Introduction
Renal cell carcinoma (RCC) is the most frequently
occurring type of kidney cancer in adults. RCC accounts for ~3% of
adult malignancies and 90–95% of neoplasms that arise from the
kidney (1). RCC may remain clinically
occult for the majority of its course, with only 10% of patients
presenting to hospital with flank pain, hematuria and a flank mass,
the classic triad of symptoms (2).
The only treatment known to be effective against localized RCC is
surgical resection, which may also be used for palliation in
metastatic disease. Nephron-sparing surgery (NSS) refers to the
complete resection of the tumor and the simultaneous effective
retainment of renal tissue in order to maximize renal function
(3). With the development of
iconography and the popularity of physical examinations, the
detection rate of incidental renal tumors and small renal
carcinomas without symptoms has increased markedly, and increasing
numbers of patients with renal tumors choose to receive NSS.
Laparoscopy technology has widespread applications in urinary
surgery. Laparoscopic NSS (LNSS) has become more common in the
treatment of RCC and has the advantages of little trauma, quick
recovery and a similar effect to open surgery (4). The core technologies of LNSS include
controlling the warm ischemia time, guaranteeing a negative margin
and avoiding the occurrence of secondary bleeding and urine leakage
(5). Based on the constantly updated
knowledge regarding renal anatomy and the anatomical structure of
the nephron, certain improvements were made to the LNSS technique,
which was then used to treat 31 patients with RCC in the present
study. The improved LNSS achieved good clinical results.
Materials and methods
Clinical data
A total of 56 patients, including 35 males and 21
females, who presented with RCC to the General Hospital of the
People's Liberation Army (Beijing, China) were treated between
January 2012 and November 2014. The mean age of the patients was
54.1±13.2 years, with a range of 28–68 years. All patients were
indicated to possess space-occupying lesions of the kidney during
the physical examinations, and were subsequently hospitalized
without clinical symptoms. Prior to surgery, an ultrasound (Pro
Focus 2202 Ultrasound Scanner, BK Ultrasound, Herlev, Denmark),
computed tomography angiography (CTA) scan (SOMATOM®
Definition AS+, Siemens AG, Munich, Germany) and magnetic resonance
imaging (MRI; MAGNETOM Avanto™ 3.0T, Siemens AG) examination were
performed, in order to evaluate the renal vessels and location of
the tumor. The average tumor size was 3.1±0.7 cm in diameter, and
ranged from 1.2–4.0 cm (Table I).
Pre-operative tumor-node-metastasis (TNM) staging (6) showed that 53 cases were T1a and 3 were
T1b, including one with an anatomically solitary kidney and two
with chronic renal insufficiencies. The pre-operative diagnoses
were all considered to be renal tumors. The complicating diseases
included 4 patients with diabetes, 2 patients with chronic renal
insufficiency and 5 patients with hypertension. Routine
examinations prior to the surgery revealed no surgical
contraindications. In addition, a control group was studied. The
control group consisted of 36 patients that received traditional
partial nephrectomy surgery, which involves a sharp incision of the
tumor with scissors, 5 mm from the tumor margin.
 | Table I.Clinical comparison of patient
features between the groups. |
Table I.
Clinical comparison of patient
features between the groups.
| Feature | Improved group
(n=56) | Control group
(n=36) | P-value |
|---|
| Age,
yearsa |
54.1±13.2 |
52.3±10.7 | 0.46 |
| Male gender, n
(%) | 35 (62.5) | 23 (63.9) | 0.80 |
| BMIa | 22.3±1.2 | 23.3±1.6 | 0.93 |
| Tumor diameter,
cma |
3.1±0.7 |
3.4±1.1 | 0.56 |
| Affected side, n
(%) |
|
|
|
| Left | 25 (44.6) | 15 (41.7) | 0.67 |
|
Right | 31 (55.4) | 21 (58.3) | 0.38 |
| Location |
|
|
|
| Upper
pole | 20 (35.7) | 14 (38.9) | 0.57 |
|
Middle | 22 (39.3) | 12 (33.3) | 0.34 |
| Inferior
pole | 14 (25.0) | 10 (27.8) | 0.76 |
|
R.E.N.A.L.a |
6.9±1.1 |
6.6±0.8 | 0.75 |
| GFR prior to surgery,
ml/mina | 45.6±6.4 | 47.6±7.4 | 0.89 |
Surgical method
All patients received LNSS under general anesthetic.
Following successful anesthesia, the patients were placed in the
lateral decubitus position. Trocars that were 5, 10 and 12 mm in
diameter were placed 1 cm above the iliac crest in the midaxillary
line, and under the 12th costal margin at the anterior and
posterior axillary lines, respectively. Next, laparoscopic and
surgical apparatus were implanted. The CO2 pressure of
the pneumoperitoneum was maintained at 10.5 mm Hg H2O.
Following the establishment of peritoneal clearance, the perirenal
fascia was opened and the renal tumor was fully exposed. The renal
arteries were dissociated and reattached in front of the psoas
major muscle, using a Delacroix-Chevalier Gregory Bulldog clamp
(115 mm; Landanger, Paris, France) to control the renal artery, and
the timer was started.
Subsequent to blocking the renal artery, the renal
capsule and renal cortex were incised using scissors with a
ring-like shape (34310-MA-D; Karl Storz, Tuttlingen, Germany) at
3–5 mm from the tumor edge. Following the incision of the renal
parenchyma, the tumor was separated from the reserved medullary and
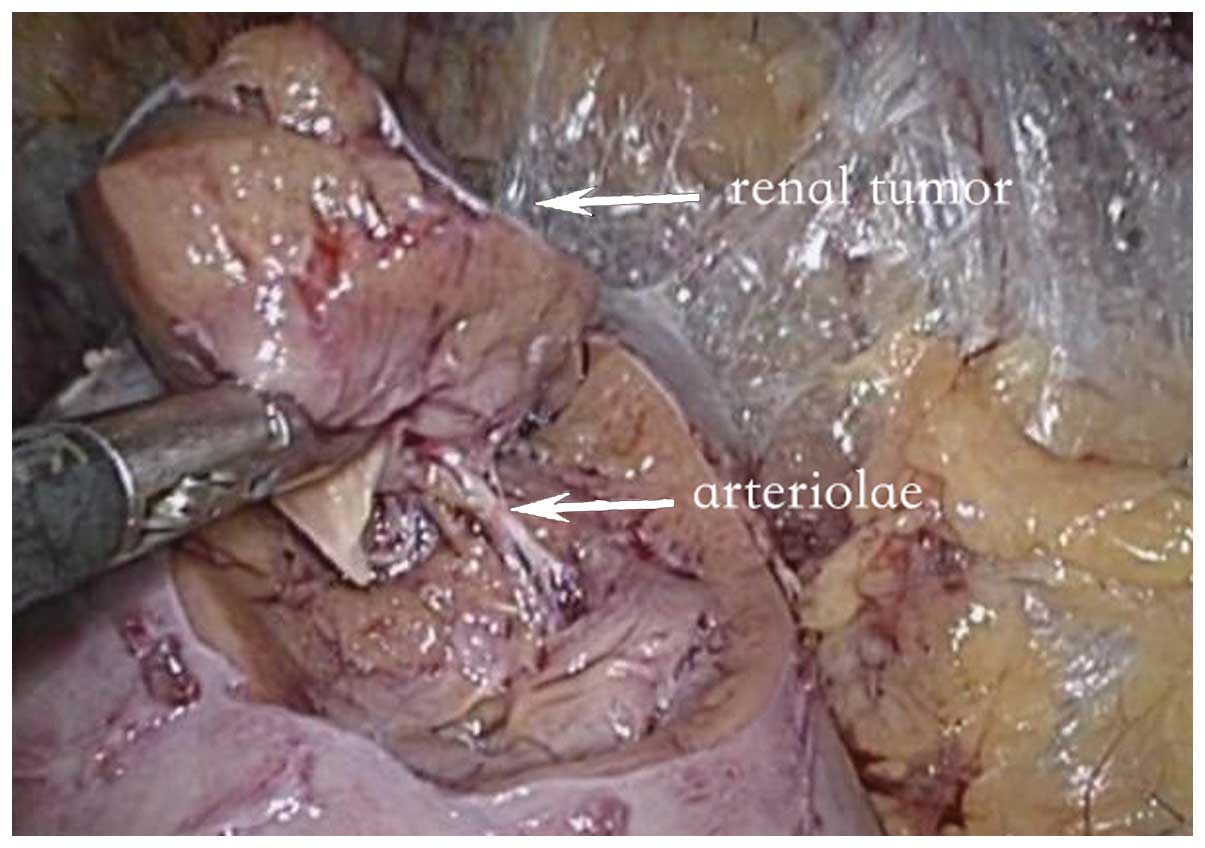
medullary ray to the depth of the basement membrane (Fig. 1). The basal blood vessels were
incised, subsequent to the hemostasis of the region using bipolar
coagulation (Fig. 2). The minor renal
calyces were carefully peeled from the structure to reveal the
clear anatomical structure of the wound (Fig. 3). The basal damaged blood vessels and
collecting systems were continuously sealed with 2–0 absorbable
sutures and the surgical wounds were continuously sealed with 1–0
absorbable sutures, using a Weck Hem-o-lok clip (Teleflex,
Morrisville, NC, USA) to maintain the tension of the sutures. The
Bulldog clamps were released, a lack of bleeding from the wound was
confirmed and the blood supply of the kidney returned to normal. A
drain was left following the surgery. The arterial occlusion time,
surgical duration, intraoperative bleeding volume, post-operative
drainage volume, pathological results, complications and
post-operative follow-up results were recorded.
All the tumor specimens collected from the surgery
were embedded in paraffin, sectioned and stained with hematoxylin
and eosin (HE). The pathological subtypes of renal cell carcinoma
were observed and analyzed by two professional pathologists, using
HE staining.
Results
Surgery
All 31 patients were operated on successfully and no
cases were converted to open surgery. The surgical duration was
80–120 min (mean, 95.5±27.1 min) and the arterial occlusion times
were 15–30 min (mean, 21.2±7.2 min). The average intraoperative
bleeding volume was 55.7±18.9 ml, with a range of 30–150 ml, while
the average post-operative drainage volume was 92.3±28.9 ml, with a
range of 50–250 ml. The average post-operative length of hospital
stay was 6.1±0.6 days, ranging from 5–7 days. No hemorrhage or
urinary leakage was observed in any patients following the surgery
(Table II).
 | Table II.Comparison of clinical data subsequent
to surgery. |
Table II.
Comparison of clinical data subsequent
to surgery.
| Feature | Improved group
(n=56) | Control group
(n=36) | P-value |
|---|
| Arterial occlusion
time, min | 21.2±7.2 | 20.1±5.7 | 0.96 |
| Surgical duration,
min |
95.5±27.1 |
90.5±21.3 | 0.47 |
| Intraoperative
bleeding, ml |
55.7±18.9 |
63.5±20.1 | 0.53 |
| Post-operative
drainage, ml |
92.3±28.9 | 112.3±34.5 | 0.16 |
| Length of hospital
stay, days |
6.1±0.6 |
6.9±0.9 |
|
| Pathological types, n
(%) |
|
|
|
| CCE | 51 (91.1) | 31 (86.1) | 0.48 |
|
Other | 5 (8.9) | 5
(13.9) | 0.11 |
| Negative margin,
% | 100.0 | 88.9 | <0.01 |
| Complications, n |
|
|
|
| Bleeding
or urine leakage | 0 | 5 | <0.01 |
|
Other | 5 | 5 | 0.13 |
| GFR, ml/min | 14.32±2.12 | 16.17±2.34 | 0.34 |
| 1-year recurrence
rate, % | 0.0 | 2.8 | 0.76 |
Post-surgery
The post-operative pathological results indicated
that 27 cases were diagnosed with suprarenal epithelioma, 2 with
chromophobe cell renal carcinoma, 1 with oxyphilic adenoma and 1
with a juxtaglomerular cell tumor. The post-operative TNM staging
revealed that 28 patients possessed T1a stage tumors and 3 patients
possessed stage T1b tumors. The Fuhrman classification (7) was used to classify 15 patients with
level 1, 8 cases with level 1–2 and 8 cases with level 2 RCC. All
tumor specimens that were removed were wedge-shaped. The tumors
were well circumscribed with negative margins. Following discharge
from the hospital, all patients were followed up for 8–28 months
(mean, 18.5±1.6 months) and no signs of local recurrence or distant
metastases were identified by renal ultrasound or computed
tomography examinations.
Discussion
Radical nephrectomies may lead to renal
decompensation. Therefore, for patients who have kidney ailments,
which may endanger renal function, or renal tumors at the T1a
clinical stage, LNSS is the recommended surgery (8). The core technologies of LNSS include
controlling the warm ischemia time, guaranteeing a negative margin
and avoiding the occurrence of secondary bleeding and urine leakage
(5). Continuous research and
improvements have been developed for these surgical aspects,
including suture techniques, renal hypothermia protection
technology, hemostatic materials and ureteral stents (9–12). These
beneficial improvements ensure that LNSS is continuously improved
and developed, and the clinical applications of LNSS are
expanding.
The direction of the laparoscopic operative channel
limits the surgery, and certain disadvantages remain unavoidable,
including the narrow operative space. Occasionally, the cutting
position is not visible and judging the base of the tumor is
challenging, which may result in cutting more renal tissue or
cutting into the tumor. Therefore, certain cases require surgeons
with increased surgical experience and no standard exists to aid
the judgment of the anatomical base of the tumor (13). At present, incisions with an
ultrasound knife or sharp cuts with scissors are in common use in
LNSS, which may easily damage the tumor capsule. In order to
prevent the recurrence of a tumor following the partial nephrectomy
of renal tumors, tumors have always been cut along the normal
tissue to guarantee a negative margin (14). Through the investigation of multiple
centers, a study conducted by Breda et al indicated that
21/855 (2.46%) patients who received LNSS had a positive margin
(15). Urinary leakage is the major
complication following LNSS, and dealing with the collecting system
may extend the surgery and the warm ischemia time; therefore,
doctors are required to be skilled in the associated surgical
techniques. Effectively closing the collecting system during
surgery may greatly decrease the possibility of urinary leakage
post-operatively (16).
The renal parenchyma is composed of the renal cortex
and the renal medulla, in a 1:2 ratio. Renal tumors grow in the
renal parenchyma under the renal capsule and show inflated growth.
Renal tumors continuously squeeze the surrounding renal parenchyma
to form a pseudocapsule, and traditionally, the excision of renal
tumors is always performed along the pseudocapsule. The renal
capsule is composed mainly of fibrous tissue, and the renal cortex
consists of a number of renal corpuscles, which are dense (17). The renal medulla includes numerous
straight renal tubules that are arranged radially to form the
medullary ray and the renal pyramids. Renal pyramids and the
corresponding renal cortex are composed of renal lobules. The
majority of the straight arterioles of the renal medulla are
parallel to the long axis of the renal pyramids (18). The amplification effects of the
laparoscope allow the easy identification of these precise
anatomical structures.
In the present study, subsequent to cutting the
renal capsule and renal parenchyma, the separation of the tumor
from the reserved medullary and medullary ray was performed to the
depth of the basement membrane, which is a simple procedure. The
tumor was isolated with visible supplying blood vessels in 27
cases. The blood vessels were cut following the hemostasis of the
region using bipolar coagulation, the wounds of which were simple
to suture and possessed a low possibility of bleeding. In addition,
for the central type of renal tumor, or ‘H’ in the R.E.N.A.L.
nephrometry scoring system, the tumors are recommended to be
stripped along the renal medulla to the renal sinus, subsequent to
opening the renal cortex, in order to avoid the accidental injury
of the renal artery and vein (19).
The tumors of 3 patients with the central type of renal tumor in
the present study were removed completely, and the renal artery and
vein were not damaged; therefore, this method may also be applied
to the treatment of central-type renal tumors. Urine collecting
systems include the minor renal calyces, renal calyces, renal
pelvis and ureteropelvic junction. The renal parenchyma encompasses
the minor renal calyces, renal calyces and the majority of the
renal pelvis. A renal calyx is composed of 2 or 3 minor renal
calyces. Carefully stripping the base of the tumor may prevent the
injury of the minor renal calyces (20). Fine processing of the blood vessels,
renal medulla and minor renal calyces may also decrease the flow of
blood from the surface of the wound, which reveals the structure
clearly and provides a good view for the suturing. Therefore, the
arterial occlusion times of the surgery reported in the present
study were similar compared with previous reports in the
literature.
Our preliminary experience of using the present
method indicated the following features: i) The integrity of the
tumor may be guaranteed during treatment for deep and basal tumors.
Since the surgery was performed along the separated medullary
space, the tumor was protected and the excision of excessive renal
tissue was avoided. ii) Blood vessels that supply the tumor may be
isolated and treated separately in order to decrease the risk of
secondary bleeding. iii) The minor renal calyces may be isolated
and treated separately to decrease the possibility of urinary
leakage. Overall, the improved LNSS for RCC, based on the precise
anatomy of the nephron, allows the excision of renal tumors
according to the renal lobules and renal pyramids. The surgery
ensures the complete resection of the tumor without the removal of
excessive renal tissue and has the effect of precisely excising the
tumor and the accumulating nephron. The LNSS method is simple, easy
to master, and has the advantages of a low positive margin rate and
fewer post-operative complications. In addition, the warm ischemia
time was not increased during surgery, which may protect renal
function to a greater extent, and the method is suitable for
promoting for clinical use. The short-term treatment effects of the
surgery are satisfactory, but the long-term effects require
additional large prospective, randomized controlled studies to
confirm the success of LNSS.
References
|
1
|
American Cancer Society, . Cancer Facts
& Figures 2014. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdfAccessed.
June 1–2014
|
|
2
|
Simon JW and Marshall FF: Kidney and
ureterClinical Oncology. Abeloff MD, Armitage J, Niederhuber J,
Kastan M and McKenna W: 2nd. Churchill Livingstone; New York, NY:
pp. 1784–1799. 2000
|
|
3
|
Margreiter M and Marberger M: Current
status of open partial nephrectomy. Curr Opin Urol. 20:361–364.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwaibold HE and Stolzenburg JU:
Laparoscopic partial nephrectomy. Arch Ital Urol Androl. 81:72–75.
2009.PubMed/NCBI
|
|
5
|
Pietzak EJ and Guzzo TJ: Advancements in
laparoscopic partial nephrectomy: Expanding the feasibility of
nephron-sparing. Adv Urol. 2012:1489522012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Belldegrun A, Tsui KH, deKernion JB and
Smith RB: Efficacy of nephron-sparing surgery for renal cell
carcinoma: Analysis based on the new 1997 tumor-node-metastasis
staging system. J Clin Oncol. 17:2868–2875. 1999.PubMed/NCBI
|
|
7
|
López JI: Comment to «Is a new
classification of the Fuhrman grade in clear cell renal cell
carcinomas feasible?». Actas Urol Esp. 36:359–360. 2012.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aron M and Turna B: Laparoscopic partial
nephrectomy: Newer trends. Indian J Urol. 25:516–522. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zorn KC, Gong EM, Orvieto MA, Gofrit ON,
Mikhail AA and Shalhav AL: Impact of collecting-system repair
during laparoscopic partial nephrectomy. J Endourol. 21:315–320.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gill IS, Ramani AP, Spaliviero M, Xu M,
Finelli A, Kaouk JH and Desai MM: Improved hemostasis during
laparoscopic partial nephrectomy using gelatin matrix thrombin
sealant. Urology. 65:463–466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nozaki T, Iida H, Morii A, Fujiuchi Y and
Fuse H: Selective renal parenchymal clamping in retroperitoneal
partial nephrectomy. J Laparoendosc Adv Surg Tech A. 22:168–172.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wen J, Li HZ, Ji ZG, Shi BB and Yan WG:
Evaluation of retroperitoneoscopic partial nephrectomy with in situ
hypothermic perfusion. Clin Transl Oncol. 14:382–385. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kong W, Zhang J, Dong B, Chen Y, Xue W,
Liu D and Huang Y: Application of a standardized anatomical
classification in a Chinese partial nephrectomy series. Int J Urol.
19:551–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Springer C, Hoda MR, Fajkovic H, Pini G,
Mohammed N, Fornara P and Greco F: Laparoscopic vs open partial
nephrectomy for T1 renal tumours: Evaluation of long-term
oncological and functional outcomes in 340 patients. BJU Int.
111:281–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Breda A, Stepanian SV, Liao J, Lam JS,
Guazzoni G, Stifelman M, Perry K, Celia A, Breda G, Fornara P, et
al: Positive margins in laparoscopic partial nephrectomy in 855
cases: A multi-institutional survey from the United States and
Europe. J Urol. 178:47–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang P, Xia D and Wang S: Multiple factor
analysis of urine leaks after retroperitoneal laparoscopic partial
nephrectomy. Urol Int. 87:411–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sampaio FJ: Renal anatomy. Endourologic
considerations. Urol Clin North Am. 27:585–607. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Preuss HG: Basics of renal anatomy and
physiology. Clin Lab Med. 13:1–11. 1993.PubMed/NCBI
|
|
19
|
Okhunov Z, Shapiro EY, Moreira DM, Lipsky
MJ, Hillelsohn J, Badani K, Landman J and Kavoussi LR: R.E.N.A.L.
nephrometry score accurately predicts complications following
laparoscopic renal cryoablation. J Urol. 188:1796–1800. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stroup SP, Palazzi K, Kopp RP, Mehrazin R,
Santomauro M, Cohen SA, Patterson AL, L'Esperance JO and Derweesh
IH: RENAL nephrometry score is associated with operative approach
for partial nephrectomy and urine leak. Urology. 80:151–156. 2012.
View Article : Google Scholar : PubMed/NCBI
|