Introduction
Asbestos has been widely used in many industrial
contexts for its extraordinary thermal insulation properties. Over
the past years, millions of tons of asbestos have been processed
worldwide. The most common asbestos used is the chrysotile (white
asbestos), followed by amphibolic asbestos (blue and brown
asbestos). However, their toxic effects have been known for 50
years (1).
It is already known that asbestos inhalation can
induce the development of mesothelioma and several other cancer
types, included that of the lung. Specifically, the risk of
developing lung cancer has been suggested to be related to the
cumulative exposure to asbestos, with an estimated increase in risk
of 1% for each fiber/ml-year of exposure (2). It has also been observed that exposure
to asbestos may be associated with non-neoplastic diseases, such as
asbestosis and pleural fibrosis (3).
The interval between first exposure and the development of
mesothelioma ranges from 20 to 60 years (4). The highest worldwide incidence is
estimated occur in the year 2020 (5).
Individuals who are at risk are those who have
previously worked in shipbuilding industries, railway construction
companies and asbestos-cement industries; however, due to the
spread of asbestos shipyards, home improvements and many other
types of uncontrolled construction work, a larger number of cases
have been identified among building craftsmen exposed accidentally
and often unknowingly. Moreover, unwitting victims of asbestos are
the wives and children of workers who carry home asbestos dust on
their clothes. The difficulty of removing asbestos already in place
represents an additional risk for the development of mesothelioma
and lung cancer (2,6).
Health surveillance in workers exposed to asbestos
is focused on the early detection of the major diseases related to
asbestos (7). Unfortunately, the
health surveillance protocols adopted for workers exposed to
asbestos do not meet the requirements of sensitivity and
specificity required to ensure early diagnosis (8). Additionally, apart from asbestos fibers,
it has been demonstrated that other fibers, such as fluoro-edenite
(FE), may have toxic effects (9).
Therefore, there is an urgent need to identify novel
markers useful for the early diagnosis of asbestos and
asbestos-like fibers-associated diseases. Although it has been
demonstrated that fibulin-3 is an important marker used for the
diagnosis of mesothelioma (10), its
role in the early stage of the disease is still under debate.
High circulating levels of fibulin-3 have been
observed in street cleaners from Biancavilla (Sicily, Italy), which
are at high risk of FE exposure, when comparing their pleural
plaques with those of a non-exposed control group (11–13).
However, if such an overexpression is due to the exposure of
asbestos-like fibers or to other environmental factors, such as
volcanic particulates, remains to be further clarified.
On the basis of these criteria, in the present
study, we analyzed circulating levels of fibulin-3 in individuals
exposed to asbestos and compared with them with those previously
detected in workers exposed to FE. Furthermore, in vitro
experiments using mesothelial cells (Met5A) were performed to
determine whether the expression of fibulin-3 correlates with the
exposure to asbestos-like fibers and/or other particulates.
Materials and methods
Subjects
The subjects enrolled in the present study included
40 workers exposed to asbestos fibers, including 30 removal and
disposal workers and 10 coating workers, employed in old pipeline
maintenance. Ten healthy subjects were also included as controls.
The subjects and healthy controls included in this study were
recruited at the Occupational Medicine Unit, School of Medicine,
University of Catania, Catania, Italy. They comprised both
categories of workers, those exposed to asbestos fibers (cases) and
those without such exposure working in different places (healthy
subjects or controls). All study participants provided a written
informed consent. The study was approved by the University of
Catania Ethics Committee.
Information on occupational history,
socio-demographic indicators, smoking habits and clinical history
were collected using validated questionnaires. Briefly, all
subjects (workers) were male, with a mean age of 52 years; 30 out
of 40 were smokers; the mean duration of exposure to asbestos was
14 years. All healthy subjects were male with a mean age of 50
years and all of them were smokers. The participants underwent
blood testing, spirometry, physical examination and chest
high-resolution computed tomography (HRCT).
Blood samples were collected into EDTA plasma tubes
and processed within 2 h. The samples were centrifuged at 950 × g
for 10 min and the supernatant was aliquoted and stored at −80°C
until analysis.
Enzyme-linked immunosorbent assay (ELISA). Fibulin-3
plasma levels were determined in the 40 cases and in 10 controls in
duplicate using a human EGF-containing fibulin-like extracellular
matrix protein 1 (EFEMP1) ELISA kit (Cusabio Biotech Co., Ltd.,
College Park, MD, USA) according to the manufacturer's
instructions. Plasma samples were diluted and the immunoassay was
carried out according to the manufacturer's instructions. Marker
concentrations for each sample were calculated from the standard
curve. All tests were assayed in duplicate. A subset of samples was
assayed 4 times in each ELISA plate for quality control. No
significant cross-reactivity to or interference with various
proteins was observed. The optical density was measured at 450 nm
using a microplate reader (Tecan ELISA Reader; Tecan Group Ltd.,
Männedorf, Switzerland). The results were compared with those
obtained from the 27 street cleaners exposed to carcinogenic fibers
analyzed in our previous study (11).
Cell culture and treatment
The non-malignant transformed human pleural
mesothelial cell line (Met-5A) was obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA) and cultured in
RPMI-1640 medium, supplemented with 10% fetal bovine serum, 1%
penicillin/streptomycin (all from Lonza, Walkersville, MD, USA) and
incubated at 37°C in humidified atmosphere with 5%
CO2.
The cells were plated at a density of 120,000
cells/well in a 6-well dish in a final volume of 2 ml per well, and
exposed to various concentrations of FE and A, B, C and D volcanic
particulates (10, 50 and 100 µg/ml) for 72 h. MeT5A cells grown in
normal medium were used as controls. Subsequently the cells were
deprived of culture medium and washed in phosphate-buffered saline
(PBS), then collected in tubes and centrifuged at 250 × g for 5 min
at 4°C. The pellets thus obtained were stored at −80°C before being
used for protein and RNA extraction. All treatments were performed
in triplicate.
FE fibers were collected in the area of Biancavilla
(Sicily) and then characterized. The volcanic particulates [A
(basalt residues), B (volcanic ash), C (mixed basalt and cement)
and D (cement)], were collected in different areas of Mount Etna
(Sicily, Italy) and underwent physical and chemical
characterizations, as previously described (14). All particles were suspended in
RPMI-1640 medium to yield stock solutions.
Reverse transcription-quantitative
(real-time) PCR (RT-qPCR)
Total RNA was isolated from the cell pellets using
an extraction kit (PureLink RNA mini kit; Ambion/Life Technologies,
Carlsbad, CA, USA) according to the manufacturer's instructions.
First-strand cDNA was reverse transcribed with 1 µg of total RNA.
The resultant cDNA was then used for quantitative PCR (qPCR)
reactions, which were performed using the Applied Biosystems 7300
Real-Time PCR system (Applied Biosystems, Foster City, CA,
USA).
The fibulin-3 primer sequences were as follows:
5′-CGAGGGGAGCAGTGCGTAGACATAG-3′ (sense) and
5′-CTTCACAGTTGAGCCTGTCACTGCT-3′ (antisense). The housekeeping gene,
phosphoglycerate kinase 1 (PGK1), was used as an internal control
for normalization of the results. The PGK1 primer sequences were as
follows: 5′-TTAAAGGGAAGCGGGTCGTT-3′ (sense) and
5′-CAGGCATGGGCACACCAT-3′ (antisense).
The amplification of fibulin-3 and PGK1 was
performed with 1 cycle at 95°C for 10 min, and 40 cycles at 95°C
for 15 sec and 60°C for 1 min. The calculation of the relative
expression of each transcript was performed using the
2−ΔΔCt method.
Western blot analysis
The cells were lysed in NP-40 cell lysis buffer (50
mM Tris pH 7.4, 250 mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM
Na3VO4, 1% Nonidet P-40, 0.02%
NaN3) containing a protease inhibitor cocktail (Sigma,
St. Louis, MO, USA). The extracted protein amounts were quantified
using the Quick Start™ Bradford 1X Dye Reagent assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Equivalent amounts of
protein (40 µg) were separated using Mini Protean TGX Precast Gels
(Bio-Rad Laboratories, Inc.; 4–15%) and transferred onto a
PVDF/nitrocellulose membrane (Bio-Rad Laboratories, Inc.). Western
blot analysis was performed using the following primary antibodies:
EFEMP1 (PA5-26104) (Thermo Fisher Scientific, Waltham, MA, USA) and
β-tubulin (ab15568) (Abcam, Cambridge, UK). The western blot
analysis signal was detected using the ChemiDoc Touch Imaging
system (Bio-Rad Laboratories, Inc.). The densitometry of the
western blot analysis results was measured using ImageJ software
(National Institutes of Health, Bethesda, MD, USA).
Computational analysis of stathmin
1
To determine whether stathmin 1 overexpression is
associated with exposure to asbestos, datasets of gene expression
profiling available on the Gene Expression Omnibus (GEO) dataset
(www.ncbi.nlm.nih.gov/geo/) were
analyzed. Among all publically available GEO datasets, only those
containing lung cancer or mesothelioma samples with stathmin 1
expression levels, clinical data relative to asbestos exposure were
analyzed. Based on these criteria, only the GSE23822 dataset
(15) was used for our analysis. This
dataset includes 26 samples of lung cancer with confirmed asbestos
exposure and 30 non-exposed samples.
No additional normalization procedure was applied.
Differential analysis of stathmin 1 expression levels between the
exposed and non-exposed groups was performed using the Student's
t-test.
Statistical analysis
Statistical analysis of fibulin-3 expression in the
plasma samples was performed using Kruskal-Wallis test. The
analysis of the expression levels of EFEMP1 in the cells was
obtained using the Student's t-test. A value of p<0.05 (by a
two-tailed test) was considered to indicate a statistically
significant difference.
Odds ratios (OR) and the corresponding 95%
confidence intervals (CI) were calculated by means of multiple
logistic regression models including terms for gender, age and
smoking habits. The test for trend was based on the
likelihood-ratio test between the models with and without linear
terms for each variable of interest. The attributable risk percent
values were computed as previously described (16). All statistical analyses were performed
using SAS 9.2 statistical software (SAS Institute, Cary, NC,
USA).
Results
Both groups of asbestos workers (disposal and
coating) enrolled in this study did not report a diagnosis of
mesothelioma or other pulmonary diseases (asbestosis and/ or
pleural plaques); only 7 workers exhibited an allergy to pollen
and/or dust and 30 out of the 40 workers were smokers. The mean
values (% predicted) of the functional respiratory tests were
within the normal range for both groups. Blood parameters were in
the normal range (data not shown).
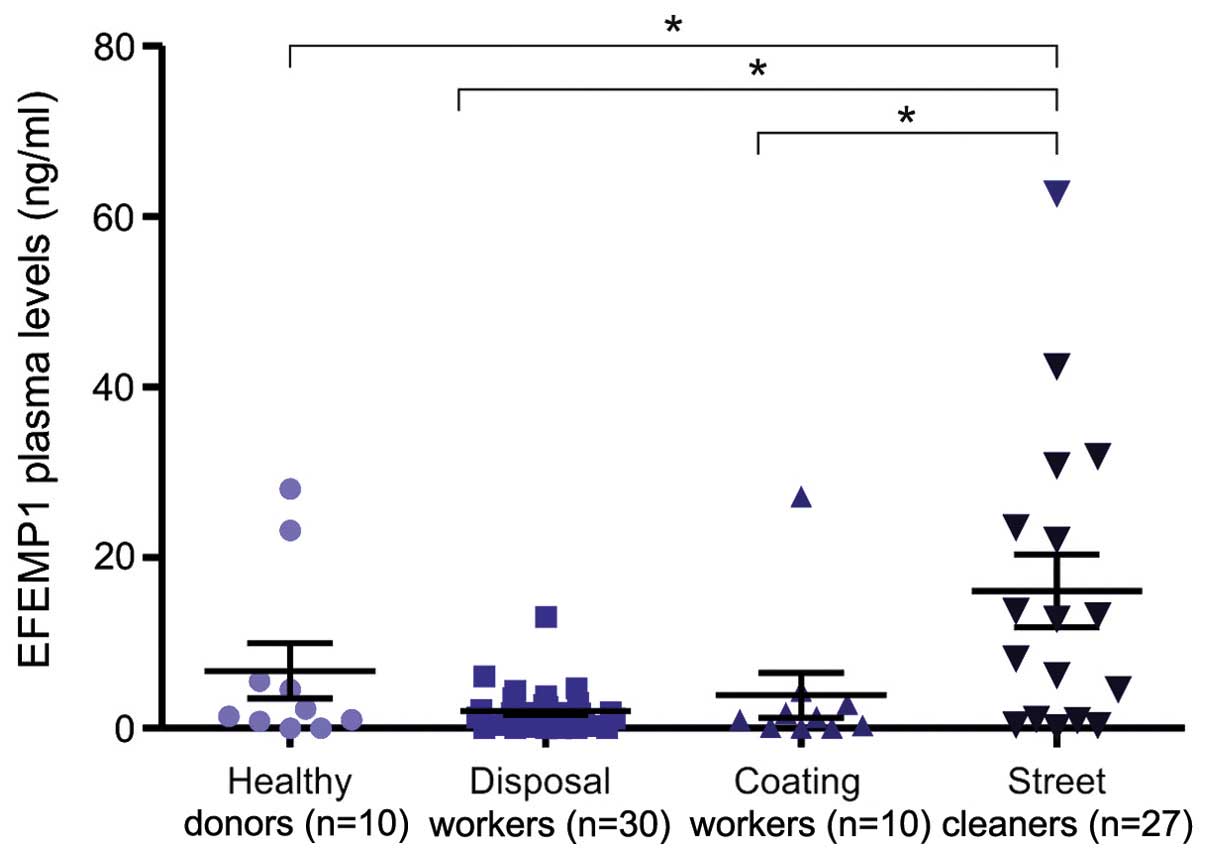
Analysis of fibulin-3 in workers
The median plasma levels of fibulin-3 were 1.51
ng/ml (0.46–3.978) and 1.73 ng/ml (1.012–3.59) in the
removal/disposal and coating worker groups, respectively. These
results were similar to those detected in the normal samples.
However, statistical differences were observed when we compared
these data with those obtained from the plasma of workers
occupationally exposed to carcinogenic fibers previously analyzed
(11) (Fig.
1). No association between fibulin-3 plasma levels and the
duration of exposure to asbestos or the radiographic score was
observed in these subjects. The fibulin-3 plasma levels were also
not influenced by the age, gender or smoking habits of the subjects
(data not shown).
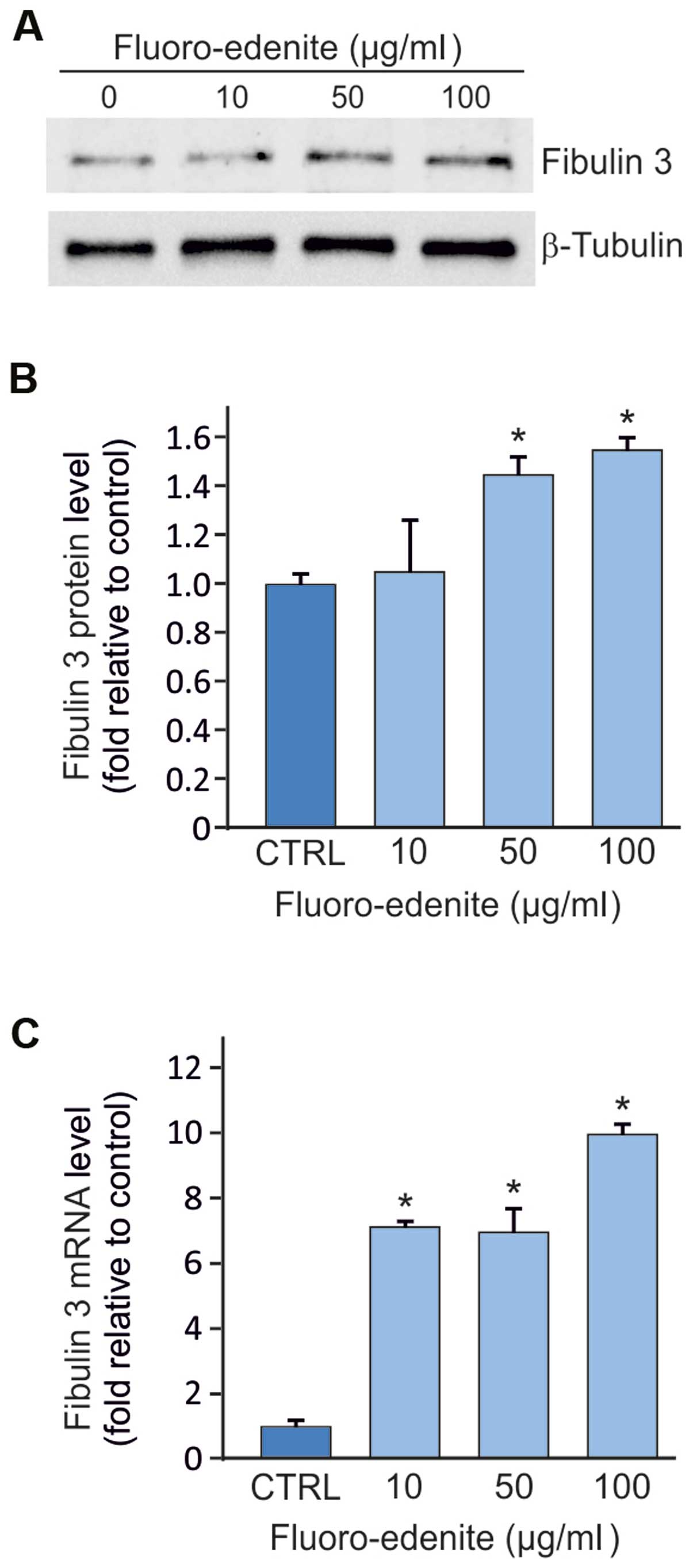
Expression of fibulin-3 detected at
the transcript and protein levels in MeT5A cells
The fibulin-3 protein and mRNA levels were evaluated
in the MeT5A cells incubated with 10, 50 and 100 µg/ml of FE fibers
and A, B, C and D volcanic particulates for 72 h. As shown in
Fig. 2A, a marked increase in
fibulin-3 protein expression was observed in the MeT5A cells
exposed to FE compared with the controls. However, the cells
exposed to the A, B, C and D volcanic particulates did not exhibit
any significant increase in fibulin-3 expression (data not
shown).
As shown in Fig. 2B,
the MeT5A cells exhibited higher protein levels of fibulin-3
following exposure to FE at the concentrations of 50 and 100 µg/ml
(p<0.05), compared with the controls; however, exposure of the
cells to 10 µg/ml of FE did not lead to any significant changes in
fibulin-3 protein expression compared with the untreated MeT5A
cells.
mRNA fibulin-3 overexpression was detected in the
cells exposed to each of the three concentrations of fluoro-edenite
(Fig. 2C). Similar to the results
obtained for the protein levels the cells exposed to the A, B, C
and D volcanic particulates did not exhibit any significant
increase in mRNA fibulin-3 expression (data not shown).
Computational analysis of stathmin
1
A publically available dataset of 56 lung cancer
samples was analyzed for the expression of stathmin 1. This dataset
comprised 26 tumor samples from lung cancer patients with a history
of exposure to asbestos. The transcript levels of stathmin were
significantly higher in the group of tumors derived from patients
exposed to asbestos compared with the other group not exposed to
asbestos (Fig. 3).
Discussion
FE, a mineral from the calcic clino-amphibole
subgroup, was found in the sputum of subjects from Biancavilla, a
city in Eastern Sicily, and in lung specimens from sheep living
nearby (12,13,17). In
particular, various types of airborne asbestos-like mineral fibers
were identified in the volcanic area of Mount Etna and may
represent the cause of the increased incidence of mesothelioma and
lung cancer and other lung diseases (18). As far back as 1987, the International
Agency of Research on Cancer emphasized that the vast majority of
asbestos-induced diseases are caused by occupational exposure, as
well as non-occupational asbestos exposure (19,20).
Starting from in the mid-1970s, the discovery of sites of endemic
mesothelioma in some rural areas (Turkey, Greece, Cyprus, Corsica,
and more recently, New Caledonia, Sicily, China and California) has
provided important information about the carcinogenicity of various
mineral fibers (6,21).
In the present study, for the first time, at least
to the best of our knowledge, the expression levels of fibulin-3 in
normal mesothelial cells following exposure to both FE and other
volcanic particulates were analyzed. These data strongly support
the notion that fibulin-3 in mesothelial cells is activated
following exposure to FE and not following exposure to the other
particulates. These results suggest the potential role of fibulin-3
in the development of neoplastic and non-neoplastic diseases of the
respiratory tract in subjects exposed to FE (11).
Surprisingly, we also observed that the plasma
levels of fibulin-3 were significantly lower in the group of
workers exposed to asbestos fibers compared to those previously
observed exposed to FE (11). These
findings suggest that asbestos disposal workers properly used the
personal protective equipment (PPE) according to the current
regulations. By contrast, other workers, such as street cleaners
should be better equipped to prevent injury caused exposure to FE
(11). According to this observation,
our in vitro experiments indicated that fibulin-3 was
overexpressed in mesothelial cells following exposure to FE and not
following exposure to volcanic particulates. These data were
observed at both the transcript and protein levels. Therefore, we
can argue that the detection of high plasma levels of fibulin-3,
observed in street cleaners from the Biancavilla area, may be due
to FE exposure (11). However,
exposure to volcanic particulates did not affect fibulin-3
expression. These data may be in agreement with those of a previous
study demonstrating the high incidence of mesotheliomas in the area
of Biancavilla associated with exposure to FE (18).
Overall, our data support the notion that the
increased levels of fibulin-3 are associated most likely with an
inflammatory mechanism caused by exposure to FE (22). Furthermore, these data indicate that
the subset of subjects exposed to FE has to be monitored for the
development of mesothelioma. Accordingly, it is already known that
chronic exposure to asbestos-like fibers may cause chronic
inflammation and cancer. Such chronic inflammation is associated
with the production of several cytokines and growth factors that
may cause cellular proliferation and apoptosis arrest (9,22). On this
matter, it has been shown that p27 is downregulated in mesothelial
cells following exposure to FE fibers (23). Notably, p27 is considered a tumor
suppressor gene due to its function as a regulator of the cell
cycle, and in cancers it is often inactivated (24). It has also been demonstrated that low
levels of p27 are associated with stathmin upregulation,
determining an aggressive phenotype of tumor cells (25). Of note, we have previously
demonstrated that stathmin expression is useful for the survival of
cancer cells carrying a p53 mutant (26) that may be also involved in the drug
resistance mechanisms of hematopoietic cells (27). On this ground, we performed a
bioinformatic evaluation to determine whether stathmin is involved
in the malignant transformation associated with exposure to
asbestos-like fibers. This computational approach showed the
overexpression of stathmin in the group of 26 lung cancer patients
with a history of asbestos exposure compared with those not exposed
to any fibers. According to these data, we can speculate that both
fibulin-3 overexpression and stathmin activation may be responsible
for the malignant transformation of mesothelial cells following
exposure to asbestos-like fibers.
References
|
1
|
Wagner JC, Sleggs CA and Marchand P:
Diffuse pleural mesothelioma and asbestos exposure in the North
Western Cape Province. Br J Ind Med. 17:260–271. 1960.PubMed/NCBI
|
|
2
|
Boffetta P: Health effects of asbestos
exposure in humans: a quantitative assessment. Med Lav. 89:471–480.
1998.PubMed/NCBI
|
|
3
|
International Agency for Research on
Cancer, . Asbestos (Chrysolite, Amosite, Crocidolite, Tremolite,
Actinolite, and Anthophyllite)IARC Monographs. Arsenic, Metals,
Fibres and Dusts. International Agency for Research on Cancer;
Lyon: pp. 147–167. 2009
|
|
4
|
Tan C and Treasure T: Mesothelioma: time
to take stock. J R Soc Med. 98:455–458. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McDonald JC and McDonald AD: The
epidemiology of mesothelioma in historical context. Eur Respir J.
9:1932–1942. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crisstaudo A, Foddis R and Guglielmi G:
Methodology and results of an experience of medical surveillance of
people previously exposed to asbestos in Tuscany. G Ital Med Lav
Ergon. 32(Suppl 4): 385–388. 2010.(In Italian). PubMed/NCBI
|
|
8
|
Marcus PM, Bergstralh EJ, Fagerstrom RM,
Williams DE, Fontana R, Taylor WF and Prorok PC: Lung cancer
mortality in the Mayo Lung Project: impact of extended follow-up. J
Natl Cancer Inst. 92:1308–1316. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loreto C, Rapisarda V, Carnazza ML,
Musumeci G, Valentino M, Fenga C and Martinez G: Fluoro-edenite
fibres induce lung cell apoptosis: an in vivo study. Histol
Histopathol. 23:319–326. 2008.PubMed/NCBI
|
|
10
|
Pass HI, Levin SM, Harbut MR, Melamed J,
Chiriboga L, Donington J, Huflejt M, Carbone M, Chia D, Goodglick
L, et al: Fibulin-3 as a blood and effusion biomarker for pleural
mesothelioma. N Engl J Med. 367:1417–1427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rapisarda V, Ledda C, Migliore M, Salemi
R, Musumeci A, Bracci M, Marconi A, Loreto C and Libra M: FBLN-3 as
a biomarker of pleural plaques in workers occupationally exposed to
carcinogenic fibers: a pilot study. Future Oncol. 11(Suppl 24):
35–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bruni BM, Pacella A, MazziottiTagliani S,
Gianfagna A and Paoletti L: Nature and extent of the exposure to
fibrous amphiboles in Biancavilla. Sci Total Environ. 370:9–16.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Putzu MG, Bruno C, Zona A, Massiccio M,
Pasetto R, Piolatto PG and Comba P: Fluoro-edenitic fibres in the
sputum of subjects from Biancavilla (Sicily): a pilot study.
Environ Health. 5:202006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ledda C, Rapisarda V, Bracci M, Proietti
L, Zuccarello M, Fallico R, Fiore M and Ferrante M: Professional
exposure to basaltic rock dust: assessment by the Vibrio fischeri
ecotoxicological test. J Occup Med Toxicol. 8:232013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wright CM, Francis SM Savarimuthu, Tan ME,
Martins MU, Winterford C, Davidson MR, Duhig EE, Clarke BE, Hayward
NK, Yang IA, et al: MS4A1 dysregulation in asbestos-related lung
squamous cell carcinoma is due to CD20 stromal lymphocyte
expression. PLoS One. 7:e349432012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Polesel J, Franceschi S, Talamini R, Negri
E, Barzan L, Montella M, Libra M, Vaccher E, Franchin G, La Vecchia
C and Serraino D: Tobacco smoking, alcohol drinking, and the risk
of different histological types of nasopharyngeal cancer in a
low-risk population. Oral Oncol. 47:541–545. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Nardo P: More attention to veterinarian
epidemiologists. Epidemiol Prev. 28:1942004.(In Italian).
PubMed/NCBI
|
|
18
|
Paoletti L, Batisti D, Bruno C, Di Paola
M, Gianfagna A, Mastrantonio M, Nesti M and Comba P: Unusually high
incidence of malignant pleural mesothelioma in a town of eastern
Sicily: an epidemiological and environmental study. Arch Environ
Health. 55:392–398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
International Agency for Research on
Cancer, . IARC Monographs on the evaluation of carcinogenic risks
to humans. Supplement 7:1-42. IARC Press; Lyon: 1987
|
|
20
|
Committee on Asbestos: Selected Health
Effects; Board on Population Health and Public Health Practices;
Institute of Medicine, . Asbestos: Selected Cancers. The National
Academies Press; Washington, DC: 2006
|
|
21
|
Goldberg M and Luce D: The health impact
of nonoccupational exposure to asbestos: what do we know? Eur J
Cancer Prev. 18:489–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cardile V, Lombardo L, Belluso E, Panico
A, Renis M, Gianfagna A and Balazy M: Fluoro-edenite fibers induce
expression of Hsp70 and inflammatory response. Int J Environ Res
Public Health. 4:195–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Musumeci G, Cardile V, Fenga C, Caggia S
and Loreto C: Mineral fibre toxicity: Expression of retinoblastoma
(Rb) and phospho-retinoblastoma (pRb) protein in alveolar
epithelial and mesothelial cell lines exposed to fluoro-edenite
fibres. Cell Biol Toxicol. 27:217–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chu IM, Hengst L and Slingerland JM: The
Cdk inhibitor p27 in human cancer: prognostic potential and
relevance to anticancer therapy. Nat Rev Cancer. 8:253–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baldassarre G, Belletti B, Nicoloso MS,
Schiappacassi M, Vecchione A, Spessotto P, Morrione A, Canzonieri V
and Colombatti A: p27(Kip1)-stathmin interaction influences sarcoma
cell migration and invasion. Cancer Cell. 7:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sonego M, Schiappacassi M, Lovisa S,
Dall'Acqua A, Bagnoli M, Lovat F, Libra M, D'Andrea S, Canzonieri
V, Militello L, et al: Stathmin regulates mutant p53 stability and
transcriptional activity in ovarian cancer. EMBO Mol Med.
5:707–722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCubrey JA, Abrams SL, Ligresti G,
Misaghian N, Wong EW, Steelman LS, Bäsecke J, Troppmair J, Libra M,
Nicoletti F, et al: Involvement of p53 and Raf/MEK/ERK pathways in
hematopoietic drug resistance. Leukemia. 22:2080–2090. 2008.
View Article : Google Scholar : PubMed/NCBI
|