Introduction
As a member of the inhibitor of apoptosis protein
family, survivin resides in both the nucleus and cytoplasm of
cells. Survivin controls both cell division (when localized to the
nucleus) and cell survival (when localized to the cytoplasm) and
thus may exhibit a dual function (1–3). In normal
adult tissues survivin is usually undetectable, however it is
overexpressed in the majority of human tumors, including lung,
colon, breast, stomach and liver cancer (4–9). Due to
its involvement in tumorigenesis, survivin has been proposed as a
prognostic marker and a molecular target for anticancer therapies
(10–13).
Survivin shuttles between the nucleus and cytoplasm,
and several studies have demonstrated that chromosomal regional
maintenance protein-1 (CRM-1) regulates its subcellular
distribution (2,3,12,13). CRM-1, a member of the importin B
superfamily, exports both proteins and mRNAs from the nucleus and
is the main nuclear exporter in humans. It recognizes a
leucine-rich nuclear export signal in proteins and exports
proteins, including survivin, via the CRM-1/Ras-related nuclear
protein-guanosine-5′-triphosphate axis (14–17). High
CRM-1 expression has been identified in several malignancies,
including stomach, pancreatic, ovarian, cervical, renal and
esophageal cancers. Its presence in tumor cells indicates that it
predicts an unfavorable prognosis (18–23).
At present, cancer is the primary cause of mortality
in Japan (24). In 2011, a total of
132,000 and 125,000 cases of gastric and colorectal cancer were
diagnosed, respectively (25).
Furthermore, gastric and colorectal cancer accounted for 47,903 and
48,500 mortalities in 2014, respectively (25). Gastric cancer remains a significant
cause of cancer-related mortality (24). Our previous study demonstrated that
nuclear and cytoplasmic survivin expression was higher in
colorectal carcinomas than gastric carcinomas (26). The levels of nuclear and cytoplasmic
survivin are directly correlated in colorectal carcinoma, but not
gastric carcinomas. However, the effect of survivin localization
remains unclear and thus, further study of survivin/CRM-1
interaction with regard to tumorigenesis is required (27–29).
The aims of the present study were to compare the
expression levels and intracellular localization of CRM-1 in human
gastric and colorectal carcinomas and to investigate the
association between CRM-1 and survivin expression. In this study,
CRM-1 expression was assessed using immunohistochemistry and to the
best of our knowledge, this method has not been used to detect this
protein previously.
Materials and methods
Human tissue samples
A total of 69 advanced gastric adenocarcinoma
specimens (33 intestinal type and 36 diffuse type) and 78
colorectal adenocarcinoma specimens (68 well- or
moderately-differentiated and 10 poorly-differentiated) collected
between April 2004 and October 2009 were obtained from the archives
of the Department of Diagnostic Pathology, Osaka Red Cross Hospital
(Osaka, Japan) and the Kobe Central Hospital of Social Insurance
(Kobe, Japan). The histological typing was classified according to
the Japanese Classification of gastric (30) or colorectal carcinoma (31). Histologically, papillary
adenocarcinomas and tubular adenocarcinomas (well- or
moderately-differentiated) were classified as intestinal type
adenocarcinoma, whereas poorly-differentiated adenocarcinomas and
signet ring cell carcinomas were classified as diffuse type
adenocarcinoma. The median ages of the gastric and colorectal
carcinoma patients were 72 (range, 41–94 years) and 67.5 years
(range, 27–86 years), respectively. The gastric carcinoma group
consisted of 48 male (70%) and 21 female (30%) patients, whereas
the colorectal carcinoma group consisted of 44 male (56%) and 34
female (44%) patients. The present study was approved by the Ethics
Committee of Kobe University Graduate School of Health Sciences
(Kobe, Japan).
Immunohistochemistry
Formalin-fixed, paraffin-embedded
surgically-resected tumor tissues were cut into 3-µm sections, and
mounted on aminopropyltriethoxysilane-coated glass slides
(Matsunami Glass, Osaka, Japan).
Slides were deparaffinized using xylene and
dehydrated using a graded ethanol series. For antigen retrieval,
the slides were immersed in 10-mM citrate (pH 6.0) and heated to
>100°C for 10 min in a pressure cooker (Groupe SEB, Écully,
France). After heating, the sections were cooled at room
temperature in soaking solution for 30 min and washed with running
tap water followed by 10 mM phosphate-buffered saline (PBS; pH
7.2). After blocking in 0.25% casein in PBS (Dako, Glostrup,
Denmark), the sections were incubated overnight in PBS (negative
control) or PBS containing a rabbit anti-CRM-1 monoclonal antibody
(dilution, 1:1,200; catalog no. ab77977; Abcam, Cambridge, UK) at
room temperature. To detect CRM-1, the sections were rinsed with
PBS and incubated with Histofine Simple Stain MAX-PO (Nichirei
Biosciences Inc., Tokyo, Japan) for 1 h at room temperature.
Reaction products were then developed with 3,3′-diaminobenzidine
and counterstained with Mayer's hematoxylin (Nacalai Tesque, Kyoto,
Japan).
Immunostaining evaluation
At least five randomly selected fields were examined
(magnification, ×400) to determine the mean percentage of tumor
cells expressing nuclear and/or cytoplasmic CRM-1. Samples in which
≥30% of the tumor cells exhibited nuclear staining or in which ≥15%
of the tumor cells exhibited cytoplasmic staining were considered
positive for CRM-1 protein expression. These cutoff values were
based on the median observed values. All slides were evaluated by
two independent investigators blinded to the experimental
design.
Statistical analysis
χ2 test and Fisher's exact test were used
to assess the CRM-1 expression levels of two groups. Spearman's
rank correlation was used to assess correlations between two
variables. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed with
Statcel 3 (OMS, Tokyo, Japan).
Results
Expression of CRM-1 in gastric
carcinoma
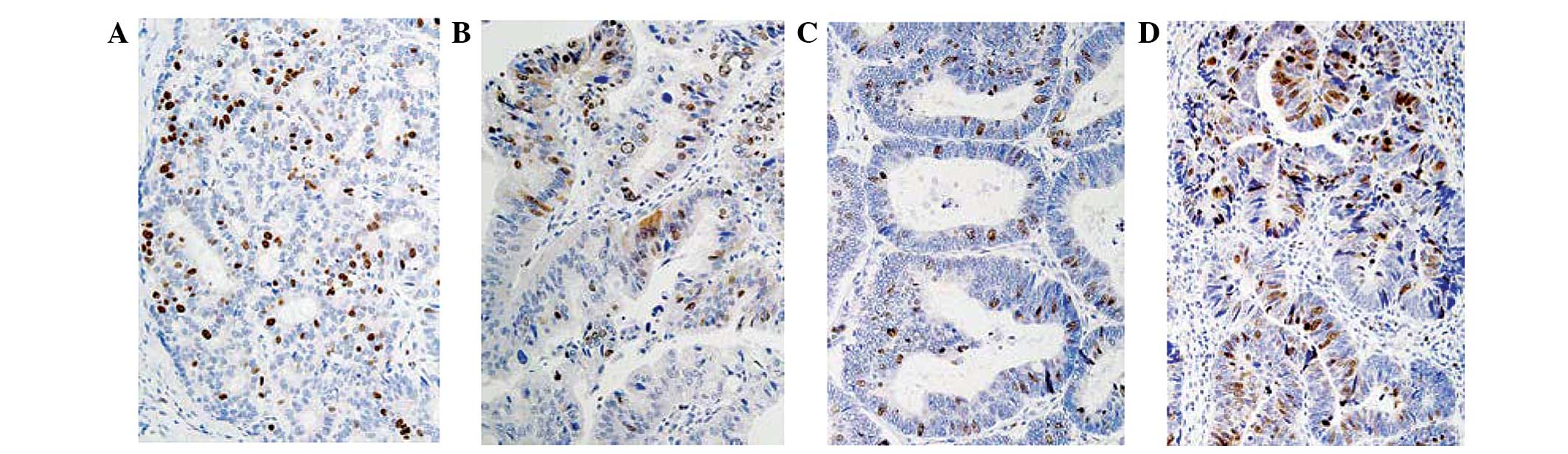
CRM-1 was predominantly localized to the nucleus in
gastric carcinoma tissue samples (Fig.
1). Nuclear expression of CRM-1 was observed in 61% (42/69) of
samples and cytoplasmic CRM-1 expression was observed in 29%
(20/69) of samples (Table I). Nuclear
CRM-1 was identified in 73% (24/33) of intestinal type tumors and
50% (18/36) of diffuse type tumors. Cytoplasmic CRM-1 was detected
in 36% (12/33) of intestinal type tumors and 22% (8/36) of diffuse
type tumors. No significant differences were identified between
CRM-1 expression in intestinal type and diffuse type tumors.
 | Table I.Nuclear and cytoplasmic expression of
CRM-1 in gastrointestinal and colorectal adenocarcinomas. |
Table I.
Nuclear and cytoplasmic expression of
CRM-1 in gastrointestinal and colorectal adenocarcinomas.
|
| CRM-1 expression |
|---|
|
|
|
|---|
| Cancer type | Nucleus, n (%) | Cytoplasm, n (%) |
|---|
| Gastric
adenocarcinoma (n=69) | 42 (61) | 20 (29) |
|
Intestinal type (n=33) | 24 (73) | 12 (36) |
| Diffuse
type (n=36) | 18 (50) | 8
(22) |
| Colorectal
adenocarcinoma (n=78) | 43 (55) | 29 (37) |
| Well- to
moderately-differentiated (n=68) | 40 (59) | 29 (43) |
|
Poorly-differentiated
(n=10) | 3
(30) | 0
(0) |
Expression of CRM-1 in colorectal
carcinoma
Nuclear CRM-1 expression was observed in 55% (43/78)
and cytoplasmic CRM-1 expression in 37% (29/78) of colorectal
carcinoma samples (Table I). Nuclear
CRM-1 was identified in 59% (40/68) of well to
moderately-differentiated tumors and 30% (3/10) of
poorly-differentiated tumors. Cytoplasmic CRM-1 was observed in 43%
(29/68) of well to moderately-differentiated tumors and none of the
poorly-differentiated tumors.
Association between CRM-1 and survivin
expression in gastric and colorectal carcinomas
The correlation between nuclear or cytoplasmic CRM-1
expression and nuclear or cytoplasmic survivin expression in
gastric carcinoma (intestinal type and diffuse type) and colorectal
carcinoma was evaluated (Table II).
In intestinal type gastric carcinoma, no correlations were
identified between any parameters. In diffuse type gastric
carcinoma, nuclear CRM-1 expression was significantly correlated
with nuclear (r=0.649; P<0.001) and cytoplasmic (r=0.371;
P=0.028) expression of survivin. In colorectal carcinoma, nuclear
and cytoplasmic CRM-1 significantly correlated with nuclear and
cytoplasmic survivin (nuclear CRM-1 and nuclear survivin, r=0.338;
P=0.003; nuclear CRM-1 and cytoplasmic survivin, r=0.299; P=0.009;
cytoplasmic CRM-1 and nuclear survivin, r=0.254; P=0.026;
cytoplasmic CRM-1 and cytoplasmic survivin, r=0.268; P=0.019).
 | Table II.Correlation between CRM-1 and survivin
expression in the nucleus and cytoplasm in gastric and colorectal
carcinoma. |
Table II.
Correlation between CRM-1 and survivin
expression in the nucleus and cytoplasm in gastric and colorectal
carcinoma.
|
| Nuclear survivin | Cytoplasmic
survivin |
|---|
|
|
|
|
|---|
| Cancer type | r | P-value | r | P-value |
|---|
| Gastric carcinoma,
total |
|
|
|
|
|
N-CRM-1 | 0.484 |
<0.001a | 0.322 | 0.008a |
|
C-CRM-1 | 0.192 | 0.113 | 0.127 | 0.296 |
| Gastric carcinoma,
intestinal type |
|
|
|
|
|
N-CRM-1 | 0.204 | 0.249 | 0.292 | 0.098 |
|
C-CRM-1 | 0.096 | 0.588 | 0.267 | 0.131 |
| Gastric carcinoma,
diffuse type |
|
|
|
|
|
N-CRM-1 | 0.649 | 0.001a | 0.371 | 0.028a |
|
C-CRM-1 | 0.245 | 0.147 | 0.078 | 0.645 |
| Colorectal
carcinoma, total |
|
|
|
|
|
N-CRM-1 | 0.338 | 0.003a | 0.299 | 0.009a |
|
C-CRM-1 | 0.254 | 0.026a | 0.268 | 0.019a |
Discussion
Previous studies have implicated CRM-1 in
carcinogenesis. CRM-1 expression has been found to correlate with a
more aggressive tumor status and it has been suggested that CRM-1
may have prognostic value in gliomas, osteosarcomas, ovarian
cancers and pancreatic cancers (18,20,32,33).
Previous studies have also demonstrated an association between
CRM-1 expression and resistance to chemotherapy and thus, potent
inhibitors of CRM-1 are currently being developed for use in cancer
therapy. At present, members of the CRM-1 inhibitor family are
novel therapeutic targets in renal cell carcinoma, non-small cell
lung carcinoma and lymphoma (19,34–36).
The present study compared CRM-1 expression in
gastric and colorectal carcinomas. To the best of our knowledge,
this is the first study to report a correlation between CRM-1 and
survivin expression using immunohistochemistry.
CRM-1 is predominantly found in the nucleus. In the
present study, no significant differences in the nuclear or
cytoplasmic expression rate of CRM-1 were identified between
gastric and colorectal carcinomas. Furthermore, no significant
differences in CRM-1 expression rate were identified between
gastric carcinomas classified according to tumor type
(differentiation status), although expression rates in both the
nucleus and cytoplasm were higher in intestinal type gastric
carcinomas than diffuse type gastric carcinomas. Zhou et al
(23) reported that CRM-1 expression
did not correlate with tumor differentiation status (poor vs. well
to moderate). The authors also reported a higher CRM-1 expression
rate in well to moderately-differentiated gastric adenocarcinomas
compared with poorly-differentiated gastric adenocarcinomas
(23). These results are consistent
with those of the present study and suggest that no association
exists between CRM-1 expression and tumor differentiation status in
gastric carcinoma. A previous immunohistochemical analysis revealed
that high CRM-1 expression was correlated with histological
differentiation in esophageal squamous cell carcinoma but not
pancreatic adenocarcinoma (18,22). The
association between CRM-1 expression level and histological tumor
differentiation remains unclear. In the present study, the CRM-1
expression rates in well or moderately versus poorly-differentiated
colorectal carcinomas could not be investigated accurately due to
the large difference in sample sizes in each group.
In the present study, the association between CRM-1
and survivin expression in both the nucleus and cytoplasm was also
investigated. In colorectal carcinoma, these parameters correlated
in all expression patterns (nuclear and nuclear, cytoplasmic and
cytoplasmic, and nuclear and cytoplasmic). In gastric carcinoma, no
association between cytoplasmic CRM-1 expression and nuclear or
cytoplasmic survivin expression was identified, whereas nuclear
CRM-1 expression was found to correlate with nuclear or cytoplasmic
survivin expression in diffuse but not intestinal type gastric
carcinoma. These results indicate that CRM-1 expression patterns
differ in gastric and colorectal carcinomas and suggest that
CRM-1-mediated nuclear export of survivin may be deregulated in
gastric carcinoma.
Recent studies have demonstrated that the
survivin/CRM-1 axis exhibits an important function in regulating
cell division and cell survival (1–3). Survivin
shuttles proteins between the nucleus and cytoplasm (37,38). When
highly expressed in the cytoplasm, survivin may protect cancer
cells by inhibiting apoptosis. In the nucleus, survivin
predominantly associates with borealin, aurora B kinase and inner
centrosome protein to form the chromosome passenger complex, which
controls multiple processes during mitosis and is essential for
genomic stability. Several studies have revealed that survivin is
upregulated in the cytoplasm of cancer cells, where it prevents
apoptosis and promotes tumor progression (3–9,13). Elucidation of the mechanism by which
CRM-1 modulates survivin expression in several tumor types would be
of great importance, and clarification of CRM-1 expression patterns
may aid in the selection of effective anticancer agents.
In conclusion, the present study revealed that CRM-1
is involved in survivin expression in gastric and colorectal
carcinomas. We hypothesize that CRM-1 exhibits different functions
in gastric and colorectal carcinoma. Further studies are required
to determine whether CRM-1 expression is a prognostic predictor in
these tumor types.
Acknowledgements
The authors would like to thank Dr Masayuki Shintaku
(Osaka Red Cross Hospital) and Dr Toshihiko Miyake (Kobe Central
Hospital of Social Insurance) for their support.
References
|
1
|
Li F, Ackermann EJ, Bennett CF, Rothermel
AL, Plescia J, Tognin S, Villa A, Marchisio PC and Altieri DC:
Pleiotropic cell-division defects and apoptosis induced by
interference with survivin function. Nat Cell Biol. 1:461–466.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li F and Ling X: Survivin study: An update
of “what is the next wave”? J Cell Physiol. 208:476–486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Knauer SK, Mann W and Stauber RH:
Survivin's dual role: An export's view. Cell Cycle. 6:518–521.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andersen MH, Svane IM, Becker JC and
Straten PT: The universal character of the tumor-associated antigen
survivin. Clin Cancer Res. 13:5991–5994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen P, Li J, Ge LP, Dai CH and Li XQ:
Prognostic value of survivin, X-linked inhibitor of apoptosis
protein and second mitochondria-derived activator of caspases
expression in advanced non-small-cell lung cancer patients.
Respirology. 15:501–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiaoyuan C, Longbang C, Jinghua W,
Xiaoxiang G, Huaicheng G, Qun Z and Haizhu S: Survivin: A potential
prognostic marker and chemoradiotherapeutic target for colorectal
cancer. Ir J Med Sci. 179:327–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Youssef NS, Hewedi IH and Abd Raboh NM:
Immunohistochemical expression of survivin in breast carcinoma:
Relationship with clinicopathological parameters, proliferation and
molecular classification. J Egypt Natl Canc Inst. 20:348–357.
2008.PubMed/NCBI
|
|
8
|
Wang TT, Qian XP and Liu BR: Survivin:
Potential role in diagnosis, prognosis and targeted therapy of
gastric cancer. World J Gastroenterol. 13:2784–2790.
2007.PubMed/NCBI
|
|
9
|
Ito T, Shiraki K, Sugimoto K, Yamanaka T,
Fujikawa K, Ito M, Takase K, Moriyama M, Kawano H, Hayashida M, et
al: Survivin promotes cell proliferation in human hepatocellular
carcinoma. Hepatology. 31:1080–1085. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miura K, Fujibuchi W, Ishida K, Naitoh T,
Ogawa H, Ando T, Yazaki N, Watanabe K, Haneda S, Shibata C and
Sasaki I: Inhibitor of apoptosis protein family as diagnostic
markers and therapeutic targets of colorectal cancer. Surg Today.
41:175–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sah NK, Khan Z, Khan GJ and Bisen PS:
Structural, functional and therapeutic biology of survivin. Cancer
Lett. 244:164–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stauber RH, Mann W and Knauer SK: Nuclear
and cytoplasmic survivin: Molecular mechanism, prognostic, and
therapeutic potential. Cancer Res. 67:5999–6002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fornerod M, Ohno M, Yoshida M and Mattaj
IW: CRM1 is an export receptor for leucine-rich nuclear export
signals. Cell. 90:1051–1060. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukuda M, Asano S, Nakamura T, Adachi M,
Yoshida M, Yanagida M and Nishida E: CRM1 is responsible for
intracellular transport mediated by the nuclear export signal.
Nature. 390:308–311. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ossareh-Nazari B, Bachelerie F and
Dargemont C: Evidence for a role of CRM1 in signal-mediated nuclear
protein export. Science. 278:141–144. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stade K, Ford CS, Guthrie C and Weis K:
Exportin 1 (Crm1p) is an essential nuclear export factor. Cell.
90:1041–1050. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH
and Sun YJ: Prognostic value of CRM1 in pancreas cancer. Clin
Invest Med. 32:E315–E321. 2009.PubMed/NCBI
|
|
19
|
Inoue H, Kauffman M, Shacham S, Landesman
Y, Yang J, Evans CP and Weiss RH: CRM1 blockade by selective
inhibitors of nuclear export attenuates kidney cancer growth. J
Urol. 189:2317–2326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noske A, Weichert W, Niesporek S, Röske A,
Buckendahl AC, Koch I, Sehouli J, Dietel M and Denkert C:
Expression of the nuclear export protein chromosomal region
maintenance/exportin 1/Xpo1 is a prognostic factor in human ovarian
cancer. Cancer. 112:1733–1743. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van der Watt PJ, Maske CP, Hendricks DT,
Parker MI, Denny L, Govender D, Birrer MJ and Leaner VD: The
Karyopherin proteins, Crm1 and Karyopherin beta1, are overexpressed
in cervical cancer and are critical for cancer cell survival and
proliferation. Int J Cancer. 124:1829–1840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang X, Cheng L, Yao L, Ren H, Zhang S,
Min X, Chen X, Zhang J and Li M: Involvement of chromosome region
maintenance 1 (CRM1) in the formation and progression of esophageal
squamous cell carcinoma. Med Oncol. 31:1552014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou F, Qiu W, Yao R, Xiang J, Sun X, Liu
S, Lv J and Yue L: CRM1 is a novel independent prognostic factor
for the poor prognosis of gastric carcinomas. Med Oncol.
30:7262013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hama H, Tabuchi T, Ito Y, Fukushima W,
Matsunaga I, Miyashiro I and Nakayama T: Smoking behavior and
participation in screening for lung, gastric, and colorectal
cancers. Nihon Koshu Eisei Zasshi. 63:126–134. 2016.(In Japanese).
PubMed/NCBI
|
|
25
|
Nishimoto H, Katanoda K, Sato N, Sobue T
and Mikami H: Cancer statistics in Japan 2014Wakao F: Center for
Cancer Control and Information Services. National Cancer Center;
2015, (In Japanese).
|
|
26
|
Shintani M, Sangawa A, Yamao N and
Kamoshida S: Immunohistochemical expression of nuclear and
cytoplasmic survivin in gastrointestinal carcinoma. Int J Clin Exp
Pathol. 6:2919–2927. 2013.PubMed/NCBI
|
|
27
|
Hutten S and Kehlenbach RH: CRM1-mediated
nuclear export: To the pore and beyond. Trends Cell Biol.
17:193–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Knauer SK, Krämer OH, Knösel T, Engels K,
Rödel F, Kovács AF, Dietmaier W, Klein-Hitpass L, Habtemichael N,
Schweitzer A, et al: Nuclear export is essential for the
tumor-promoting activity of survivin. FASEB J. 21:207–216. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stauber RH, Rabenhorst U, Rekik A, Engels
K, Bier C and Knauer SK: Nucleocytoplasmic shuttling and the
biological activity of mouse survivin are regulated by an active
nuclear export signal. Traffic. 7:1461–1472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Japanese Gastric Cancer Association, .
Japanese classification of gastric carcinoma. 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Japanese Society for Cancer of the Colon
and Rectum, . Japanese Classification of Colorectal Carcinoma. 2nd
English edition. Kanehara & Co., Ltd.; Tokyo: 2009
|
|
32
|
Shen A, Wang Y, Zhao Y, Zou L, Sun L and
Cheng C: Expression of CRM1 in human gliomas and its significance
in p27 expression and clinical prognosis. Neurosurgery. 65:153–159.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yao Y, Dong Y, Lin F, Zhao H, Shen Z, Chen
P, Sun YJ, Tang LN and Zheng SE: The expression of CRM1 is
associated with prognosis in human osteosarcoma. Oncol Rep.
21:229–235. 2009.PubMed/NCBI
|
|
34
|
Wang S, Han X, Wang J, Yao J and Shi Y:
Antitumor effects of a novel chromosome region maintenance 1 (CRM1)
inhibitor on non-small cell lung cancer cells in vitro and in mouse
tumor xenografts. PLoS One. 9:e898482014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yashiroda Y and Yoshida M:
Nucleo-cytoplasmic transport of proteins as a target for
therapeutic drugs. Curr Med Chem. 10:741–748. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang K, Wang M, Tamayo AT, Shacham S,
Kauffman M, Lee J, Zhang L, Ou Z, Li C, Sun L, et al: Novel
selective inhibitors of nuclear export CRM1 antagonists for therapy
in mantle cell lymphoma. Exp Hematol. 41:67–78.e4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Knauer SK, Bier C, Habtemichael N and
Stauber RH: The Survivin-Crm1 interaction is essential for
chromosomal passenger complex localization and function. EMBO Rep.
7:1259–1265. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nguyen KT, Holloway MP and Altura RA: The
CRM1 nuclear export protein in normal development and disease. Int
J Biochem Mol Biol. 3:137–151. 2012.PubMed/NCBI
|