibility
between the cells and the material was good (Fig. 1E). SEM revealed that the cells were
adhered on the scaffolds tightly (Fig.
1F). After the MV4-11 cells were added, the cells stretched out
long tentacle-like pseudopods to contact with the osteoblasts in
the niches (Fig. 1G), while the cells
in the 2D system were flat and produced less extracellular matrix
(Fig. 1H).
To detect the level of ALP expression, ELISA was
used for the supernatant samples. For the 2D and 3D culture
systems, the ALP level increased with the culture period (Fig. 1I). In the 2D culture system, if the
relative ALP activity of the 7th day was 100±10%, that of the 14th
day was 130±10% and that of the 21st day was 132±3%. In the 3D
culture system, the relative activity of the 7th day was 135±5%,
that of the 14th day was 172±12% and that of the 21st day was
168±7%. The activity of Opn was also assessed and similar effects
were found. In the 2D culture system, if the relative Opn activity
of the 7th day was 100±18%, that of the 14th day was 148±10% and
that of the 21st day was 146±17%. In the 3D culture system, the
relative activity of the 7th day was 135±8.6%, that of the 14th day
was 191±10% and that of the 21st day was 190±10%. MSCs
differentiated into osteoblasts, which was accompanied with the
rise of Opn levels. These experiments indicated that the Opn level
reached its highest level at 14 days and then began to level off
(Fig. 1J). The 3D scaffolds were
determined to be more suitable for cell growth.
c(RGDfV) induces disruption of
leukemia cell migration and adhesion to leukemia osteoblasts in the
3D and 2D culture systems
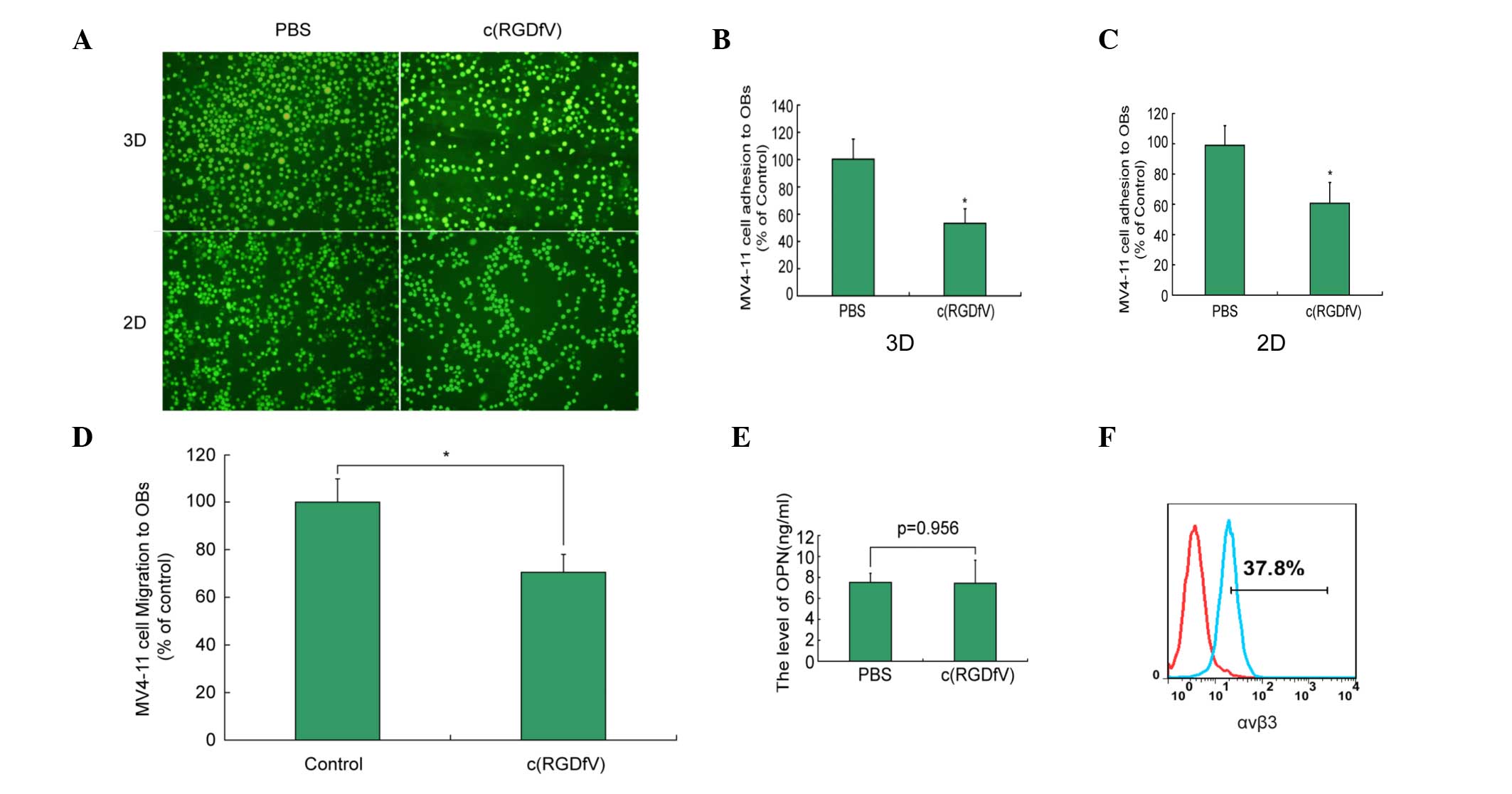
As shown in Fig. 2A and
B, the adhesion index of c(RGDfV) in the scaffolds was 52±12%
compared with that of the control group (P<0.05). The migration
index of c(RGDfV) in the scaffolds was 70±8% compared with that of
the control group (P<0.05) (Fig.
2C). c(RGDfV) induced the disruption of leukemia cell migration
in the 3D culture systems (Fig. 2D).
The adhesion and migration of the 2D culture system was similar to
that of the 3D culture system. In the in vitro studies,
c(RGDfV) did not affect the level of Opn (Fig 2E). The MV4–11 cells exhibited the
expression of αvβ3 (Fig. 2F).
c(RGDfV) has distinct effects on the
cell cycle
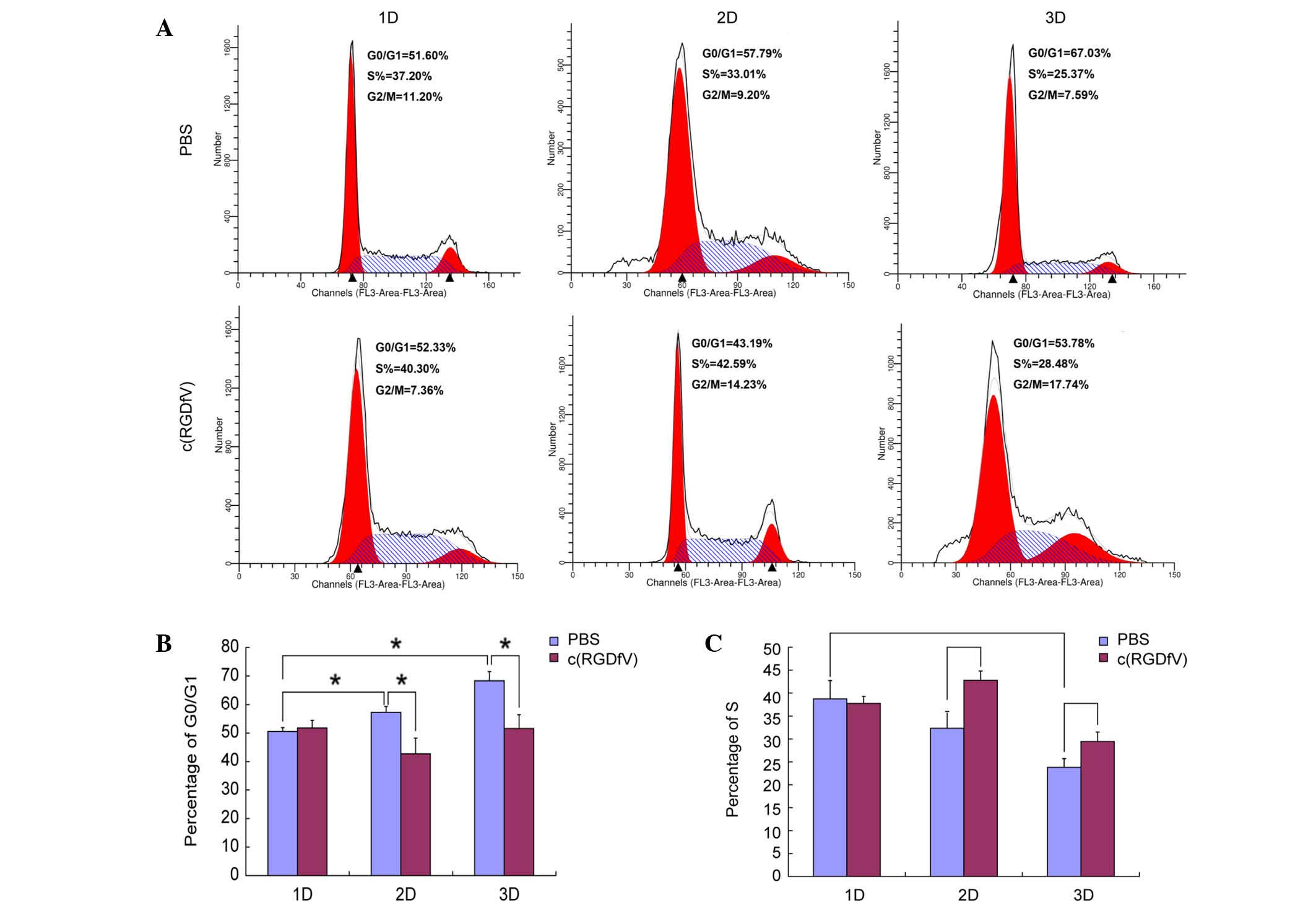
In the present study, the leukemia osteoblasts
induced the cell cycle arrest of the MV4-11 cells in the G0/G1
phase (69.67±3.2% in the 3D scaffolds, 57.26±2.05% in the 2D
culture system and 50.53±1.36% in the system in which the cells
were cultured alone) (P<0.001 for the 3D versus 1D culture
system; P=0.012 for the 2D versus 1D culture system). c(RGDfV) did
not affect the percentage of cells in the G0/G1 phase (phase rate)
when leukemia cells were cultured alone. c(RGDfV) induced the cells
to enter the cycle in the presence of osteoblasts in the 2D and 3D
culture systems. In the 2D culture system, G0/G1-phase rates
induced by the c(RGDfV) and control groups were 43.39±1.51 and
57.26±2.05%, respectively (P=0.013). The S-phase rates were
42.81±2.02 and 32.33±2.08%, respectively (P=0.003). In the 3D
culture system, the G0/G1-phase rates induced by the c(RGDfV) and
control groups were 52.92±4.88 and 69.67±3.2%, respectively
(P=0.008). The S-phase rates were 27.82±2.01 and 23.79±1.69%,
respectively (P=0.045). The 3D culture system had a higher arrest
effect on MV4-11 compared with that of the 2D culture system
(Fig. 3).
c(RGDfV) overcame drug resistance
induced by osteoblasts in Co-culture with leukemia cell lines
To investigate the effects of osteoblast cells on
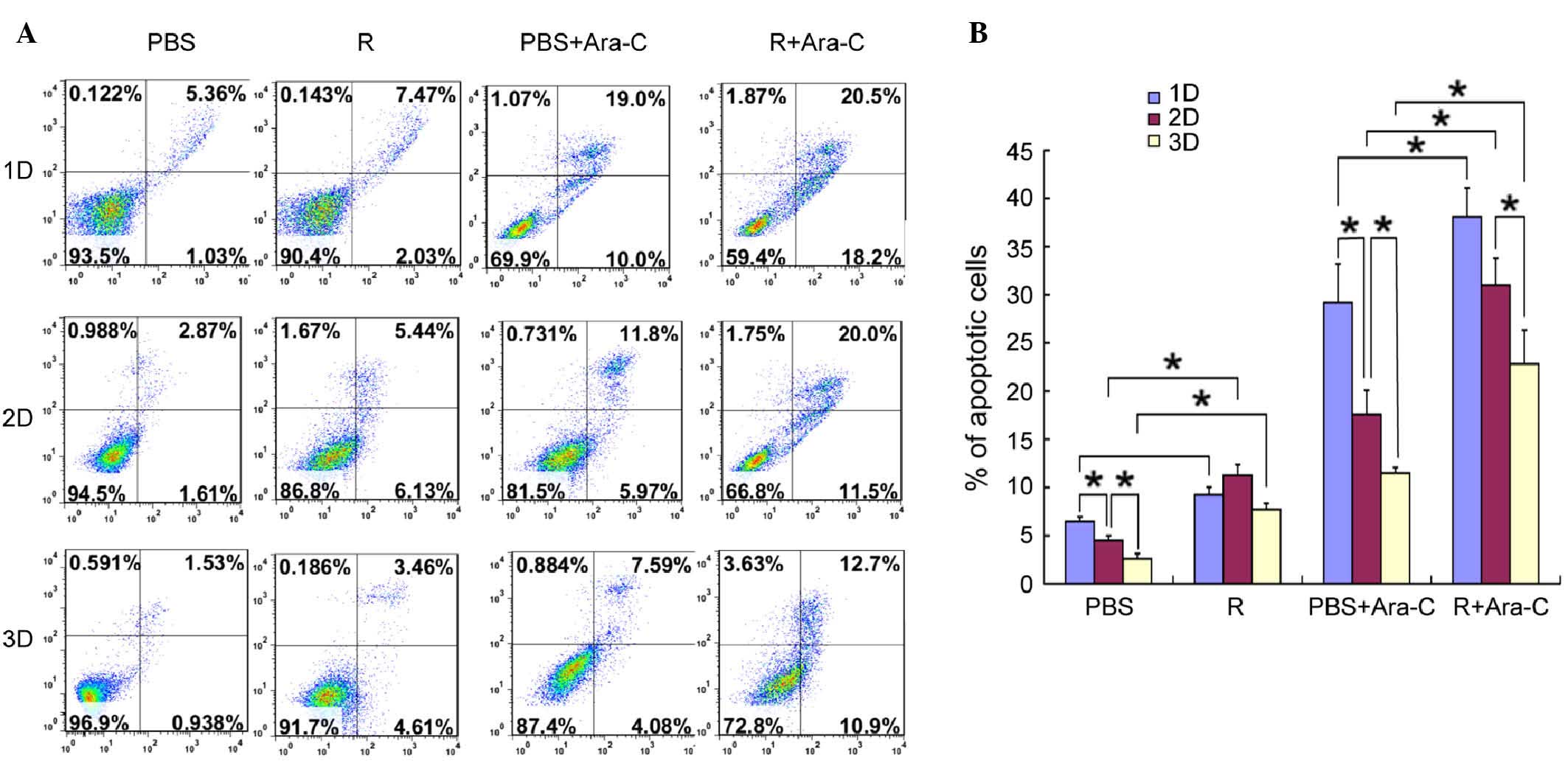
the apoptotic potential of MV4-11 cells, co-cultured leukemia cells
were stained and measured by flow cytometry. Fig. 4 shows that c(RGDfV) increased the
apoptosis rate induced by Ara-C in the absence of leukemia
osteoblasts, while in the presence of leukemia osteoblasts, the
apoptosis rate of the leukemia cells decreased. The percentage of
apoptotic cells was significantly decreased to 2.6±0.55% from
6.46±0.5% when the leukemia cells and osteoblasts were cultured on
the scaffolds compared with MV4-11 cells cultured alone
(P<0.05). c(RGDfV) increased the apoptosis rates in the presence
or absence of Ara-C when the leukemia cells were co-cultured with
the osteoblasts. Prior to Ara-C being administrated in the
scaffolds, the apoptosis rates induced by the c(RGDfV) and control
groups were 7.71±0.63 and 2.6±0.55%, respectively. After Ara-C was
administered, the apoptosis rates were 22.83±3.51 and 11.52±0.56%,
respectively. In contrast to the 2D culture system, the scaffolds
exhibited lower apoptosis rates and more leukemia cells were alive,
which indicated that the 3D scaffolds had higher drug resistance
and that the 3D culture system was favorable to cell growth (only
the 0.2 µg/ml dosage of Ara-C is displayed, however, the other two
doses exhibited similar effects). The experiments were repeated
twice and produced identical results (Fig. 4).
Discussion
The bone marrow niche contains a large variety of
cell types, including HSCs, MSCs, osteoblasts, macrophages, fat
cells, reticular cells and endothelial cells, and it regulates stem
cell quiescence, self-renewal and differentiation (9). Osteoblasts are the major compartments of
the endosteal niche and they are differentiated from MSCs.
Osteoblasts are bone-forming cells that play an important role in
the osteoblastic niche (10). The
endosteal niche resides under the endosteum. The area has a low
level of oxygen and the HSCs maintain it in a quiescent state (low
cycle or G0 phase) (11). In
addition, the osteoblasts maintain the balance between HSC homing
and trans-marrow migration (12,13). Under
the condition of normal hematopoiesis, the endosteal niche can
support the long-term survival of HPCs and long-term
culture-initiating cells (14).
To date, a large amount of 3D biological materials
have been used to test whether they could promote the growth and
osteogenic differentiation of MSCs. It was found that 3D Insert
polystyrene (PS) scaffolds promoted the adhesion and growth of
MSCs. Furthermore, they induced osteogenic differentiation with
osteogenic supplements (15). The 3D
PS scaffold relies on a solid polymer porous material. The scaffold
possesses 100% pore interconnectivity. The cylinders are 150 µm in
diameter, with 200-µm spaces between the fibers. The cultivation
method for the 3D scaffolds is similar to that of the 2D culture
system. In addition, it can enhance the efficiency of cell culture.
Cytokines and growth factors secreted by cells are easy to
separate. Cells can grow in the PS scaffolds in a relatively well
distributed manner and they stick tightly to the scaffolds. The
scaffolds are beneficial for cell adhesion and growth, and provide
sufficient space and surface area for the secretion of
extracellular matrix.
In the present study, a 3D bioengineered scaffold
coated with leukemia osteoblasts was created. MSCs were employed
and induced to differentiate into osteoblasts as feeder cells, and
the 2D and 3D culture system was constructed to imitate the
endosteal niche. In the experiments, the induced MSCs were
identified as osteoblasts according to their morphology and
expression of ALP. This showed that a biomimetic osteoblast niche
had successfully been constructed. Next, a leukemia cell line was
inoculated in it and the cells grew in the mimicked
microenvironment. It was found that the scaffolds could promote
cell proliferation. After 14 days of culture, the cell number had
reached its maximum, which may be a result of the little remaining
space for the growth of the cells. Compared with the 2D culture
system, the cells possessed more viability, more extracellular
matrix and longer proliferation periods in the 3D scaffold. The 3D
Inserts increased the surface area and space for cell adhesion and
growth compared with the 2D system. For example, the growth surface
of the 3D Insert-PS 24-well was 10.2 cm2, while the 2D
surface was 1.9 cm2. The scaffolds offer a large number
of cell binding sites and space for cell growth. 3D bioengineered
scaffolds caused the osteoblasts to form an elongated, highly
branched morphology, while the osteoblasts in the 2D system were
flat in shape.
The abilities of the leukemia osteoblasts to
decrease the apoptosis rate and their effects on the cell cycle in
the FLT3-ITD-positive myeloid leukemia MV4-11 cell line were then
tested. From the scanning electron microscopy, it was apparent that
the leukemia cells had adhered to the bone marrow stroma and
gradually embedded in the bone marrow stromal layer to form a
shelter structure. A few of the leukemia cells migrated to the
marrow stromal layer and others penetrated into the cytoplasm,
which constituted a shield phase association. In addition, the
apoptotic leukemia cell rate was lower in the presence of the
osteoblasts. It was also found that the co-culture of primary
cultured leukemia osteoblasts and MV4-11 cells made the leukemia
cells arrest in the G0/G1 phase. The bone marrow niche protected
the stem cells from the deleterious effects of Ara-C. The leukemia
microenvironment is believed to be a key factor of drug resistance.
Resistance may be caused by the release of soluble growth factors
or by cell-cell (16) or
cell-extracellular matrix (17)
interactions. Cell adhesion-mediated drug resistance is the
resistance to chemotherapy caused by malignant cells with stroma.
In AML, the bone marrow microenvironment offers a vital shelter of
minimal residual disease following chemotherapy (18). These data suggest that stromal cells
can protect the AML cells from the damage caused by chemotherapy
drugs. The present results are consistent with these results and
those of another previous study (19). Furthermore, the MV4-11 cells in the 3D
system displayed higher percentages of G0/G1 phase cells and fewer
apoptotic cells compared with the 2D culture system, indicating
that the 3D system better represented in vivo drug
resistance.
Osteopontin is a secreted phosphorylated
glycoprotein that is synthesized and secreted by various tissues
and cells. In the bone marrow, osteoblasts are the main cells to
secrete Opn. When binding with its receptor (αvβ integrin family or
CD44), Opn can mediate the adhesion between cells and stroma, and
activate the second messengers of the signal transduction pathways.
In addition, Opn can inhibit the apoptosis of cells (20). The levels of Opn in the tissue and
blood are the indices of the diagnosis and prognosis of cancer. Lee
et al (21) found that in
newly diagnosed AML patients, the higher the Opn level the worse
the prognosis. Opn is an independent adverse prognostic factor in
AML. The high expression of Opn in the leukemia bone marrow niche
is the pivotal factor for resistant and residual leukemia cells.
Opn may act as a ‘molecular switch’ in the adhering and sheltering
of AML cells by bone marrow microenvironment. In the present study,
it was found that Opn was expressed in the niche.
Opn can combine with αvβ3 and CD44, and regulate the
expression of the downstream effector genes. The combination plays
a key role in HSC adhesion and induces them to stay in the
endosteal niche and maintain the G0 period (22,23). αvβ3
is a membrane receptor protein with a specially recognized RGD
sequence (Arg-Gly-Asp). αvβ3 is highly expressed in tumor cells,
with low expression in normal cells, so it is an ideal target for
tumor therapy. If there are RGD domain mutations, Opn will lose the
function of promoting adhesion, and at the same time, it will
induce the apoptosis of the cells (24). Mitjans et al (25) administered the antagonists of αvβ3,
namely αvβ3-specific monoclonal antibody 17E6 and cyclic RGD
peptide, to melanoma cells and found that each inhibited cell
adhesion.
In the present study, c(RGDfV), which blocks αvβ3,
was administered, and it was found that this had no effect on the
cell cycle when leukemia cells were cultured alone. However,
c(RGDfV) caused additional effects to spontaneous and Ara-C-induced
apoptosis in leukemia cells and induced the cells to pass the
stationary phase in the presence of osteoblasts. Furthermore, the
leukemia cells in the 3D and 2D culture systems showed similar
effects. The 3D system also showed fewer apoptotic cells and a
greater percentage of G0/G1 phase cells, which indicated that the
3D scaffolds possessed a greater percentage of drug resistance. The
present study also tested the effects of c(RGDfV) on adhesion and
migration. The results showed that the drug decreased adhesion and
induced migration to the osteoblasts. Overall, c(RGDfV) may promote
the leukemia cells to leave the protective endosteal niche and
enhance their chemotherapeutic sensitivity.
In conclusion, the interaction of leukemia cells
with the bone marrow niche provides a protective environment and
resistance to chemotherapy agents such as Ara-C. An increasing
amount of research is being focused on disrupting the interaction
of leukemia cells and stromal cells to enhance the killing effect
of chemotherapy drugs. c(RGDfV) can be used to mobilize leukemia
cells through the disrupting the Opn/αvβ3 axis to make the myeloid
leukemia cells more sensitive to chemotherapy agents. The present
experiments indicated that such administration may be used to
enhance the sensitivities to chemotherapy drugs by interfering with
the adhesion between leukemia cells and the endosteal niche.
Therefore, improving the hematopoietic microenvironment to clear
minimal residual disease may be worthwhile and may provide a novel
method for the treatment of leukemia. 3D scaffolds may also be a
novel tool to study the hematopoietic microenvironment.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation, the Key Discipline of Medical
Science of China (grant no. 81000195).
References
|
1
|
Schofield R: The relationship between the
spleen colony-forming cell and the haemopoietic stem cell. Blood
Cell. 4:7–25. 1978.
|
|
2
|
Saito Y, Kitamura H, Hijikata A,
Tomizawa-Murasawa M, Tanaka S, Takagi S, Uchida N, Suzuki N, Sone
A, Najima Y, et al: Identification of therapeutic targets for
quiescent, chemotherapy-resistant human leukemia stem cells. Sci
Transl Med. 2:17ra92010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lane SW, Scadden DT and Gilliland DG: The
leukemic stem cell niche: Current concepts and therapeutic
opportunities. Blood. 114:1150–1157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bissell MJ, Rizki A and Mian IS: Tissue
architecture: the ultimate regulator of breast epithelial function.
Curr Opin Cell Biol. 15:753–762. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Breslin S and O'Driscoll L:
Three-dimensional cell culture: the missing link in drug discovery.
Drug Discov Today. 18:240–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nilsson SK, Johnston HM, Whitty GA,
Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ,
Simmons PJ and Haylock DN: Osteopontin, a key component of the
hematopoietic stem cell niche and regulator of primitive
hematopoietic progenitor cells. Blood. 106:1232–1239. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kluza E, van der Schaft DW, Hautvast PA,
Mulder WJ, Mayo KH, Griffioen AW, Strijkers GJ and Nicolay K:
Synergistic targeting of alphavbeta3 integrin and galectin-1 with
heteromultivalent paramagnetic liposomes for combined MR imaging
and treatment of angiogenesis. Nano Lett. 10:52–58. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kassem M, Risteli L, Mosekilde L, Melsen F
and Eriksen EF: Formation of osteoblast-like cells from human
mononuclear bone marrow cultures. APMIS. 99:269–274. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Locatelli F, Maccario R and Frassoni F:
Mesenchymal stromal cells, from indifferent spectators to principal
actors. Are we going to witness a revolution in the scenario of
allograft and immune-mediated disorders? Haematologica. 92:872–877.
2007.PubMed/NCBI
|
|
10
|
Mendez-Ferrer S, Michurina TV, Ferraro F,
Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A,
Enikolopov GN and Frenette PS: Mesenchymal and haematopoietic stem
cells form a unique bone marrow niche. Nature. 466:829–834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eliasson P and Jönsson JI: The
hematopoietic stem cell niche: Low in oxygen but a nice place to
be. J Cell Physiol. 221:17–22. 2010. View Article : Google Scholar
|
|
12
|
Balduino A, Hurtado SP, Frazão P, Takiya
CM, Alves LM, Nasciutti LE, El-Cheikh MC and Borojevic R: Bone
marrow subendosteal microenvironment harbours functionally distinct
haemosupportive stromal cell populations. Cell Tissue Res.
319:255–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Visnjic D, Kalajzic Z, Rowe DW, Katavic V,
Lorenzo J and Aguila HL: Hematopoiesis is severely altered in mice
with an induced osteoblast deficiency. Blood. 103:3258–3264. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calvi LM, Adams GB, Weibrecht KW, Weber
JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P,
Bringhurst FR, et al: Osteoblastic cells regulate the
haematopoietic stem cell niche. Nature. 425:842–846. 2003.
View Article : Google Scholar
|
|
15
|
Rodríguez JP, González M, Ríos S and
Cambiazo V: Cytoskeletal organization of human mesenchymal stem
cells (MSC) changes during their osteogenic differentiation. J Cell
Biochem. 93:721–731. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paraguassú-Braga FH, Borojevic R, Bouzas
LF, Barcinski MA and Bonomo A: Bone marrow stroma inhibits
proliferation and apoptosis in leukemic cells through gap
junction-mediated cell communication. Cell Death Differ.
10:1101–1108. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moqattash S and Lutton JD: Leukemia cells
and the cytokine network. Proc Soc Exp Biol Med. 219:8–27. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meads MB, Hazlehurst LA and Dalton WS: The
bone marrow microenvironment as a tumor sanctuary and contributor
to drug resistance. Clin Cancer Res. 14:2519–2526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tabe Y, Konopleva M, Munsell MF, Marini
FC, Zompetta C, McQueen T, Tsao T, Zhao S, Pierce S, Igari J, et
al: PML-RARalpha is associated with leptin-receptor induction: The
role of mesenchymal stem cell-derived adipocytes in APL cell
survival. Blood. 103:1815–1822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hijiya N, Setoguchi M, Matsuura K, Higuchi
Y, Akizuki S and Yamamoto S: Cloning and characterization on of the
human osteopontin gene and its promoter. Biochem J. 303:255–262.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee CY, Tien HF, Hou HA, Chou WC and Lin
LI: Marrow osteopontin level as a prognostic factor in acute
myeloid leukemia. Br J Haematol. 141:736–739. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nilsson SK, Johnston HM, Whitty GA,
Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ,
Simmons PJ and Haylock DN: Osteopontin, a key component of the
hematopoietic stem cell niche and regulator of primitive
hematopoietic progentior cells. Blood. 106:1232–1239. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haylock DN and Nilsson SK: Osteopontin: A
bridge between bone and blood. Br J Haematol. 134:467–474. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Courter D, Cao H, Kwok S, Kong C, Banh A,
Kuo P, Bouley DM, Vice C, Brustugun OT, Denko NC, et al: The RGD
domain of human osteopontin promotes tumor growth and metastasis
through activation of survival pathways. PLoS One. 5:e96332010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitjans F, Meyer T, Fittschen C, Goodman
S, Jonczyk A, Marshall JF, Reyes G and Piulats J: In vivo therapy
of malignant melanoma by means of antagonists of alphav integrins.
Int J Cancer. 87:716–723. 2000. View Article : Google Scholar : PubMed/NCBI
|