Introduction
Ovarian cancer is one of the most common
gynecological malignant tumors with a high mortality rate; it
remains the most common cause of gynecological cancer-associated
mortality (1). Epithelial ovarian
cancer (EOC) constitutes >90% of ovarian malignancies (2), and is often found in the advanced stage
of ovarian cancer (3). Although
traditional tumor cytoreduction and platinum-based chemotherapy can
relieve the disease, ~70% of patients with advanced ovarian cancer
relapse, and the majority succumb to severe side effects and
chemotherapy resistance (4). The
5-year survival rate for ovarian cancer has not improved
significantly over time, and it remains a serious threat to the
lives and health of women (5).
In the last two decades, immunotherapy for EOC has
emerged as an attractive auxiliary treatment method. It is commonly
accepted that ovarian tumors are immunogenic (6). Previous studies have associated
antitumor immune responses with a significant improvement in
overall survival (7,8), which may be due to their ability to
increase antitumor immunological activity, thereby inhibiting and
killing tumor cells. Among the various types of immunotherapy,
adoptive cellular immunotherapy involving the infusion of
immunocompetent cells with antitumor activity into patients in
order to directly kill tumor cells or stimulate the body's immune
response is one of the hotspots in cancer treatment (9,10). An
important issue of immunotherapy is the selection of appropriate
tumor killing cells: Cytotoxic T lymphocytes (CTLs) are the first
to respond to tumor antigens and the most effective of the effector
cells in the killing of tumor cells (11). CTLs are able to eliminate minimal
residual nidi and may even entirely attenuate advanced cancer;
thus, they have an important role in antitumor immunotherapy
(11,12).
CTL activation requires a double-signal stimulus.
Tumor antigen peptides are processed as polypeptides by
antigen-presenting cells (APCs), combined with major
histocompatibility complex (MHC) molecules and transported to the
APC surface to generate a T-cell receptor (TCR) activation signal
(13). The combination of antigen and
relevant receptors on the surface of T lymphocytes generates the
co-stimulatory signal, which is the second signal (14). Only upon stimulation with both the TCR
activation signal and the co-stimulatory signal can T lymphocytes
be activated (13). Adjuvants are
able to non-specifically alter or enhance the body's specific
immune response to antigens, enhance the immunogenicity of the
corresponding antigen or change the type of immune response
(15).
Human epidermal growth factor receptor 2 (HER2/neu)
is an attractive immunological target, since HER2/neu activates
signaling pathways involved in cellular differentiation,
proliferation, migration and apoptosis (16). It has previously been demonstrated
that HER2/neu is recurrently overexpressed in ovarian cancer, which
may lead to enhanced cell proliferation and malignant phenotype
transformation (17,18). In addition, previous studies have
reported that HER2/neu is an important surface biomarker of ovarian
cancer cells and is associated with a poor prognosis and
chemotherapy resistance (19–21). Oligodeoxynucleotides with CpG motifs
(CpG oligodeoxynucleotide; CpGODN) have shown strong immune
activation effects (22), adjuvant
effects and a low toxicity; they are suitable for artificial
synthesis and are one of the strongest novel adjuvants to date
(23). Ginsenoside has previously
shown a strong activity in the prevention and inhibition of the
growth and metastasis of tumors (24). The ginsenoside Rg1 monomer is an
effective immune adjuvant that can stimulate the proliferation of T
and B lymphocytes and differentiation of antigen-specific
lymphocytes (25,26).
The present study aimed to establish a novel method
for inducing large numbers of CTLs via the double-signal activation
pathway, in order to develop an immune-based therapy for the
inhibition and killing of ovarian cancer cells with a high safety
and efficacy. CpGODN and ginsenoside Rg1 were united as an immune
adjuvant and, combined with the HER2/neu antigen peptide, were used
to establish a specific CTL culture system in vitro.
Subseuqently, the inhibitory and lethal effects of the CTL culture
system on SKOV3 ovarian cancer cells were assessed. The results of
the present study may provide the experimental foundation for
further research on the function and mechanism of CTLs in tumor
immunotherapy, and provide a theoretical basis for their clinical
promotion and application.
Materials and methods
Ethics statement
This study was performed in accordance with
institutional and national guidelines and regulations, and with
approval from the Ethics Committee of The Second Hospital of Jilin
University (Changchun, China). Written informed consent was
obtained from all patients.
Isolation of peripheral blood
mononuclear cells (PBMCs) and cell culture
Peripheral blood (20 ml) was obtained from healthy
donors by venipuncture at The Second Hospital of Jilin University
between June 2013 and December 2014, and PBMCs were isolated by
density gradient centrifugation (27)
using lymphocyte separation medium (Beijing Dingguo Biological
Technology, Co., Ltd., Beijing, China). Cell counts of living cells
were determined by trypan blue staining exclusion assays. The SKOV3
human ovarian cancer cell line was purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
PBMCs and SKOV3 cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (FBS; Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) at 37°C in a humidified 5% CO2
incubator. SKOV3 cells in the logarithmic growth phase were used in
the study.
Specific antigen epitopes
CTL antigen epitopes were predicted using GenBank
(https://www.ncbi.nlm.nih.gov/genbank/), CLC Protein
Workbench 3 (CLC bio, Waltham, MA, USA) and the SignalP 3.0 server
(http://www.cbs.dtu.dk/services/SignalP-3.0/). A
9-amino acid human leukocyte antigen-A2-restricted CTL epitope
corresponding to a cleavage site of the HER2/neu tumor antigen was
also predicted by the above mentioned software. Finally, the
P369-377 peptide epitope, which has previously been shown to be
immunocompetent (28–31), was elected as the antigen peptide from
the antigen epitope prediction results. The amino acid sequence of
the P369-377 peptide epitope was as follows:
Lysine-isoleucine-phenylalanine (Phe)-glycine-serine-leucine
(Leu)-alanine-Phe-Leu. The peptide was synthesized by Sangon
Biotech, Co., Ltd. (Shanghai, China).
Viability of SKOV3 cells treated with
PBMCs activated by various concentrations of stimulators
The concentration of PBMCs was adjusted to
1×106/ml and the cells were inoculated into 96-well
plates (100 µl/well). HER2/neu antigen peptide (Sangon Biotech,
Co., Ltd.) was added into five groups of CpGODN (Sangon Biotech
Co., Ltd.) or ginsenoside Rg1 (purity >99%; Jilin University) at
different concentrations (3.75, 7.5, 15, 30 or 60 µg/ml), making
the final concentration of HER2/neu 10 µg/ml. Subsequently, the
mixtures containing various concentrations of CpGODN or ginsenoside
Rg1 were added to the PBMCs and incubated for 7 days; each group
consisted of three parallel wells. SKOV3 cells in the logarithmic
growth phase were digested with 0.25% trypsin and diluted in
RPMI-1640 medium containing 10% FBS. The single cell suspension
(5×104/ml; 100 µl) was inoculated into 96-well plates,
and the cells were cultured at 37°C in 5% CO2 overnight.
After the cells had adhered to the well, the supernatant was
discarded and the PBMCs stimulated with the mixtures were added to
SKOV3 cells. After 48 h of incubation, 20 µl MTT (5 mg/ml;
Sigma-Aldrich; EMD Millipore, Darmstadt, Germany) was added to each
well and incubated at 37°C in a 5% CO2 incubator for an
additional 4 h. The supernatant was then removed and 150 µl
dimethyl sulfoxide (Sigma-Aldrich; EMD Millipore) was added to each
well and the cells were gently shaken for 10 min until the crystals
had dissolved. Absorbance values were determined using an ELISA
reader at 490 nm.
Establishment of CTL cultures in vitro
and cell growth curves
A mixture of CpGODN (30 µg/ml), ginsenoside Rg1 (30
µg/ml) and HER2/neu antigen peptide (10 µg/ml), termed the specific
mixture, was added to PBMCs (1×104/ml) in 24-well plates
and cultured for 34 days at 37°C. The medium was replaced and the
cells were adjusted to 1×106/ml every 3 days. On day 34,
the cells were collected and named HER2/neu-specific CTLs. Growth
curves were drawn according to cell counts on days 1, 4, 7, 10, 13,
16, 19, 22, 25, 28, 31 and 34.
Karyotype analysis
Karyotypes were analyzed by conventional Giemsa
staining. Conventional cytogenetic analysis of PBMCs was performed
prior to and following stimulation with the specific mixture. PBMCs
(1×105 cells/well) were treated with 0.05 ml colcemid
(25 µg/ml; Sigma-Aldrich; EMD Millipore) for 4 h at 37°C, after
which the cells were harvested and resuspended in 8 ml 37°C
hypotonic solution, and then cultured at 37°C in a humidified 5%
CO2 incubator for 30 min. Following incubation, cells
were harvested and fixed in freshly prepared fixative (volume ratio
of methanol:acetic acid, 3:1), and slides were prepared by
hot-plate drying for 15 min. Metaphase chromosomes were banded
using trypsin-Giemsa and karyotyped according to the International
System for Human Cytogenetic Nomenclature (ISCN 2005) (32).
Flow cytometry
PBMCs were harvested following incubation with
specific mixture for 10, 20 or 30 days. The expression of cluster
of differentiation (CD)3, CD4 and CD8 on the surface of PBMCs was
measured using flow cytometry. Briefly, the cells were washed in
fluorescence-activated cell sorting (FACS) medium
[phosphate-buffered saline (PBS) containing 1% bovine serum albumin
(Sigma-Aldrich; EMD Millipore)] and stained at 4°C for 20 min with
the following antibodies: Anti-CD3-phycoerythrin (PE)-cyanine 5
(1:20; cat. no. ab157300; Abcam, Cambridge, UK), CD4-fluorescein
isothiocyanate (1:20; cat. no. ab59474; Abcam) and CD8-PE (1:20;
cat. no. ab210327; Abcam). Thereafter, cells were washed three
times with PBS and analyzed by FACS (FCM-500; Beckman Coulter,
Inc., Brea, CA, USA).
Inhibition of SKOV3 cells treated with
HER2/neu-specific CTLs
PBMCs were divided into three groups, as follows: i)
The effect cell control group; ii) the HER2/neu group (positive
control group); and iii) the specific mixture group (experimental
group). PBMCs (1×106/ml) were seeded into 96-well plates
in triplicate; each well had a volume of 100 µl. Subsequently, the
cells were treated with 10 µg/ml HER2/neu or the specific mixture
(30 µg/ml CpGODN, 30 µg/ml ginsenoside Rg1 and 10 µg/ml HER2/neu)
and cultured at 37°C in a 5% CO2 incubator. Cells
collected on day 19 were used as the effect cells. SKOV3 cells in
the logarithmic growth phase were digested with 0.25% trypsin and
the single cell suspension was inoculated into 96-well plates at
5×104/ml. The cells were cultured at 37°C in 5%
CO2 overnight, after which the supernatant was
discarded, such that the adherent cells became the target cells.
SKOV3 cells were added to the three groups of effect cells at
effect cell-to-target cell ratios of 5:1, 10:1, 20:1 and 40:1. The
group with no effect cells was the target cell control group. Cells
were cultured at 37°C in a 5% CO2 incubator for 48 h.
MTT assays were performed at 6, 12, 18, 24, 30, 36, 42 and 48 h,
and absorbance values were determined using an ELISA reader at 490
nm. The CTL inhibition rate on SKOV3 cells was calculated as
follows: CTL inhibitory rate (%) = [target cell control group
A490 - (experimental group A490 - effect cell
control group A490)] / target cell control group
A490 × 100. The inhibitory effect of HER2/neu-specific
CTLs on SKOV3 cells at different time points was tested in the
following two groups: i) The experimental group treated with
specific mixture at the effect-to-target ratio of 20:1; and ii) the
control group added into equal medium with no effect cells.
Statistical analysis
Data are presented as the mean ± standard deviation.
Comparisons among the groups were performed using one way analysis
of variance and Tukey's test. Between group comparisons were
performed using Student's t-tests. Statistical analyses were
conducted using SPSS 19.0 statistical software (IBM SPSS, Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Stimulation of PBMCs with various
concentrations of immunological adjuvant and HER/neu antigen
peptide
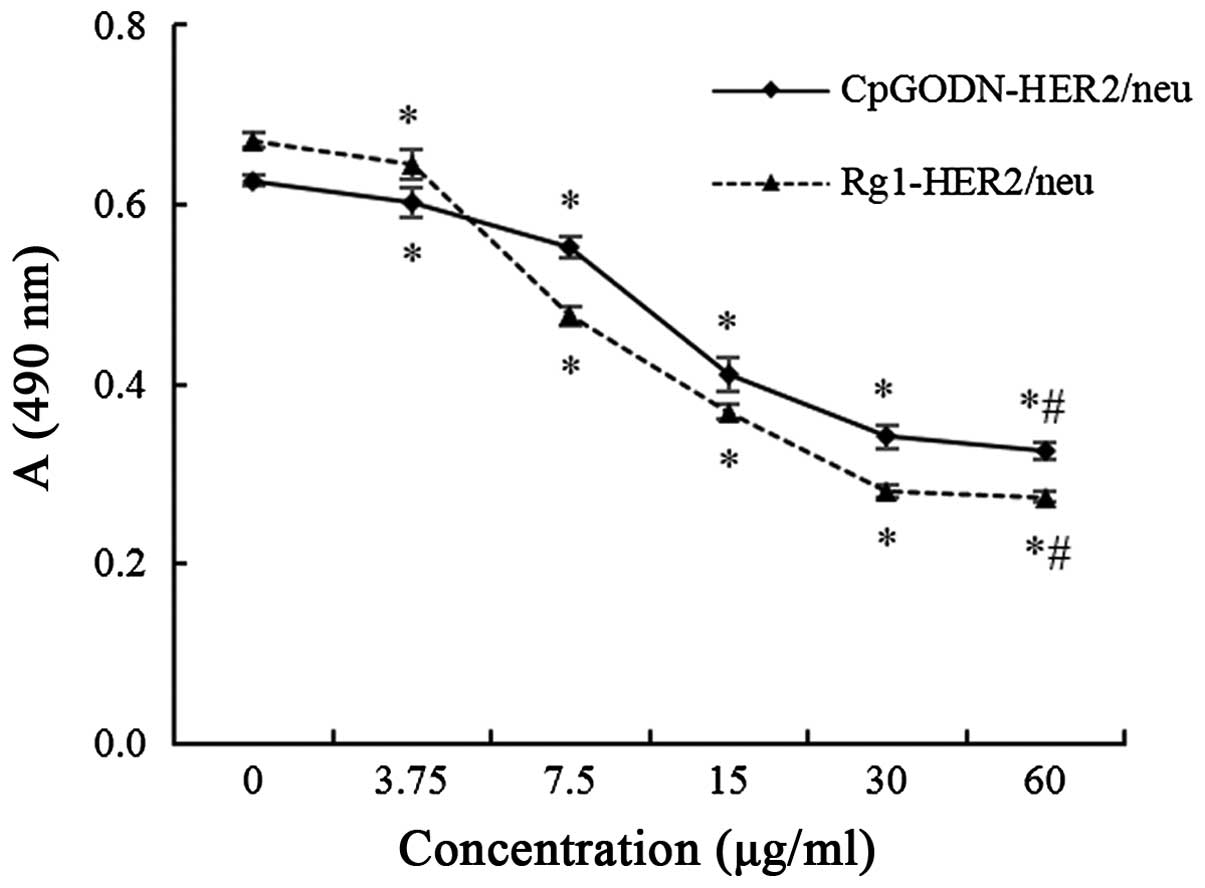
To observe the common effects of CpGODN and HER2/neu
antigen peptide and ginsenoside Rg1 and HER2/neu antigen peptide
stimulation on PBMC activation, MTT assays were performed to assess
the viability of SKOV3 cells treated with stimulated PBMCs. As
shown in Fig. 1, the number of viable
SKOV3 cells was significantly decreased as the concentration of
CpGODN or ginsenoside Rg1 was increased. These results suggested
that increasing the number of stimulated PBMCs significantly
reduced the viability of SKOV3 cells (P=0.047, <0.001,
<0.001, <0.001 and <0.001 for the 3.75, 7.5, 15, 30 and 60
µg/ml CpGODN-HER2/neu groups vs. the group without CpGODN,
respectively; and P=0.040, <0.001, <0.001, <0.001 and
<0.001 for the 3.75, 7.5, 15, 30 and 60 µg/ml vs. the group
without Rg1, respectively). However, the viability of SKOV3 cells
stimulated with 60 µg/ml CpGODN or ginsenoside Rg1 was not
significantly different compared with those stimulated with 30
µg/ml CpGODN or ginsenoside Rg1 (P=0.688 for the CpGODN-HER2/neu
group and P=0.953 for the Rg1-HER2/neu group). Therefore, 30 µg/ml
CpGODN and ginsenoside Rg1 were used for subsequent
experiments.
Karyotype analysis of
HER2/neu-specific CTLs
To assess the safety of the specific CTL culture
system in vitro, the PBMC karyotype was analyzed in order to
detect whether PBMCs were stable following stimulation with the
specific mixture. As shown in Fig.
2A, there was no change in the PBMC karyotype following
stimulation with the specific mixture.
 | Figure 2.Characterization of the specific
cytotoxic T lymphocyte (CTL) culture system in vitro. (A)
Karyotype analysis of peripheral blood mononuclear cells (PBMCs)
was performed using conventional Giemsa staining. (a) The karyotype
of PBMCs following stimulation with specific mixture (the
experimental group). (b) The karyotype of PBMCs without stimulation
(the control group). (B) Cell proliferation was determined by
counting total cell numbers at 1, 4, 7, 10, 13, 16, 19, 22, 25, 28,
31 and 34 days of culture. Values are the mean ± standard deviation
(SD) of three independent experiments. *P<0.01,
∆P>0.05 vs. the cell number on day 1.
#P>0.05 vs. the cell number on day 19. (C and D) The
percentages of CD3+, CD3+CD4+ and
CD3+CD8+ PBMCs were assayed by flow cytometry
prior to stimulation and on days 10, 20 and 30 following
stimulation with the specific mixture. Values are the mean ± SD of
three independent experiments. *P<0.05, **P<0.01 vs. day 0.
CD, cluster of differentiation; PE-Cy5, phycoerythrin-cyanine 5;
FITC, fluorescein isothiocyanate. |
Proliferation of specific
mixture-treated PBMCs
The number of PBMCs was counted on days 1, 4, 7, 10,
13, 16, 19, 22, 25, 28, 31 and 34, in order to detect the effect of
the specific mixture on the growth of PBMCs. As shown in Fig. 2B, the number of cells increased as the
culture time was extended. Cell proliferation was not detected
prior to day 4 (P=1.000 for 4th day group vs. 1st day group).
However, the number of PBMCs was significantly increased after day
7 (P<0.01 for groups on days 7, 10, 13, 16, 19, 22, 25, 28, 31
and 34 vs. 1st day group), although there was no significant
difference in the cell number after day 19 (P=0.986, 0.230, 0.094,
0.195 and 1.000 for groups on days 22, 25, 28, 31 and 34 vs. 19th
day group). Therefore, the stimulated cells collected on day 19
were used as the effector cells in the subsequent experiments.
Immunophenotype of PBMCs
T-cells are characterized by the CD3+
molecular phenotype. CTLs are CD8+ T-cells, while
CD4+ T-cells constitutes another molecular phenotype
(33,34). To identify whether the stimulated
PBMCs were CTLs, flow cytometry was performed to examine the
immunophenotype. As shown in Fig. 2C and
D, the immunophenotypes of PBMCs were changed following
stimulation with the specific mixture. The proportion of cells that
expressed CD3 increased from 28.17±5.14 to 79.30±3.17% following
stimulation (P<0.01 for 30th day group vs. group on day 0).
Furthermore, the cells expressing both CD3 and CD4 increased from
16.40±5.16 to 54.53±1.85% after 30 days of stimulation (P=0.002 for
30th day group vs. group on day 0), and the number of cells
expressing CD3 and CD8 were significantly increased (P<0.01 for
30th day group vs. group on day 0). These results suggested that
specific CTL cells had been successfully induced by the specific
mixture.
Lethal and inhibitory effects of
HER2/neu-specific CTLs on SKOV3 cells
Following the establishment and identification of
the specific CTL system, the lethal and inhibitory effects of the
HER2/neu-specific CTLs on SKOV3 ovarian cancer cells was assessed
using MTT assays. As shown in Fig.
3A, as compared with the positive control group, the
experimental group appeared to have a stronger lethal effect on
SKOV3 cells, which increased as the ratio of effect cells-to-target
cells was increased (P=0.049, 0.034, 0.023 and 0.022 for
experimental groups with effect-target ratio of 5:1, 10:1, 20:1 and
40:1 vs. positive control groups, respectively). These results
suggested that the HER2/neu-specific CTLs exerted lethal effects on
ovarian cancer cells, which were positively related to the number
of activated PBMCs. Therefore, the effect-to-target ratio of 20:1
was used in the subsequent experiments.
 | Figure 3.Inhibitory effect of
HER2/neu-specific CTLs on SKOV3 cells. (A) SKOV3 cells were treated
with HER2/neu-specific CTLs at different effect-to-target cell
ratios (5:1, 10:1, 20:1 or 40:1), and the inhibitory effect was
analyzed using MTT assays. Inhibition rates were calculated
according to the formula: Inhibitory rate (%) = [target cell
control group A490 - (experimental group A490
- effect cell control group A490)] / target cell control
group × 100. Values are the mean ± standard deviation (SD) of six
independent experiments. *P<0.05 vs. the positive control group
(HER2/neu). (B) SKOV3 cells were treated with HER2/neu-specific
CTLs at the effect-to-target cell ratio of 20:1, and analyzed using
MTT assays after 6, 12, 18, 24, 30, 36, 42 and 48 h of treatment.
Values are the mean ± SD of six independent experiments. *P<0.01
vs. the control group. CTL, cytotoxic T lymphocytes; HER2/neu,
human epidermal growth factor receptor 2. |
The inhibitory effect of the effect-to-target ratio
of 20:1 on SKOV3 cells is shown in Fig.
3B. The growth curve of SKOV3 cells treated with the control
group PBMCs (without HER2/neu, CpGODN and ginsenoside Rg1) showed
an increasing trend during the first 48 h. Conversely, the growth
curve of the experimental group treated with HER2/neu-specific CTLs
showed a decreasing trend during the first 48 h. The number of
SKOV3 cells in the experimental group was significantly decreased
compared with the control group at the same time point (P<0.01
for experimental groups at 12, 18, 24, 30, 36, 42 and 48 h vs. the
corresponding control groups).
Discussion
Ovarian cancer is the most lethal gynecological
malignancy. Primary treatment involves surgical debulking of the
visible disease, followed by adjuvant chemotherapy (7). However, even though >70% of patients
initially respond to cisplatin, the prognosis is still poor, due to
chemoresistance or high proportion of advance-stage cases (4). Currently, tumor biological therapies are
receiving increasing attention as an alternative effective
therapeutic strategy to operation, radiotherapy and
chemotherapy.
As a novel adjuvant treatment method, immunotherapy
has been shown to improve the antitumor immune response in the
body, so as to eliminate residual metastatic lesions and delay the
development of cancer following the standard cytoreductive surgery
and platinum-based chemotherapy (35). In addition, immunotherapy was reported
to effectively improve patients' autonomic anticancer immunity,
strengthen their physique, reduce chemotherapy resistance, enhance
the effect of chemotherapy on ovarian cancer and extend the valid
period of survival (10).
Antigen-specific active immunotherapy aims to induce
tumor-antigen-specific antitumor immune responses, and has emerged
as an alternative treatment for ovarian cancer (36). In the process of an immune response,
tumor antigens are processed by APCs and presented to CTLs
(14). Adjuvants are able to
non-specifically alter or enhance the body's immune response to
specific antigens, and enhance the immunogenicity of the
corresponding antigen or change the type of immune response
(37).
In the present study, HER2/neu was used as the tumor
antigen adjuvant to enhance the immunogenicity of HER2/neu and the
specificity of the immune response. The HER2/neu antigen peptide
was combined with CpGODN and ginsenoside Rg1, and the mixture was
used to stimulate T lymphocytes in peripheral blood via the
double-signal pathway. Subsequently, CTLs were efficiently
proliferated in vitro. By controlling a single variable, the
optimum ratio of CpGODN, ginsenoside Rg1 and HER2/neu antigen
peptide was found. MTT assays demonstrated that, when the
concentration of CpGODN and ginsenoside Rg1 was 30 µg/ml, and that
of HER2/neu was 10 µg/ml, the lethal effect of specific CTL on
SKOV3 cells was the most potent. Therefore, a specific CTL in
vitro culture system was established. Following treatment with
the specific mixture, PBMCs underwent a 4-day adaptive phase,
followed by proliferation; the logarithmic growth phase was reached
on day 7. From day 19, the growth of cells began to plateau,
although PBMCs were shown to remain viable up until day 34. As a
result of active metabolism in the logarithmic phase, the cell
condition was fine. Therefore, the cells harvested on day 19 were
used as effector cells in the subsequent experiments to ensure both
a good condition and quantity of cells. Flow cytometry was used to
asses the immunophenotypes of the PBMCs. The results indicated
that, following treatment with the specific mixture, the
percentages of CD3+, CD3+CD4+ and
CD3+CD8+ PBMCs were significantly increased
compared with the control group, which demonstrated that a large
quantity of CTL cells were induced successfully. Through chromosome
karyotype analyses, it was shown that the lymphocyte karyotype did
not change following stimulation with the specific mixture. These
results suggested that HER2/neu-specific CTLs may be safely
reinfused into the human body. The high survival rate and strong
proliferative capacity of the HER2/neu-specific CTLs were the basis
for their strong cytotoxic activity, thereby solving the lack of
effector cells obtained by amplification in vitro (38).
The rapid proliferation of cells is one of the most
important characteristics of malignant tumors. Therefore,
effectively inhibiting the growth and proliferation of tumor cells
is one of the main purposes of antitumor therapies. In the present
study, the MTT method (39) was
adopted to investigate the inhibitory effect of HER2/neu-specific
CTLs on SKOV3 cells at different effect cell-to-target cell ratios.
HER2/neu-specific CTLs were diluted into various concentrations
according to the different effect-to-target cell ratios and added
to SKOV3 cells. The results showed that, compared with the control
group, the inhibitory effect on SKOV3 cells was significantly
increased for all experimental groups. The inhibition rate was
76.75% when the effect-to-target cell ratio was 40:1. The influence
of HER2/neu-specific CTLs on SKOV3 cell viability was assessed
using the MTT method, and growth curves for SKOV3 cells in the
control group and experimental groups were constructed. The result
indicated that, compared with the control group, the growth of
SKOV3 cells treated with HER2/neu-specific CTLs was significantly
inhibited. These results suggested that HER2/neu-specific CTLs were
effective across the entire growth processes of SKOV3 cells.
In conclusion, the present study united CpGODN and
ginsenoside Rg1 as an immune adjuvant, which, combined with the
HER2/neu antigen peptide, was used to rapidly induce a large number
of PBMCs to form CTL cells, thereby establishing a large-scale
specific CTL culture system in vitro. This system was shown
to satisfy the safety of clinical reinfusion. The high rate of
proliferation of the HER2/neu-specific CTLs solved the problem of
obtaining sufficient effector cells by amplification in
vitro. In addition, the present study demonstrated the high
antigen specificity of the specific CTL in vitro, which had
significant inhibitory and lethal effects on ovarian cancer cells.
The results of the present study may serve as an experimental
foundation for the systematic analysis of the function and
mechanisms of HER2/neu-specific CTLs in tumor immunotherapy.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81302242 and
81272875), the Ministry of Education for Young Teacher Foundation
of China (grant no. 20110061120084), the Jilin province Science and
Technology Funds (grant nos. 20120957, 20130102094JC and
20140204022YY), and the Jilin province Development and Reform
Commission Funds (grant no. 2013C026-3).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bell DA: Origins and molecular pathology
of ovarian cancer. Mod Pathol. 18(Suppl 2): S19–S32. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu KH, Skates S, Hernandez MA, Bedi D,
Bevers T, Leeds L, Moore R, Granai C, Harris S, Newland W, et al: A
2-stage ovarian cancer screening strategy using the Risk of Ovarian
Cancer Algorithm (ROCA) identifies early-stage incident cancers and
demonstrates high positive predictive value. Cancer. 119:3454–3461.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai Y, Tan X, Liu J, Shen Y, Wu D, Ren M,
Huang P and Yu D: Inhibition of PI3K/Akt/mTOR signaling pathway
enhances the sensitivity of the SKOV3/DDP ovarian cancer cell line
to cisplatin in vitro. Chin J Cancer Res. 26:564–572.
2014.PubMed/NCBI
|
|
5
|
Lenhard SM, Bufe A, Kümper C, Stieber P,
Mayr D, Hertlein L, Kirschenhofer A, Friese K and Burges A: Relapse
and survival in early-stage ovarian cancer. Arch Gynecol Obstet.
280:71–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mantia-Smaldone GM, Corr B and Chu CS:
Immunotherapy in ovarian cancer. Hum Vaccin Immunother.
8:1179–1191. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adams SF, Levine DA, Cadungog MG, Hammond
R, Facciabene A, Olvera N, Rubin SC, Boyd J, Gimotty PA and Coukos
G: Intraepithelial T cells and tumor proliferation: Impact on the
benefit from surgical cytoreduction in advanced serous ovarian
cancer. Cancer. 115:2891–2902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Conejo-Garcia JR, Katsaros D,
Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H,
Schlienger K, Liebman MN, et al: Intratumoral T cells, recurrence
and survival in epithelial ovarian cancer. N Engl J Med.
348:203–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosenberg SA, Restifo NP, Yang JC, Morgan
RA and Dudley ME: Adoptive cell transfer: A clinical path to
effective cancer immunotherapy. Nat Rev Cancer. 8:299–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berek J, Taylor P, McGuire W, Smith LM,
Schultes B and Nicodemus CF: Oregovomab maintenance
monoimmunotherapy does not improve outcomes in advanced ovarian
cancer. J Clin Oncol. 27:418–425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dadmarz RD, Ordoubadi A, Mixon A, Thompson
CO, Barracchini KC, Hijazi YM, Steller MA, Rosenberg SA and
Schwartzentruber DJ: Tumor-infiltrating lymphocytes from human
ovarian cancer patients recognize autologous tumor in an MHC class
II-restricted fashion. Cancer J Sci Am. 2:263–272. 1996.PubMed/NCBI
|
|
12
|
Santin AD, Bellone S, Ravaggi A, Pecorelli
S, Cannon MJ and Parham GP: Induction of ovarian tumor-specific
CD8+ cytotoxic T lymphocytes by acid-eluted peptide-pulsed
autologous dendritic cells. Obstet Gynecol. 96:422–430. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smith KA: Interleukin-2: Inception,
impact, and implications. Science. 240:1169–1176. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gri G, Gallo E, Di Carlo E, Musiani P and
Colombo MP: OX40 ligand-transduced tumor cell vaccine synergizes
with GM-CSF and requires CD40-Apc signaling to boost the host T
cell antitumor response. J Immunol. 170:99–106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coumes F, Huang CY, Huang CH, Coudane J,
Domurado D, Li S, Darcos V and Huang MH: Design and development of
immunomodulatory antigen delivery systems based on peptide/PEG-PLA
conjugate for tuning immunity. Biomacromolecules. 16:3666–3673.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Preston CC, Goode EL, Hartmann LC, Kalli
KR and Knutson KL: Immunity and immune suppression in human ovarian
cancer. Immunotherapy. 3:539–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meden H and Kuhn W: Overexpression of the
oncogene c-erbB-2 (HER2/neu) in ovarian cancer: A new prognostic
factor. Eur J Obstet Gynecol Reprod Biol. 71:173–179. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang SC and Hung MC: HER2 overexpression
and cancer targeting. Semin Oncol. 28:115–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tuefferd M, Couturier J, Penault-Llorca F,
Vincent-Salomon A, Broët P, Guastalla JP, Allouache D, Combe M,
Weber B, Pujade-Lauraine E and Camilleri-Broët S: HER2 status in
ovarian carcinomas: A multicenter GINECO study of 320 patients.
PLoS One. 2:e11382007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Høgdall EV, Christensen L, Kjaer SK,
Blaakaer J, Bock JE, Glud E, Nørgaard-Pedersen B and Høgdall CK:
Distribution of HER-2 overexpression in ovarian carcinoma tissue
and its prognostic value in patients with ovarian carcinoma: From
the Danish MALOVA ovarian cancer study. Cancer. 98:66–73. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teplinsky E and Muggia F: Targeting HER2
in ovarian and uterine cancers: Challenges and future directions.
Gynecol Oncol. 135:364–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klinman DM, Klaschik S, Sato T and Tross
D: CpG oligonucleotides as adjuvants for vaccines targeting
infectious diseases. Adv Drug Deliv Rev. 61:248–255. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia Y, Gupta GK, Castano AP, Mroz P, Avci
P and Hamblin MR: CpG oligodeoxynucleotide as immune adjuvant
enhances photodynamic therapy response in murine metastatic breast
cancer. J Biophotonics. 7:897–905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qi LW, Wang CZ and Yuan CS: American
ginseng: Potential structure-function relationship in cancer
chemoprevention. Biochem Pharmacol. 80:947–954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee EJ, Ko E, Lee J, Rho S, Ko S, Shin MK,
Min BI, Hong MC, Kim SY and Bae H: Ginsenoside Rg1 enhances CD4(+)
T-cell activities and modulates Th1/Th2 differentiation. Int
Immunopharmacol. 4:235–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su F, Yuan L, Zhang L and Hu S:
Ginsenosides Rg1 and Re act as adjuvant via TLR4 signaling pathway.
Vaccine. 30:4106–4112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Posevitz-Fejfár A, Posevitz V, Gross CC,
Bhatia U, Kurth F, Schütte V, Bar-Or A, Meuth SG and Wiendl H:
Effects of blood transportation on human peripheral mononuclear
cell yield, phenotype and function: Implications for immune cell
biobanking. PLoS One. 9:e1159202014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weidanz JA, Nguyen T, Woodburn T,
Neethling FA, Chiriva-Internati M, Hildebrand WH and Lustgarten J:
Levels of specific peptide-HLA class I complex predicts tumor cell
susceptibility to CTL killing. J Immunol. 177:5088–5097. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Conrad H, Gebhard K, Krönig H, Neudorfer
J, Busch DH, Peschel C and Bernhard H: CTLs directed against HER2
specifically cross-react with HER3 and HER4. J Immunol.
180:8135–8145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mittendorf EA, Holmes JP, Ponniah S and
Peoples GE: The E75 HER2/neu peptide vaccine. Cancer Immunol
Immunother. 57:1511–1521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vertuani S, Triulzi C, Roos AK, Charo J,
Norell H, Lemonnier F, Pisa P, Seliger B and Kiessling R: HER-2/neu
mediated down-regulation of MHC class I antigen processing prevents
CTL-mediated tumor recognition upon DNA vaccination in HLA-A2
transgenic mice. Cancer Immunol Immunother. 58:653–664. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shaffer LG and Tommerup N: An
international system for human cytogenetic nomenclature (2005). S
Karger AG; Basel: 2005
|
|
33
|
Stuge TB, Holmes SP, Saharan S,
Tuettenberg A, Roederer M, Weber JS and Lee PP: Diversity and
recognition efficiency of T cell responses to cancer. PLoS Med.
1:e282004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yee C, Gilbert MJ, Riddell SR, Brichard
VG, Fefer A, Thompson JA, Boon T and Greenberg PD: Isolation of
tyrosinase-specific CD8+ and CD4+ T cell clones from the peripheral
blood of melanoma patients following in vitro stimulation with
recombinant vaccinia virus. J Immunol. 157:4079–4086.
1996.PubMed/NCBI
|
|
35
|
Zsiros E, Tanyi J, Balint K and Kandalaft
LE: Immunotherapy for ovarian cancer: Recent advances and
perspectives. Curr Opin Oncol. 26:492–500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leffers N, Daemen T, Helfrich W, Boezen
HM, Cohlen BJ, Melief CJ and Nijman HW: Antigen-specific active
immunotherapy for ovarian cancer. Cochrane Database Syst Rev.
9:CD0072872014.PubMed/NCBI
|
|
37
|
Powell BS, Andrianov AK and Fusco PC:
Polyionic vaccine adjuvants: Another look at aluminum salts and
polyelectrolytes. Clin Exp Vaccine Res. 4:23–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sachamitr P, Hackett S and Fairchild PJ:
Induced pluripotent stem cells: Challenges and opportunities for
cancer immunotherapy. Front Immunol. 5:1762014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Montoro E, Lemus D, Echemendia M, Martin
A, Portaels F and Palomino JC: Comparative evaluation of the
nitrate reduction assay, the MTT test, and the resazurin microtitre
assay for drug susceptibility testing of clinicalisolates of
Mycobacterium tuberculosis. J Antimicrob Chemother. 55:500–505.
2005. View Article : Google Scholar : PubMed/NCBI
|