Introduction
As the leading cause of mortality due to
gynecological malignancies in women worldwide, epithelial ovarian
cancer (EOC) originates from the ovarian surface, inclusion cysts
in the ovarian parenchyma or the nearby distal fallopian tube
epithelium, and is characterized by a response to cytotoxic
chemotherapy at an early stage, followed by recurrence and disease
progression frequently (1,2). According to the reports of the National
Cancer Institute (Rockville, MD, USA), EOC caused an estimated
15,500 mortalities in the USA in 2012 (3). In China, it has also been a major
problem over the past few decades, with high mortality (4). Since >70% of EOC patients are
diagnosed in advanced stages, with distant metastases at the time
of diagnosis, the overall five-year survival rate is 20–30%
(5,6).
Given this scenario, it is of great clinical significance to
develop novel and more efficient therapeutic strategies to combat
EOC.
MicroRNAs (miRNAs or miRs) represent a diverse class
of evolutionarily conserved, small (21–23 nucleotides in length),
non-protein-coding RNAs (7).
Functionally, miRNAs execute post-transcriptional regulation by
binding to the 3′-untranslated region of their target genes
(8). Recent evidence has shown that
each miRNA can regulate multiple target genes, leading to the
potential influence of miRNAs on various cellular activities
(9). Pathologically, half of the
human miRNAs have been identified to be located in
cancer-associated genomic regions, and can act as either tumor
suppressors or oncogenes according to the functions of their target
genes (10). Growing evidence
suggests that the aberrant expression of miRNAs may be involved in
the pathogenesis of numerous cancers (11). Particularly in EOC, there have been a
number of studies that have reported the differentially regulated
miRNAs in cancer cells compared with normal controls, and have
confirmed their critical role in cancer progression (12–14). For
instance, Wang et al (12)
revealed that miR-182 was upregulated in ovarian cancer tissues and
cell lines, and could function as an oncogene to promote cancer
cell growth, invasion and chemoresistance by targeting programmed
cell death 4. Zhang et al (13) reported that miR-124 was downregulated
in ovarian cancer specimens as well as in cell lines, and
suppressed cancer cell migration and invasion by targeting
sphingosine kinase 1. Wu et al (14) demonstrated that miR-145 was
downregulated in human ovarian cancer, and inhibited cancer cell
growth and invasion by targeting p70S6 kinase 1 and mucin 1.
Collectively, these studies suggest the involvement of miRNAs in
the carcinogenesis of EOC. A recent study has shown that miR-298
was downregulated and could regulate the expression of the polycomb
protein enhancer of zeste 2 (EZH2) in recurrent EOC (15). To date, no functional evidence of a
miR-298-EZH2 axis in EOC has been documented.
In the current study, the potential involvement of
the miR-298-EZH2 axis in EOC was investigated. The expression
levels of miR-298 and EZH2 messenger (m) RNA in human EOC tissues
were detected, and the associations of miR-298 and/or EZH2
expression with clinicopathological features of EOC patients were
analyzed. In addition, a potential role of the miR-298-EZH2 axis on
cell motility was also investigated on EOC cell lines. The present
study provides an improved understanding of the molecular
mechanisms responsible for the aggressive nature of human EOC.
Materials and methods
Patients and ethics
In total, 100 EOC tissue specimens and 20 normal
ovarian tissue specimens were collected from surgery during January
2008 to December 2012, snap-frozen in liquid nitrogen and then
stored at −80°C, at the Department of Obstetrics and Gynecology,
Huai'an First People's Hospital, Nanjing Medical University
(Huai'an, China). Written informed consent was obtained from all
patients, and the study was approved by the Ethics Committee of
Huai'an First People's Hospital, Nanjing Medical University.
None of the EOC patients were treated with
radiotherapy, chemotherapy or hormonal therapy prior to surgery.
Surgical staging was established based on the International
Federation of Gynecology and Obstetrics system (16). The clinicopathological features of the
100 EOC patients were summarized in Table
I. All 20 normal ovarian tissues were obtained from women who
underwent hysterectomies for benign disease.
 | Table I.Association of miR-298 downregulation
and/or polycomb protein EZH2 upregulation with clinicopathological
features of epithelial ovarian cancer tissues. |
Table I.
Association of miR-298 downregulation
and/or polycomb protein EZH2 upregulation with clinicopathological
features of epithelial ovarian cancer tissues.
| Features | No. of patients | miR-298-low | P-value |
miR-298-low/EZH2-high | P-value | EZH2-high | P-value |
|---|
| Age |
|
|
|
|
|
|
|
| <55
years | 45 | 23 (51.11) | 0.380 | 20 (44.44) | 0.260 | 18 (40.00) | 0.500 |
| ≥55
years | 55 | 30 (54.55) |
| 31 (56.36) |
| 22 (40.00) |
|
| Histological
type |
|
|
|
|
|
|
|
|
Serous | 28 | 13 (46.43) | 0.290 | 15 (53.57) | 0.180 | 8 (28.57) | 0.230 |
|
Non-serous | 82 | 40 (48.78) |
| 36 (43.90) |
| 32 (39.02) |
|
| Residual tumor upon
surgery |
|
|
|
|
|
|
|
| <1
cm | 35 | 16 (45.71) | 0.210 | 15 (42.86) | 0.160 | 15 (42.86) | 0.320 |
| ≥1
cm | 65 | 37 (56.92) |
| 36 (55.38) |
| 25 (38.46) |
|
| Clinical stage |
|
|
|
|
|
|
|
|
I–II | 80 | 35 (43.75) | 0.010 | 33 (41.25) | 0.010 | 24 (30.00) | 0.008 |
|
III–IV | 20 | 18 (90.00) |
| 17 (85.00) |
| 16 (80.00) |
|
| Pathological
grade |
|
|
|
|
|
|
|
|
1–2 | 70 | 28 (40.00) | 0.020 | 26 (37.14) | 0.020 | 15 (21.43) | 0.010 |
| 3 | 30 | 25 (83.33) |
| 25 (83.33) |
| 25 (83.33) |
|
Cell culture
Two EOC cell lines, SKOV3 and OVCAR3, were obtained
from the Shanghai Institute of Cell Biology, China Academy of
Sciences (Shanghai, China), and were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal calf serum (Gibco; Thermo Fisher
Scientific, Inc.) in a humidified atmosphere of 5% CO2
at 37°C.
RNA and miRNA extraction
Total RNAs were extracted from tissues and cells
with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. In addition, total miRNA
was extracted from tissues and cells using the mirVana miRNA
Isolation kit (Ambion; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
RT-qPCR assay was performed to detect the expression
levels of miR-298 and EZH2 mRNA in EOC tissues and cells. In brief,
10 µg of small nuclear RNA and 20 µg of total RNA were subjected to
RT. Single-stranded complementary (c) DNA was synthesized using the
PrimeScript RT Reagent kit (Promega Corporation, Madison, WI, USA).
Subsequently, the single-stranded cDNA was used for the
amplification of mature miR-298, EZH2 and the endogenous controls
[U6 small nuclear RNA B and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH)] by PCR. The PCR primers used were as
follows: miR-298 forward, 5′-ACACTCAGCTGGGAGCAGAAGCAGGGAG-3′ and
reverse, 5′-GGTGTCGTGGAGTCG-3′; U6 forward,
5′-CGCTTCGGCAGCACATATAC-3′ and reverse, 5′-CAGGGGCCATGCTAATCTT-3′;
EZH2 forward, 5′-GGGAGACTATTCTTGATGGGAAG-3′ and reverse,
5′-ACTGCAACGTAGGTCCCTGA-3′; and GAPDH forward,
5′-GGCGGCACCACCATGTACCCT-3′ and reverse,
5′-AGGGGCCGGACTCGTCATACT-3′. The PCR conditions were: Initial
denaturation at 95°C for 3 min, followed by 40 cycles of 95°C for
15 sec, 62°C for 30 sec and 72°C for 30 sec.
qPCR was performed using SYBR Green PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) on an ABI
7300HT Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Standard curves were generated, and the relative
amount of miR-298 or EZH2 was normalized to the amount of U6 or
GAPDH, respectively. Gene expression was normalized to endogenous
controls, and fold-changes were calculated using the
2−ΔΔCq method (17).
Plasmid construct and
transfection
miR-298 mimics and mimic controls were purchased
from Shanghai GenePharma Co., Ltd. (Shanghai, China). According to
the previous report of Zhang et al (18), the full-length EZH2 cDNA was obtained
by PCR using an expressed sequence tag clone as template, and
constructed into the pCEP4 expression vector to express EZH2. The
cells were transfected using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
transfection protocol.
Western blot analysis
Upon 72 h of transient transfection, EOC cells were
harvested and lysed using radioimmunoprecipitation assay buffer [50
mM Tris-HCl (pH 8.8), 298 mM NaCl, 1% NP-40, 1% sodium deoxycholate
and 0.1% sodium dodecyl sulfate (SDS)]. Then, western blot analysis
was performed to detect the expression levels of EZH2 protein. The
proteins were resolved on a 10% SDS denaturing polyacrylamide gel
and transferred onto a nitrocellulose membrane. The membranes were
blocked with 5% nonfat milk in Tris-buffered saline with Tween-20
for 1 h at room temperature, and next incubated with anti-EZH2
antibody (dilution 1:250; sc-25383; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) or anti-GAPDH antibody (dilution 1:250; sc-25778;
Santa Cruz Biotechnology, Inc.) overnight at room temperature.
Horseradish peroxidase-conjugated anti-rabbit secondary antibody
(dilution 1:1,000; sc-2030; Santa Cruz Biotechnology, Inc.) was
used for detection following incubation for 2 h at room
temperature. GAPDH was used as an internal control for the
normalization of candidate proteins. Protein expression was
assessed by enhanced chemiluminescence with SuperSignal West Pico
Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.),
followed by exposure to a chemiluminescent film (Pierce
Biotechnology, Inc., Rockford, IL, USA).
In vitro migration assay
Wound healing assay was performed to evaluate the
cell migration abilities of EOC cells transfected with miR-298
mimics/mimic controls or EZH2 expression vector/negative control
(NC) vector, according to previous studies (19,20).
Briefly, 48 h after transfection, EOC cells were scraped with a
200-µl pipette tip to create an artificial wound, and incubated
with fresh medium containing mitomycin C (5 µg/ml; Applied
Biosystems; Thermo Fisher Scientific, Inc.) for 12 h. The fraction
of cell coverage across the line was measured as the migration
rate. All experiments were performed in triplicate.
In vitro invasion assay
Transwell assay was performed to evaluate the cell
invasion ability of EOC cells transfected with miR-298 mimics/mimic
controls or EZH2 expression vector/NC vector using Matrigel-coated
cell culture chambers (8-µm pore size; EMD Millipore, Billerica,
MA, USA). Briefly, 48 h after transfection, confluent EOC cells
were resuspended in 200 µl serum-free RPMI 1640 medium and placed
into the upper chamber of the insert with Matrigel. Medium with 5%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) was
added into the lower chamber as a chemoattractant. After 24 h of
incubation, the cells remaining on the upper membrane were
carefully removed. Cells that had invaded through the membrane were
manually counted at ×200 magnification from 10 different fields of
each filter. All experiments were conducted in triplicate.
Statistical analysis
Statistical analyses were performed using SPSS
version 19.0 software (IBM SPSS, Armonk, NY, USA). Data were
expressed as the mean ± standard deviation of ≥3 independent
experiments. Group differences were compared using one-way analysis
of variance or two-tailed Student's t test. The association between
miR-298 and EZH2 mRNA expression was evaluated by Spearman's
correlation. Comparisons between groups were performed using the
χ2 test for categorical variables. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-298 is downregulated and EZH2 mRNA
is upregulated in human EOC tissues
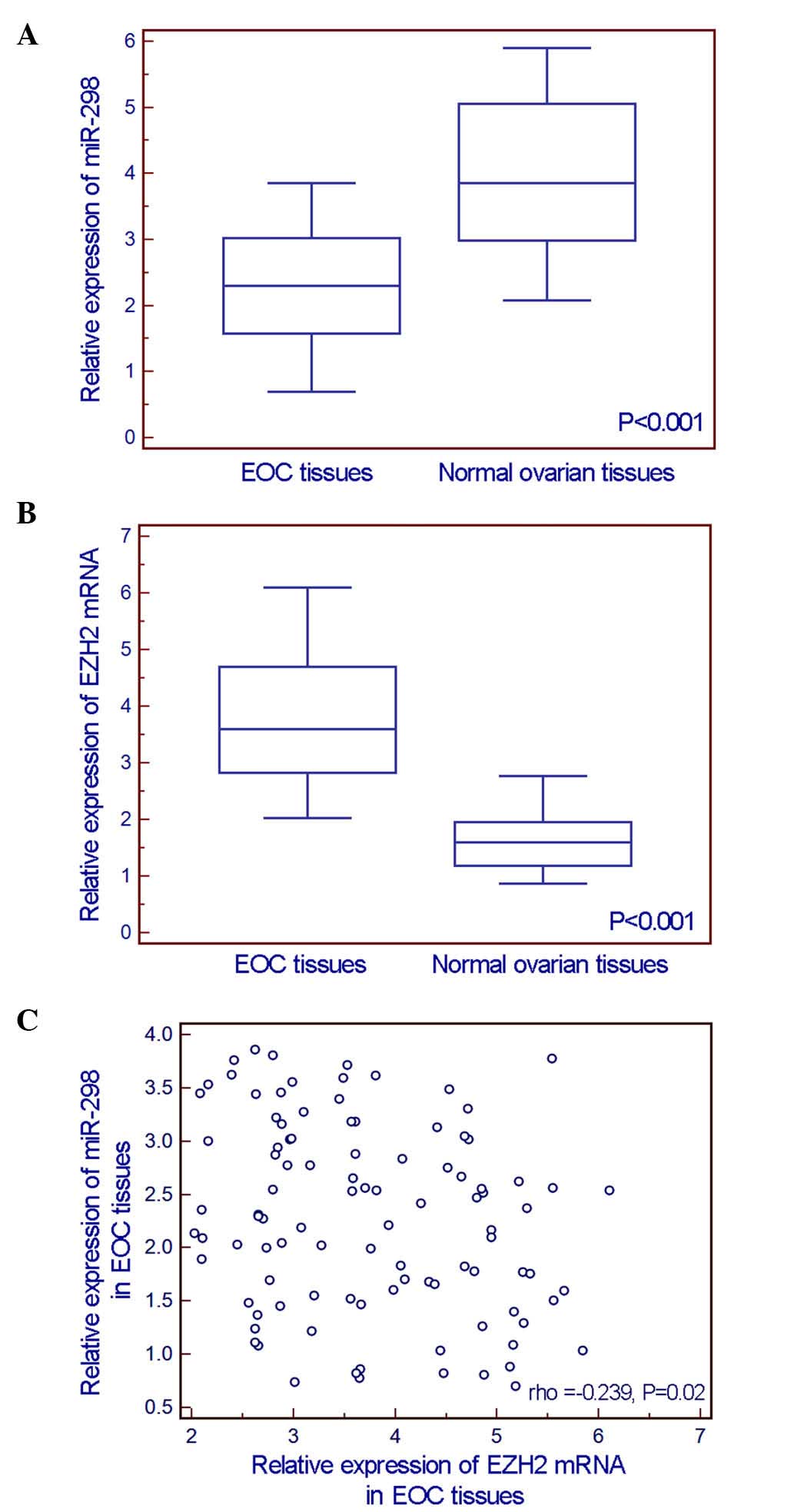
The comparisons of miR-298 and EZH2 expression
detected by RT-qPCR assay between 100 EOC tissue specimens and 20
normal ovarian tissue specimens indicated that the expression level
of miR-298 in EOC tissues was significantly lower than that in
normal ovarian tissues (EOC vs. normal: 2.29±0.88 vs. 4.02±1.19,
P<0.001, Fig. 1A), while EZH2 mRNA
expression was significantly upregulated in EOC tissues (EOC vs.
normal: 3.73±1.07 vs. 1.65±0.58, P<0.001, Fig. 1B). In addition, an inverse correlation
existed between miR-298 and EZH2 mRNA expression levels (Spearman's
correlation coefficient =−0.239, P=0.020, Fig. 1C).
miR-298 downregulation and EZH2
upregulation are associated with aggressive tumor progression of
human EOC
To assess the associations of miR-298 and/or EZH2
mRNA expression with the clinicopathological features of human EOC,
the 100 patients were divided into miR-298-low (n=53), miR-298-high
(n=47), EZH2-low (n=49) and EZH2-high (n=51) groups using the
median values of miR-298 (2.31) or EZH2 (3.61) expression in EOC
tissues, respectively. Of the 100 EOC patients, 13 (13.00%), 40
(40.00%), 36 (36.00%), and 11 (11.00%) patients belonged to the
miR-298-low/EZH2-low, miR-298-low/EZH2-high, miR-298-high/EZH2-low
and miR-298-high/EZH2-high groups, respectively.
As shown in Table I,
miR-298 downregulation and EZH2 upregulation were significantly
associated with high clinical stage (both P=0.01) and pathological
grade (both P=0.02) of EOC patients. The EOC tissues with advanced
clinical stage (III–IV) more frequently exhibited low miR-298
expression (P=0.010, Table I) or high
EZH2 expression (P=0.010, Table I)
than those with low clinical stage (I–II). Additionally, miR-298
expression exhibited a trend that correlated with pathological
grade (P=0.020, Table I). It was also
observed that EZH2 was aberrantly upregulated in tumor tissues with
high pathological grade compared with those with low pathological
grade (P=0.020, Table I). Of note,
the associations of combined miR-298-low/EZH2-high expression with
high clinical stage (P=0.008, Table
I) and high pathological grade (P=0.010, Table I) of EOC patients were both more
remarkable than those of miR-298-low or EZH2-high alone.
miR-298 inhibits cell migration and
invasion of EOC cells in vitro
To evaluate the effect of miR-298 on malignant
phenotypes in EOC cells, miR-298 mimics were transfected into two
EOC cell lines (SKOV3 and OVCAR3) to overexpress this miRNA
(Fig. 2). From the wound healing
assay, it was observed that enforced expression of miR-298
inhibited the migration activity of both SKOV3 and OVCAR3 cells
(Fig. 3). Notably, in the transwell
invasion assay, using Matrigel to simulate the extracellular
matrix, the invasion activities of the two cell lines in
vitro were suppressed following the transfection of miR-298
mimics (Fig. 4).
Overexpression of EZH2 can rescue the
inhibitory effect induced by miR-298
To further validate whether EZH2 could mediate the
tumor-suppressive effects of miR-298 on EOC cells, pCEP4/EZH2 was
constructed to overexpress this gene. The data shown in Fig. 5A and B confirmed that EZH2 expression
was significantly upregulated and could rescue the inhibition of
the EZH2 protein levels caused by miR-298 mimics. Notably, the
wound healing assay and the transwell invasion assay respectively
demonstrated that the overexpression of EZH2 could efficiently
alleviate the miR-298 mimics-induced inhibition of migration and
invasion in both SKOV3 and OVCAR3 cells (Fig. 6).
Discussion
Increasing evidence has revealed that miRNAs
function as important players and therapeutic targets in various
human cancers, including EOC (12–14). The
expression of miRNAs is remarkably deregulated in EOC, implying the
involvement of miRNAs in the initiation and progression of this
disease (12–14). In the current study, the data
indicated that miR-298 expression in human EOC tissues was
significantly downregulated compared with that in normal controls,
and negatively correlated with the expression of EZH2 mRNA. miR-298
downregulation and EZH2 upregulation were identified to be
significantly associated with high clinical stage and pathological
grade of EOC patients. In addition, the ectopic expression of
miR-298 could efficiently inhibit cell migration and invasion.
Notably, the overexpression of EZH2 could restore the cell
migration and invasion abilities suppressed by miR-298. These
results linked EZH2 to the miRNA miR-298, and demonstrated their
roles in regulating the malignant phenotypes of EOC cells and the
aggressive cancer progression of EOC patients.
Aberrant expression of miR-298 has been observed in
ovarian cancer tissues (15), serum
from men with metastatic castration-resistant prostate cancer
(21) and breast cancer cell lines
(22). However, the function and
mechanism of this miRNA regulation in human cancers remain unclear.
Gui et al (15) previously
reported that EZH2 expression could be concomitantly and
significantly reduced by miR-298 overexpression in EOC cells. As a
catalytic subunit of polycomb repressive complex 2, EZH2 exhibits
intrinsic histone lysine methyltransferase activity on histone H3
Lys-9 and −27 or histone H1 Lys-26 (23). EZH2 acts as a recruitment platform for
DNA methyltransferases, and is essential for DNA methylation of
EZH2-target promoters (24). Notably,
EZH2 also can silence gene expression by binding to the promoter of
its target genes directly (25).
Previous studies have reported the overexpression of EZH2 in
several types of human cancers and its association with
aggressiveness and poor prognosis in breast (26) and prostate cancer (27). EZH2 is often upregulated in EOC cells,
and can promote cell proliferation, inhibit apoptosis and enhance
angiogenesis in EOC (28,29). Thus, the present authors speculated
that the dysregulation of miR-298 may contribute to EZH2-mediated
malignant phenotypes in EOC cells. The present study confirmed the
inverse correlation existing between miR-298 and EZH2 mRNA
expression levels in EOC tissues based on a large cohort of
clinical samples. Notably, statistical analysis revealed remarkable
associations between miR-298 downregulation and/or EZH2
upregulation and aggressive clinical phenotypes of EOC patients. In
essence, this provided the possibility that the dysregulation of
the miR-298-EZH2 axis may lead to occurrence and progression in
EOC.
Migration and invasion, resulting in high cell
mortality, represent two major attributes of malignant tumors
(30). In the current study, it was
validated that miR-298 downregulation may result in the inhibition
of the migration and invasion of EOC cells. To the best of our
knowledge, there are no published data on the role of miR-298
regarding migration and invasion in cancer cells. Additionally, via
functional studies on EZH2, it was noticed that the overexpression
of EZH2 could restore the inhibitory effects of miR-298 on protein
levels, cell migration and cell invasion abilities, further
confirming that miR-298 exhibited an EZH2-dependent stimulatory
activity in the regulation of migration and invasion in EOC
cells.
In conclusion, our data offer convincing evidence
that the dysregulation of the miR-298-EZH2 axis may be important in
tumor progression of EOC patients. The present findings also
confirmed a tumor-suppressive role of miR-298 in modulating EOC
cell motility by regulating the expression of EZH2, implying its
potential as a novel miRNA-based therapeutic target for the
treatment of human EOC.
References
|
1
|
Vargas-Hernández VM, Moreno-Eutimio MA,
Acosta-Altamirano G and Vargas-Aguilar VM: Management of recurrent
epithelial ovarian cancer. Gland Surg. 3:198–202. 2014.PubMed/NCBI
|
|
2
|
Xu L, Cai J, Yang Q, Ding H, Wu L, Li T
and Wang Z: Prognostic significance of several biomarkers in
epithelial ovarian cancer: A meta-analysis of published studies. J
Cancer Res Clin Oncol. 139:1257–1277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yeh YM, Chuang CM, Chao KC and Wang LH:
MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis
by targeting SOX4 and HIF-1α. Int J Cancer. 133:867–878. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang L, Lv W and Zhao X: CD24 as a
molecular marker in ovarian cancer: A literature review. Cancer
Transl Med. 2:29–32. 2016. View Article : Google Scholar
|
|
5
|
Coleman RL, Monk BJ, Sood AK and Herzog
TJ: Latest research and treatment of advanced-stage epithelial
ovarian cancer. Nat Rev Clin Oncol. 10:211–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minig L, Otaño L, Diaz-Padilla I, Gallego
R Alvarez, Patrono MG and de Bernabé J Valero: Therapeutic
management of epithelial ovarian cancer during pregnancy. Clin
Transl Oncol. 15:259–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hata A and Lieberman J: Dysregulation of
microRNA biogenesis and gene silencing in cancer. Sci Signal.
8:re32015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen J and Hung MC: Signaling-mediated
regulation of MicroRNA processing. Cancer Res. 75:783–791. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zaman MS, Maher DM, Khan S, Jaggi M and
Chauhan SC: Current status and implications of microRNAs in ovarian
cancer diagnosis and therapy. J Ovarian Res. 5:442012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mansoori B, Mohammadi A, Shirjang S and
Baradaran B: Micro-RNAs: The new potential biomarkers in cancer
diagnosis, prognosis and cancer therapy. Cell Mol Biol
(Noisy-le-grand). 61:1–10. 2015.PubMed/NCBI
|
|
12
|
Wang YQ, Guo RD, Guo RM, Sheng W and Yin
LR: MicroRNA-182 promotes cell growth, invasion, and
chemoresistance by targeting programmed cell death 4 (PDCD4) in
human ovarian carcinomas. J Cell Biochem. 114:1464–1473. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Wang Q, Zhao Q and Di W: MiR-124
inhibits the migration and invasion of ovarian cancer cells by
targeting SphK1. J Ovarian Res. 6:842013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu H, Xiao Z, Wang K, Liu W and Hao Q:
MiR-145 is downregulated in human ovarian cancer and modulates cell
growth and invasion by targeting p70S6K1 and MUC1. Biochem Biophys
Res Commun. 441:693–700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gui T, Bai H, Zeng J, Zhong Z, Cao D, Cui
Q, Chen J, Yang J and Shen K: Tumor heterogeneity in the recurrence
of epithelial ovarian cancer demonstrated by polycomb group
proteins. Onco Targets Ther. 7:1705–1716. 2014.PubMed/NCBI
|
|
16
|
Pereira A, Pérez-Medina T, Magrina JF,
Magtibay PM, Rodríguez-Tapia A, Peregrin I, Mendizabal E and
Ortiz-Quintana L: International Federation of Gynecology and
Obstetrics staging classification for cancer of the ovary,
fallopian tube, and peritoneum: Estimation of survival in patients
with node-positive epithelial ovarian cancer. Int J Gynecol Cancer.
25:49–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Q, Padi SK, Tindall DJ and Guo B:
Polycomb protein EZH2 suppresses apoptosis by silencing the
proapoptotic miR-31. Cell Death Dis. 5:e14862014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Semaan A, Qazi AM, Seward S, Chamala S,
Bryant CS, Kumar S, Morris R, Steffes CP, Bouwman DL, Munkarah AR,
et al: MicroRNA-101 inhibits growth of epithelial ovarian cancer by
relieving chromatin-mediated transcriptional repression of
p21(waf1/cip1). Pharm Res. 28:3079–3090. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim TH, Song JY, Park H, Jeong JY, Kwon
AY, Heo JH, Kang H, Kim G and An HJ: miR-145, targeting
high-mobility group A2, is a powerful predictor of patient outcome
in ovarian carcinoma. Cancer Lett. 356:937–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Selth LA, Townley S, Gillis JL, Ochnik AM,
Murti K, Macfarlane RJ, Chi KN, Marshall VR, Tilley WD and Butler
LM: Discovery of circulating microRNAs associated with human
prostate cancer using a mouse model of disease. Int J Cancer.
131:652–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bao L, Hazari S, Mehra S, Kaushal D, Moroz
K and Dash S: Increased expression of P-glycoprotein and
doxorubicin chemoresistance of metastatic breast cancer is
regulated by miR-298. Am J Pathol. 180:2490–2503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han Li C and Chen Y: Targeting EZH2 for
cancer therapy: Progress and perspective. Curr Protein Pept Sci.
16:559–570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Verma SK: Inhibition of the histone lysine
methyltransferase EZH2 for the treatment of cancer. Curr Top Med
Chem. 15:714–719. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li LY: EZH2: Novel therapeutic target for
human cancer. Biomedicine (Taipei). 4:12014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gong Y, Huo L, Liu P, Sneige N, Sun X,
Ueno NT, Lucci A, Buchholz TA, Valero V and Cristofanilli M:
Polycomb group protein EZH2 is frequently expressed in inflammatory
breast cancer and is predictive of worse clinical outcome. Cancer.
117:5476–5484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang YA and Yu J: EZH2, an epigenetic
driver of prostate cancer. Protein Cell. 4:331–341. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li T, Cai J, Ding H, Xu L, Yang Q and Wang
Z: EZH2 participates in malignant biological behavior of epithelial
ovarian cancer through regulating the expression of BRCA1. Cancer
Biol Ther. 15:271–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu L, Guo J, Yu L, Cai J, Gui T, Tang H,
Song L, Wang J, Han F, Yang C, et al: miR-101 regulates expression
of EZH2 and contributes to progression of and cisplatin resistance
in epithelial ovarian cancer. Tumour Biol. 35:12619–12626. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martin TA, Ye L, Sanders AJ, Lane J and
Jiang WG: Cancer invasion and metastasis: Molecular and cellular
perspectiveMetastatic Cancer: Clinical and Biological Perspectives.
Jandial R: Madame Curie Bioscience Database. Landes Bioscience;
Austin, TX: pp. 2000–2013
|