Introduction
Lung cancer is the leading cause of cancer-related
mortality in the world, with non-small cell lung cancer (NSCLC)
accounting for ~80% of cases (1).
Despite advances in the management of NSCLC, improvements in
survival are marginal and the overall prognosis of patients remains
poor (2,3). Defects in the normal apoptosis machinery
have been implicated in the resistance of cancer cells to a wide
variety of current anticancer drugs (4). Therefore, identifying new agents that
induce apoptosis in cancer cells offers novel and potentially
useful approaches to improve patient responses to conventional
chemotherapy (5,6).
Osthole, 7-methoxy-8-(3-methyl-2-butenyl)coumarin, a
natural compound, may be extracted from Cnidium monnieri and
other medicinal plants (7). Previous
studies have revealed that Osthole exhibits various pharmacological
activities, including anti-inflammation (8), anti-allergy (9), anti-oxidation (10), estrogen-like (11) and anti-hepatitis (12) effects. Furthermore, accumulating
evidence indicates that Osthole confers antitumor effects by
inhibiting tumor cell growth and inducing apoptosis (13–15).
However, the effects of Osthole on the apoptosis of NSCLC and the
possible mechanisms behind it remain unclear.
The inhibitor of apoptosis proteins (IAPs) are
significant intrinsic cellular inhibitors of apoptosis (16–20). The
human IAP family contains eight proteins: c-IAP1, c-IAP2, NAIP,
Survivin, X-chromosome-encoded IAP (XIAP), Bruce, ILP-2 and Livin
(21). To date, the overexpression or
dysfunction of IAPs have been detected in various cancers (22–24).
Therefore, identifying new agents targeting IAPs is essential for
cancer drug development. Embelin is one such promising compound
targeting XIAP. Embelin, a plant-based benzoquinone derivative
(25), has been identified as a
cell-permeable, small molecular weight inhibitor of XIAP by virtue
of its interaction with the BIR3 domain (26). A number of cancers, including NSCLC
(27), express elevated levels of
XIAP and become refractory to apoptosis (23,28);
however, treatment with Embelin alone or in combination with other
anticancer drugs was observed to sensitize them towards apoptosis
(26,29).
The present study was performed to evaluate the
effects of Osthole on cell viability and apoptosis in NSCLC cells
and to determine whether Osthole-mediated apoptosis is dependent on
IAP proteins. Furthermore, we evaluated the combined effects of two
herbal medicines, Osthole and Embelin, on the apoptosis of NSCLC
cells in vitro, exploring the possibility of a combined
clinical application.
Materials and methods
Reagents
RPMI-1640, trypsin, penicillin and streptomycin were
purchased from Biological Industries (Kibutz Beit Haemek, Israel).
Fetal bovine serum (FBS) was purchased from Solarbio Science &
Technology (Beijing, China). 3-(4,5-dimethyl
thiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl
sulfoxide (DMSO), propidium iodide (PI), and Hoechst 33342 were
purchased from Sigma-Aldrich (St. Louis, MO, USA). An Annexin
V-fluorescein isothiocyanate (FITC) and PI double staining kit were
purchased from Key Gene (Nanjing, China). Osthole and Embelin were
purchased from the National Institute for the Control of
Pharmaceutical and Biological Products (Beijing, China), Stock
solution (50 mM) was prepared by dissolving Osthole or Embelin in
DMSO and stored at −20°C. Antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). All other reagents were
procured locally.
Cell culture
The human lung cancer cell line A549 was purchased
from China Center for Type Culture Collection (Wuhan, China) and
cultured in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in 5% CO2.
Cells were grown on sterile tissue culture dishes and digested
using 0.25% trypsin.
MTT assay
A549 cells (5×103/well) were plated in
96-well plates, cultured overnight, and then treated with Osthole
(100 µM) and Embelin (50 µM) alone or in combination for 24 h,
respectively. Corresponding DMSO or culture medium was used as an
empty control. Briefly, 20 µl 5 mg⁄ml MTT solution was added to
each well and incubated for 4 h at 37°C, then the supernatant was
removed from each well, and DMSO (150 µl) was added to dissolve the
formazan crystals. Absorbance was measured at 570 nm. Data were
obtained from triplicate wells per condition and the results are
representative of at least three independent experiments.
Flow cytometry
A549 cells (5×105/well) were seeded in
six-well plates and allowed to attach overnight. Then, cells were
treated with Osthole (100 µM) and Embelin (50 µM) alone or in
combination for 24 h, respectively, and harvested by 0.25% trypsin.
The cells were then washed twice with chilled phosphate-buffered
saline (PBS), then resuspended in 250 µl binding buffer and
adjusted to 1×106/ml. Staining solution containing
Annexin V/FITC and PI was added to the cell suspension. Following
incubation in the dark for 30 min, the cells were analyzed by a
FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA).
Fluorescence microscopy
A549 cells (5×105) were seeded into
six-well plates and cultured overnight, and then incubated with
Osthole (100 µM) and Embelin (50 µM) alone or in combination for 24
h, respectively. Cells were washed twice with PBS and fixed with
cold methanol and acetic acid (3/1, v/v) at 4°C. Then, cell
preparations were washed in PBS and stained with Hoechst 33342 (1
mg/ml) for 30 min in the dark before being washed again in PBS and
finally observed with a fluorescence microscope (×400, Nikon
Corporation, Tokyo, Japan).
Western blot analysis
Treated cells were analyzed by western blot
analysis. Briefly, the cell pellets were resuspended in lysis
buffer at 4°C for 1 h. Following centrifugation at 12,000 × g for
20 min, the supernatant was collected and stored at −80°C. A total
of 50 µg protein was separated using 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis and then transferred to
a polyvinylidene fluoride membrane. The membrane was blocked with
5% non-fat milk and incubated overnight at 4°C with antibodies
against Bcl-2, BAX, caspase-3, caspase-9, cleaved caspase-3,
cleaved caspase-9, XIAP, c-IAP1, c-IAP2, survivin and Smac.
Following incubation with peroxidase-conjugated anti-mouse/rabbit
IgG (Santa Cruz Biotechnology, Inc.) at 37°C for 2 h, proteins were
visualized using enhanced chemiluminescence (Pierce Biotechnology,
Inc., Rockford, IL, USA) and detected using a bioimaging system
(UVP Inc., Upland, CA, USA).
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for all statistical analyses. Data are expressed as the
means ± standard deviation. Statistical correlation of data was
checked for significance by analysis of variance and Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Combined effect of Osthole and Embelin
on viability of A549 cells
To evaluate the cytotoxicity of Osthole and Embelin
alone and in combination on lung cancer cells, A549 cells were
treated with Osthole (100 µM) and Embelin (50 µM) alone or in
combination for 24 h, respectively, and the proliferation rate was
examined using MTT assay (Fig. 1).
Our results revealed that Osthole and Embelin treatment alone
inhibited cell proliferation. Notably, however, the combination
treatment of Osthole and Embelin inhibited cell proliferation more
significantly compared with monotherapy.
Combined effect of Osthole and Embelin
on apoptosis
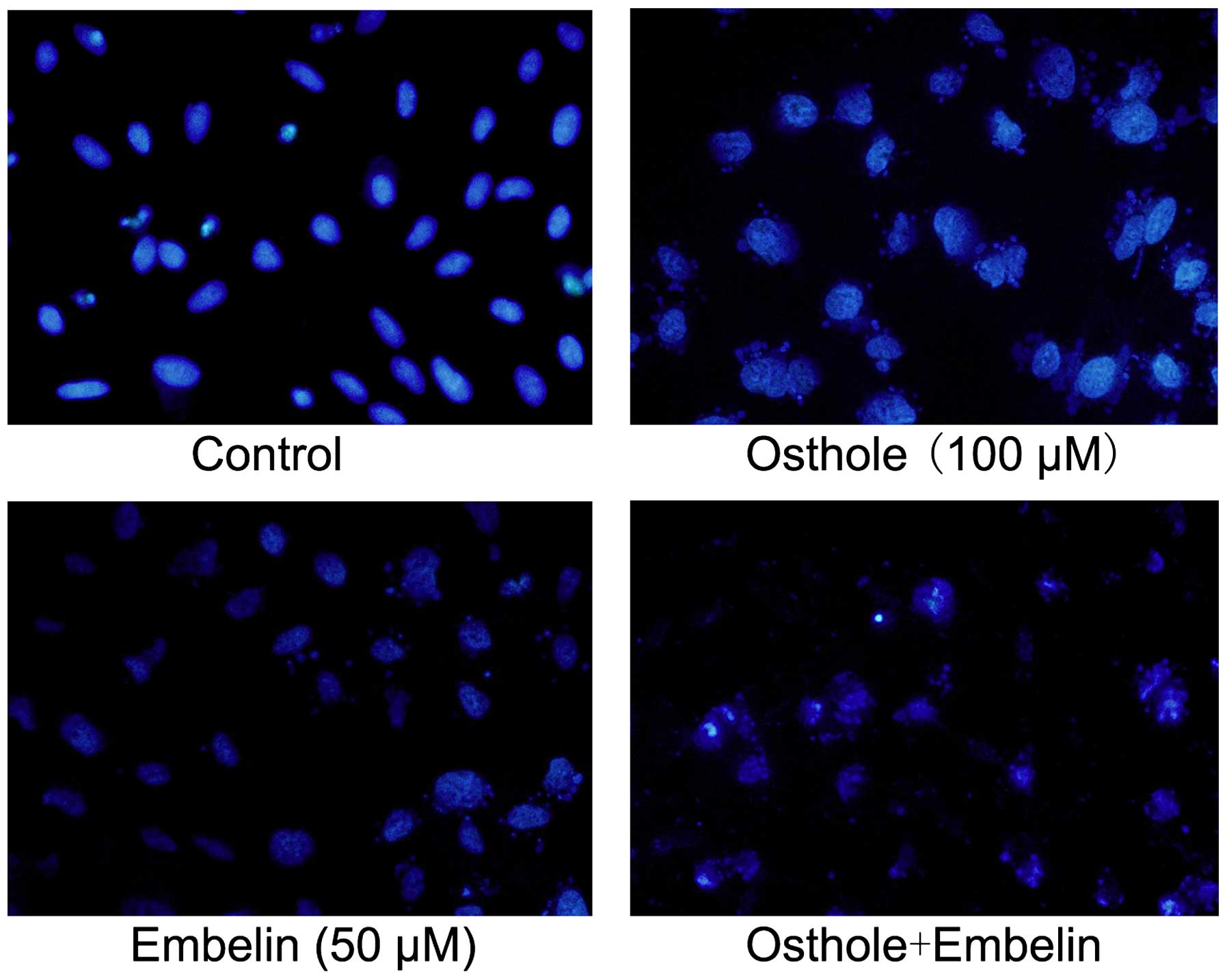
To investigate the effect of Osthole and Embelin
alone and in combination on the apoptosis of lung cancer cells,
A549 cells were treated with Osthole (100 µM) and Embelin (50 µM)
alone or in combination as indicated above. Morphological changes
were detected using fluorescence microscopy and the apoptosis rate
was evaluated by Annexin V/PI staining.
As shown in Fig. 2,
Osthole and Embelin alone caused morphological alteration in A549
cells. Typical morphological changes including condensation of
chromatin, karyopyknosis and nuclear fragmentation were observed.
Notably, compared with cells treated with a single agent, cells
exposed to the combined Osthole and Embelin treatment exhibited a
higher apoptosis rate.
Annexin V/PI flow cytometry analysis indicated that
cells receiving the combined treatment had higher levels of
apoptosis compared with cells treated with Osthole or Embelin
alone. As shown in Fig. 3, both
Osthole or Embelin increased cell apoptosis. The percentage of
apoptotic cells induced by Osthole and Embelin was 4.26±0.41% in
the control group, 18.31±2.67% in the Osthole group and 14.76±1.05%
in the Embelin group. In contrast, the apoptosis percentage induced
by the combination treatment was 34.36±2.98%. These results
demonstrated that Osthole and Embelin had a synergistic effect on
apoptosis in lung cancer cells.
Osthole regulates the IAP pathway in a
dose-dependent manner
The effect of Osthole was further investigated on
IAP proteins, which play a significant role in intrinsic programmed
cell death. A549 cells were treated with various concentrations of
Osthole (0, 50, 100 and 150 µM) for 24 h, and then the expression
levels of IAP family members including XIAP, c-IAP1, c-IAP2,
Survivin and Smac were determined by western blot analysis. The
protein levels of XIAP, c-IAP1, c-IAP2 and Survivin were decreased,
while Smac was increased following Osthole treatment (Fig. 4). Notably, the inhibitory effect of
Osthole on XIAP, c-IAP1, c-IAP2 and Survivin increased as the dose
increased. Conversely, the expression of Smac increased as the dose
of Osthole increased.
Combined effect of Osthole and Embelin
on apoptosis-related proteins
To explore the possible mechanisms by which Osthole
and Embelin regulate apoptosis, a panel of apoptosis-related
proteins were screened following treatment with Osthole (100 µM)
and Embelin (50 µM), alone or in combination. Compared with the
control group, Osthole or Embelin alone increased the expression of
BAX, caspase-3, caspase-9, cleaved caspase-3 and cleaved caspase-9,
while Bcl-2 levels were decreased following treatment (Fig. 5). Notably, the Osthole and Embelin
combination treatment had a synergistic effect on the regulation of
these proteins.
Discussion
Despite therapeutic advances, the high mortality
rate of patients with NSCLC has not been substantially reduced over
the past years. In order to improve the prognosis and survival
rate, intensive efforts have been made to identify novel anticancer
agents, and much attention has been drawn to herbal medicines,
owing to their wide range of biological activities, low toxicity
and minimal side effects. In the present study, we identified
Osthole, a natural derivative of coumarin, as a novel antitumor
agent in NSCLC. Moreover, we observed that Osthole had a
synergistic effect on Embelin, which is another promising antitumor
agent extracted from herbal medicines.
Osthole has long been used in traditional Chinese
medicine for the treatment of eczema, cutaneous pruritus,
trichomonas vaginalis infection and sexual dysfunction. Numerous
previous studies have confirmed that Osthole possesses antitumor
activity. It was reported that Osthole inhibited migration and
invasion of breast cancer cells via suppression of matrix
metalloproteinase (MMP)-2 (30). In
addition, Osthole suppresses the migratory ability of human
glioblastoma cells via the inhibition of focal adhesion
kinase-mediated MMP-13 expression (31). Moreover, several studies have
suggested that Osthole suppresses cell growth and induces apoptosis
in leukemia and hepatocellular and cervical carcinoma cells
(13–15,32). We
have previously reported that Osthole suppressed migration and
invasion, and induced apoptosis in A549 lung cancer cells (33,34). In
addition, we observed that Osthole enhanced the anticancer effect
of cisplatin in lung cancer cells in vitro (35). However, the possible mechanisms behind
this remained unclear. In the present study, we demonstrated that
Osthole induced apoptosis of A549 lung cancer cells via IAP
inhibition.
IAPs are a group of structurally related proteins
that were initially identified in baculoviruses (36). Mammalian IAPs block apoptosis either
by binding and inhibiting caspases or through caspase-independent
mechanisms (22). c-IAPs, XIAP and
melanoma IAP bind caspase-3, −7 and −9 via the BIR domains
(37–40), and induce their ubiquitination or
neddylation via the RING domain (41,42).
Moreover, c-IAPs are positive regulators of cell proliferation
(43), and the nuclear expression of
c-IAP1 has been associated with advanced disease stages and poor
patient prognosis in human cervical and esophageal squamous cell
carcinomas and bladder cancers (44–46). To
date, the overexpression of several IAPs has been detected in
various cancers including NSCLC (22–24,27,47),
and IAPs are significant targets for therapeutic intervention. It
was previously reported that IAP-targeting therapy induces
apoptosis and enhances chemotherapeutic activity against human lung
cancer cells in vitro and in vivo (27,47). In
the present study, we evaluated the effect of Osthole on the IAPs
by measuring the protein levels of XIAP, c-IAP1, c-IAP2 and
Survivin. We observed that treatment of A549 lung cancer cells with
various concentration of Osthole decreased the protein expression
of XIAP, c-IAP1, c-IAP2 and Survivin, and increased Smac expression
in a dose-dependent manner. These results indicate that Osthole
induced apoptosis via regulation of IAP family proteins in a
dose-dependent manner in NSCLC.
Embelin, a plant-based benzoquinone derivative,
serves as a novel antitumor compound by inhibiting the activity of
XIAP (25,26,48–51). More
recently, it was reported that Embelin induced apoptosis in NSCLC
cells (52). Considering that Osthole
and Embelin are low-toxicity natural compounds regulating the
apoptosis of NSCLC, we questioned whether these two agents would
have a synergistic effect on cancer therapy. We hence evaluated the
combination effect of Osthole and Embelin on A549 cell apoptosis,
and revealed that combination treatment exhibited a stronger
apoptosis-inducing effect compared with monotherapy. In addition,
compared with single-agent treatment, Osthole and Embelin
combination treatment caused a greater change in apoptosis-related
proteins including Bcl-2, BAX, caspase-3, caspase-9, cleaved
caspase-3 and cleaved caspase-9. These results indicated that
Osthole and Embelin have a synergistic effect on NSCLC
treatment.
In conclusion, the present study demonstrated that
Osthole inhibited proliferation and induced apoptosis in A549 lung
cancer cells via the IAP family proteins in a dose-dependent
manner. In addition, Osthole enhances the antitumor effect of
Embelin. The present study indicates that the combination of
Osthole and Embelin has potential clinical significance in the
treatment of NSCLC.
Acknowledgements
The present study was supported by the Doctoral
Scientific Research Foundation of Liaoning Province (Shenyang,
China; grant no. 20121127). The study was approved by the ethics
committee of Shengjing Hospital of China Medical University
(Shenyang, China).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pérez-Soler R: Individualized therapy in
non-small-cell lung cancer: future versus current clinical
practice. Oncogene. 28(Suppl 1): S38–S45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Igney FH and Krammer PH: Death and
anti-death: tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Los M, Burek CJ, Stroh C, Benedyk K, Hug H
and Mackiewicz A: Anticancer drugs of tomorrow: apoptotic pathways
as targets for drug design. Drug Discov Today. 8:67–77. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reed JC: Apoptosis-based therapies. Nat
Rev Drug Discov. 1:111–121. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liao PC, Chien SC, Ho CL, Wang EI, Lee SC,
Kuo YH, Jeyashoke N, Chen J, Dong WC, Chao LK and Hua KF: Osthole
regulates inflammatory mediator expression through modulating
NF-κB, mitogen-activated protein kinases, protein kinase C, and
reactive oxygen species. J Agric Food Chem. 58:10445–10451. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu JH, Zschocke S, Reininger E and Bauer
R: Inhibitory effects of Angelica pubescens f. biserrata on
5-lipoxygenase and cyclooxygenase. Planta Med. 64:525–529. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuda H, Tomohiro N, Ido Y and Kubo M:
Anti-allergic effects of cnidii monnieri fructus (dried fruits of
Cnidium monnieri) and its major component, osthol. Biol Pharm Bull.
25:809–812. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu WB, Zhou J, Qu Y, Li X, Lu CT, Xie KL,
Sun XL and Fei Z: Neuroprotective effect of osthole on MPP+-induced
cytotoxicity in PC12 cells via inhibition of mitochondrial
dysfunction and ROS production. Neurochem Int. 57:206–215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuo PL, Hsu YL, Chang CH and Chang JK:
Osthole-mediated cell differentiation through bone morphogenetic
protein-2/p38 and extracellular signal-regulated kinase 1/2 pathway
in human osteoblast cells. J Pharmacol Exp Ther. 314:1290–1299.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang RL, Chen CC, Huang YL, Hsieh DJ, Hu
CP, Chen CF and Chang C: Osthole increases glycosylation of
hepatitis B surface antigen and suppresses the secretion of
hepatitis B virus in vitro. Hepatology. 24:508–515. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang LL, Wang MC, Chen LG and Wang CC:
Cytotoxic activity of coumarins from the fruits of Cnidium monnieri
on leukemia cell lines. Planta Med. 69:1091–1095. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chou SY, Hsu CS, Wang KT, Wang MC and Wang
CC: Antitumor effects of Osthol from Cnidium monnieri: an in vitro
and in vivo study. Phytother Res. 21:226–230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riviere C, Goossens L, Pommery N, Fourneau
C, Delelis A and Henichart JP: Antiproliferative effects of
isopentenylated coumarins isolated from Phellolophium
madagascariense Baker. Nat Prod Res. 20:909–916. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deveraux QL and Reed JC: IAP family
proteins - suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
LaCasse EC, Baird S, Korneluk RG and
MacKenzie AE: The inhibitors of apoptosis (IAPs) and their emerging
role in cancer. Oncogene. 17:3247–3259. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deveraux QL, Takahashi R, Salvesen GS and
Reed JC: X-linked IAP is a direct inhibitor of cell-death
proteases. Nature. 388:300–304. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roy N, Deveraux QL, Takahashi R, Salvesen
GS and Reed JC: The c-IAP-1 and c-IAP-2 proteins are direct
inhibitors of specific caspases. EMBO J. 16:6914–6925. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salvesen GS and Duckett CS: IAP proteins:
blocking the road to death's door. Nat Rev Mol Cell Biol.
3:401–410. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liston P, Fong WG and Korneluk RG: The
inhibitors of apoptosis: there is more to life than Bcl2. Oncogene.
22:8568–8580. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nachmias B, Ashhab Y and Ben-Yehuda D: The
inhibitor of apoptosis protein family (IAPs): an emerging
therapeutic target in cancer. Semin Cancer Biol. 14:231–243. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tamm I, Kornblau SM, Segall H, Krajewski
S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, et al:
Expression and prognostic significance of IAP-family genes in human
cancers and myeloid leukemias. Clin Cancer Res. 6:1796–1803.
2000.PubMed/NCBI
|
|
24
|
Bertrand MJ, Milutinovic S, Dickson KM, Ho
WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ and
Barker PA: cIAP1 and cIAP2 facilitate cancer cell survival by
functioning as E3 ligases that promote RIP1 ubiquitination. Mol
Cell. 30:689–700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chitra M, Sukumar E, Suja V and Devi CS:
Antitumor, anti-inflammatory and analgesic property of embelin, a
plant product. Chemotherapy. 40:109–113. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nikolovska-Coleska Z, Xu L, Hu Z, Tomita
Y, Li P, Roller PP, Wang R, Fang X, Guo R, Zhang M, et al:
Discovery of embelin as a cell-permeable, small-molecular weight
inhibitor of XIAP through structure-based computational screening
of a traditional herbal medicine three-dimensional structure
database. J Med Chem. 47:2430–2440. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu Y, Cherton-Horvat G, Dragowska V, Baird
S, Korneluk RG, Durkin JP, Mayer LD and LaCasse EC: Antisense
oligonucleotides targeting XIAP induce apoptosis and enhance
chemotherapeutic activity against human lung cancer cells in vitro
and in vivo. Clin Cancer Res. 9:2826–2836. 2003.PubMed/NCBI
|
|
28
|
Asselin E, Mills GB and Tsang BK: XIAP
regulates Akt activity and caspase-3-dependent cleavage during
cisplatin-induced apoptosis in human ovarian epithelial cancer
cells. Cancer Res. 61:1862–1868. 2001.PubMed/NCBI
|
|
29
|
Lu J, Huang Y, Zhao W, Marquez RT, Meng X,
Li J, Gao X, Venkataramanan R, Wang Z and Li S: PEG-derivatized
embelin as a nanomicellar carrier for delivery of paclitaxel to
breast and prostate cancers. Biomaterials. 34:1591–1600. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang D, Gu T, Wang T, Tang Q and Ma C:
Effects of osthole on migration and invasion in breast cancer
cells. Biosci Biotechnol Biochem. 74:1430–1434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsai CF, Yeh WL, Chen JH, Lin C, Huang SS
and Lu DY: Osthole suppresses the migratory ability of human
glioblastoma multiforme cells via inhibition of focal adhesion
kinase-mediated matrix metalloproteinase-13 expression. Int J Mol
Sci. 15:3889–3903. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okamoto T, Kobayashi T and Yoshida S:
Chemical aspects of coumarin compounds for the prevention of
hepatocellular carcinomas. Curr Med Chem Anticancer Agents.
5:47–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu XM, Zhang Y, Qu D, Feng XW, Chen Y and
Zhao L: Osthole suppresses migration and invasion of A549 human
lung cancer cells through inhibition of matrix metalloproteinase-2
and matrix metallopeptidase-9 in vitro. Mol Med Rep. 6:1018–1022.
2012.PubMed/NCBI
|
|
34
|
Xu X, Zhang Y, Qu D, Jiang T and Li S:
Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells
by modulating PI3K/Akt pathway. J Exp Clin Cancer Res. 30:332011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu XM, Zhang Y, Qu D, Liu HB, Gu X, Jiao
GY and Zhao L: Combined anticancer activity of osthole and
cisplatin in NCI-H460 lung cancer cells in vitro. Exp Ther Med.
5:707–710. 2013.PubMed/NCBI
|
|
36
|
Duckett CS, Nava VE, Gedrich RW, Clem RJ,
Van Dongen JL, Gilfillan MC, Shiels H, Hardwick JM and Thompson CB:
A conserved family of cellular genes related to the baculovirus iap
gene and encoding apoptosis inhibitors. EMBO J. 15:2685–2694.
1996.PubMed/NCBI
|
|
37
|
Srinivasula SM, Hegde R, Saleh A, Datta P,
Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y
and Alnemri ES: A conserved XIAP-interaction motif in caspase-9 and
Smac/DIABLO regulates caspase activity and apoptosis. Nature.
410:112–116. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Z, Sun C, Olejniczak ET, Meadows RP,
Betz SF, Oost T, Herrmann J, Wu JC and Fesik SW: Structural basis
for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature.
408:1004–1008. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eckelman BP, Drag M, Snipas SJ and
Salvesen GS: The mechanism of peptide-binding specificity of IAP
BIR domains. Cell Death Differ. 15:920–928. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tenev T, Zachariou A, Wilson R, Ditzel M
and Meier P: IAPs are functionally non-equivalent and regulate
effector caspases through distinct mechanisms. Nat Cell Biol.
7:70–77. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang HK, Joazeiro CA, Bonfoco E, Kamada
S, Leverson JD and Hunter T: The inhibitor of apoptosis, cIAP2,
functions as a ubiquitin-protein ligase and promotes in vitro
monoubiquitination of caspases 3 and 7. J Biol Chem.
275:26661–26664. 2000.PubMed/NCBI
|
|
42
|
Choi YE, Butterworth M, Malladi S, Duckett
CS, Cohen GM and Bratton SB: The E3 ubiquitin ligase cIAP1 binds
and ubiquitinates caspase-3 and −7 via unique mechanisms at
distinct steps in their processing. J Biol Chem. 284:12772–12782.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Samuel T, Okada K, Hyer M, Welsh K, Zapata
JM and Reed JC: cIAP1 localizes to the nuclear compartment and
modulates the cell cycle. Cancer Res. 65:210–218. 2005.PubMed/NCBI
|
|
44
|
Imoto I, Tsuda H, Hirasawa A, Miura M,
Sakamoto M, Hirohashi S and Inazawa J: Expression of cIAP1, a
target for 11q22 amplification, correlates with resistance of
cervical cancers to radiotherapy. Cancer Res. 62:4860–4866.
2002.PubMed/NCBI
|
|
45
|
Tanimoto T, Tsuda H, Imazeki N, Ohno Y,
Imoto I, Inazawa J and Matsubara O: Nuclear expression of cIAP-1,
an apoptosis inhibiting protein, predicts lymph node metastasis and
poor patient prognosis in head and neck squamous cell carcinomas.
Cancer Lett. 224:141–151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Che X, Yang D, Zong H, Wang J, Li X, Chen
F, Chen X and Song X: Nuclear cIAP1 overexpression is a tumor
stage- and grade-independent predictor of poor prognosis in human
bladder cancer patients. Urol Oncol. 30:450–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang L, Mashima T, Sato S, Mochizuki M,
Sakamoto H, Yamori T, Oh-Hara T and Tsuruo T: Predominant
suppression of apoptosome by inhibitor of apoptosis protein in
non-small cell lung cancer H460 cells: therapeutic effect of a
novel polyarginine-conjugated Smac peptide. Cancer Res. 63:831–837.
2003.PubMed/NCBI
|
|
48
|
Danquah M, Li F, Duke CB III, Miller DD
and Mahato RI: Micellar delivery of bicalutamide and embelin for
treating prostate cancer. Pharm Res. 26:2081–2092. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sreepriya M and Bali G: Chemopreventive
effects of embelin and curcumin against
N-nitrosodiethylamine/phenobarbital-induced hepatocarcinogenesis in
Wistar rats. Fitoterapia. 76:549–555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dai Y, Qiao L, Chan KW, Yang M, Ye J, Ma
J, Zou B, Gu Q, Wang J, Pang R, et al: Peroxisome
proliferator-activated receptor-gamma contributes to the inhibitory
effects of Embelin on colon carcinogenesis. Cancer Res.
69:4776–4783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Heo JY, Kim HJ, Kim SM, Park KR, Park SY,
Kim SW, Nam D, Jang HJ, Lee SG, Ahn KS, et al: Embelin suppresses
STAT3 signaling, proliferation, and survival of multiple myeloma
via the protein tyrosine phosphatase PTEN. Cancer Lett. 308:71–80.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Avisetti DR, Babu KS and Kalivendi SV:
Activation of p38/JNK pathway is responsible for embelin induced
apoptosis in lung cancer cells: transitional role of reactive
oxygen species. PloS One. 9:e870502014. View Article : Google Scholar : PubMed/NCBI
|