Introduction
Epithelial ovarian cancer is highly invasive and has
the highest mortality rate among the various types of gynecological
malignancy (1). At the time of
diagnosis, >70% of patients have advanced stage disease
(1). Currently, the standard
treatment is cytoreductive surgery followed by platinum- and
paclitaxel-based chemotherapy. However, the efficacy of
chemotherapy is challenged by chemoresistance and tumor recurrence
(2). Therefore, molecular targeted
therapy for ovarian cancer has become a novel field of research in
recent years (3). Existing molecular
targeting agents are predominantly monoclonal antibodies that
target proteins that are abnormally expressed in tumor cells, or
small molecule protein kinase inhibitors that regulate cell growth
or inhibit angiogenesis; these agents have a higher specificity and
lower toxicity compared with traditional chemotherapeutic agents
(4).
Poly (ADP-ribose) polymerase 1 (PARP-1) is expressed
in the nuclei of most eukaryotic cells and participates in DNA
damage repair, gene transcription, the cell cycle, chromosome
function, genomic stability and cell death (5). PARP-1 contains three structural domains:
A DNA binding domain, an auto-modification domain and a catalytic
domain (6). Once activated by DNA
damage, PARP-1 rapidly forms homodimers that recognize and bind DNA
nicks, whereupon it catalyzes ADP ribosylation of itself and
histones (7), leading to chromosomal
relaxation and thereby recruitment of DNA polymerase β, X-ray
repair cross-complementing protein 1 and DNA ligase III to sites of
DNA damage to initiate DNA repair (8). When DNA damage is severe, PARP-1 becomes
over-activated, which can lead to the depletion of NAD+
and ATP and subsequently induce cell death (9).
Our previous research demonstrated that PARP-1
inhibitors can enhance the chemosensitivity of ovarian cancer cells
in vitro (10). Notably, the
PARP-1 inhibitor PJ34 can also inhibit angiogenesis in the
chorioallantoic membrane assay (11).
In order to investigate whether PARP-1 may be involved in
angiogenesis in ovarian cancer, the present study examined the
expression of PARP-1 and its association with markers of
angiogenesis in human epithelial ovarian cancer. Furthermore, the
effect of PARP-1 on the angiogenic capacity of ovarian cancer cells
in vitro was investigated.
Materials and methods
Patients and samples
Tissue samples from 60 patients with epithelial
ovarian cancer treated at the Department of Gynecology, Provincial
Hospital Affiliated to Shandong University (Jinan, China) between
January 2013 and June 2014 were used. The mean age of the patients
was 58 years (range, 38–77 years). No patients had a prior history
of chemotherapy, radiotherapy or immunotherapy. All patients were
staged at the time of surgery according to the International
Federation of Gynecology and Obstetrics (FIGO) staging guidelines
(FIGO 2000). The tissues were collected from patients after
obtaining informed consent from the patients' families. The study
was approved by the ethics committee of the Provincial Hospital
Affiliated to Shandong University.
Immunohistochemistry
Formalin-fixed, paraffin-embedded epithelial ovarian
cancer tissues were sectioned (4 µm), deparaffinized, and incubated
with 3% hydrogen peroxide followed by rabbit monoclonal anti-PARP-1
(Cell Signaling Technology, Inc., Danvers, MA, USA; 9532S;
dilution, 1:1,000), mouse monoclonal anti-VEGF-A (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA; sc-57496; dilution, 1:50)
or mouse monoclonal anti-CD34 (a marker for vascular endothelial
cells) (Santa Cruz Biotechnology, Inc.; sc-19621; dilution, 1:100)
at 4°C overnight. Subsequently, the sections were processed using a
secondary biotinylated antibody kit (SP-9000 detection kit; OriGene
Technologies, Inc., Beijing, China), following the manufacturer's
instructions. For negative controls, primary antibody was replaced
with PBS.
All sections were examined by two independent
pathologists who were blinded to the clinical data. PARP-1 was
predominantly localized to the tumor cell nuclei. Samples in which
>10% of cells were positive were considered PARP-1-positive. A
VEGF-A staining score was calculated by multiplying the score for
the percentage of positive cells (0, no positive tumor cells; 1,
≤25% positive tumor cells; 2, 26–50% positive tumor cells; 3,
51–75% positive tumor cells; and 4, ≥76% positive tumor cells) by
the staining intensity score (0, negative; 1, weak; 2, moderate; 3,
strong). A score of 0–3 was considered to indicate low expression
and a score of ≥4 was considered to indicate high expression.
Measurement of microvessel density
(MVD)
MVD was measured by assessing the CD34-positive
vessels in five fields of view. In each case, the most vascularized
area was selected and the microvessels within a high-power
magnification (×200) field of vision were counted three times.
Macrovascular structures with smooth muscle cells were excluded.
The mean of the three highest counts per tumor was used for
analysis.
Knockdown of PARP-1
SKOV3 human ovarian cancer cells and human umbilical
vein endothelial cells (HUVECs) were obtained from the Central
Laboratory of the Provincial Hospital Affiliated to Shandong
University, and were cultured in Hyclone RPMI-1640 medium (GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS) (Hyclone; GE Healthcare Life Sciences) at
37°C in a humidified incubator with 5% CO2. A small
interfering RNA (siRNA) (5′-AAGATAGAGCGTGAAGGCGAA-3′) that
specifically targets PARP-1 (GenBank accession number,
NM_001618) and negative control (NC) siRNA that does not target any
known human gene (5′-TTCTCCGAACGTGTCACGT-3′) were designed and
inserted into lentiviral vectors (pGCL green fluorescent protein
vector) by Shanghai Genechem Co., Ltd. (Shanghai, China).
SKOV3 cells (~5×104) were seeded into
12-well plates, and cultured for 24 h in RPMI-1640 medium
supplemented with 10% FBS. The media was then replaced with 500 µl
of suspension solution containing 5 µg/ml polybrene, 250 µl
Enhanced Infection Solution (GeneChem Co., Ltd.) and the lentiviral
constructs (25 µl). After 16 h, the suspension was replaced with
complete medium containing puromycin (1 µg/ml) to select stably
transfected cells.
HUVEC tubule formation assay
Matrigel (BD Biosciences, Bedford, MA, USA) was
added to 96-well culture plates (60 µl/well) and allowed to
polymerize at 37°C for 30 min. Conditioned media was collected from
SKOV3 cells transfected with NC-siRNA or PARP-1-siRNA, and
was centrifuged for 5 min at 1,000 × g at room temperature
to remove cells. HUVECs were resuspended in the conditioned media
(2.5×105 cells/ml), and 100-µl aliquots were seeded onto
the Matrigel, incubated at 37°C for 18 h, and imaged using an
inverted phase contrast microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from SKOV3 cells using
Invitrogen TRIzol reagent (Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA) according to the manufacturer's protocol. The
purified RNA was suspended in diethyl pyrocarbonate-treated water.
Total RNA (10 µl) obtained from each of the cell cultures was
converted into cDNA using oligo-dT15 primers and M-MLV reverse
transcriptase (Promega Corporation, Madison, WI, USA). Primers
specific to PARP-1 (forward, 5′-GCCCTAAAGGCTCAGAACGAC-3′,
and reverse, 5′-CACCATGCCATCAGCTACTCG-3′), VEGF-A (forward,
5′-TCGAGACCCTGGTGGACATC-3′, and reverse,
5′-CTATGTGCTGGCCTTGGTGAG-3′) and β-actin (forward,
5′-AGCGAGCATCCCCCAAAGTT-3′, and reverse,
5′-GGGCACGAAGGCTCATCATT-3′) were designed and synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China). qPCR was performed in 20-µl
reaction mixtures containing 10 µl SYBR Green Realtime PCR Master
Mix (Toyobo, Osaka, Japan), 4 µl of each forward and reverse primer
(10 µmol/l) and 2 µl cDNA in a Lightcycler 2.0 (Roche Diagnostics,
Indianapolis, IN, USA). The thermal conditions were 5 min at 95°C,
followed by 45 cycles of 5 min at 95°C, 10 sec at 60°C and 10 sec
at 72°C. The expression levels of PARP-1 and VEGF-A
were normalized to that of β-actin using the 2−ΔΔCq
method (12).
Western blotting and ELISA
Total cellular protein was extracted and protein
concentrations were determined using Protein Assay Dye Reagent
(Bio-Rad Laboratories, Inc., Cambridge, MA, USA). Subsequently,
equal amounts of total cellular protein extracts were separated
using 10% SDS polyacrylamide gel electrophoresis and transferred to
a nitrocellulose membrane (Pierce; Thermo Fisher Scientific, Inc.,
Rockford, IL, USA). Membranes were incubated for 2 h in TBS
containing 0.1% Tween-20 and 5% bovine serum albumin to block
non-specific binding. Membranes were then incubated overnight with
primary antibodies at 4°C, and then with the appropriate secondary
antibodies for 2 h at room temperature. The primary antibodies used
were against PARP-1 (9532S, Cell Signaling Technology, Inc.;
dilution, 1:1,000), VEGF-A (sc-57496, Santa Cruz Biotechnology,
Inc.; dilution, 1:300) and β-actin (sc-47778, Santa Cruz
Biotechnology, Inc.; dilution, 1:1,000). The secondary antibodies
used were as follows: Horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin (Ig)G (Santa Cruz Biotechnology, Inc.;
sc-2004; dilution, 1:8,000) and goat anti-mouse IgG (Santa Cruz
Biotechnology, Inc.; sc-2005; dilution, 1:8,000). Signals were
detected using a Pierce enhanced chemiluminescence kit (Thermo
Fisher Scientific, Inc.), and protein expression levels were
quantified using Gel-Pro Analyzer software v6.0 (Media Cybernetics,
Inc., Rockville, MD, USA). Results were normalized to the β-actin
content in the samples.
The VEGF-A content of cell supernatants was measured
using a VEGF-A ELISA (R&D Systems, Inc., Minneapolis, MN, USA)
following the manufacturer's instructions.
Statistical analysis
All in vitro experiments were performed in
triplicate. Data were analyzed using SPSS software version 19.0
(IBM SPSS, Armonk, NY, USA). The associations between PARP-1 and
clinicopathological features were assessed using the χ2
test or Fisher's exact probability test. The Student's
t-test was used to compare experimental groups. P<0.05
was considered to indicate a statistically significant difference
and all data are expressed as the mean ± standard error of the
mean.
Results
Expression of PARP-1 and association
with VEGF-A, MVD and clinicopathological features of human ovarian
cancer
The rate of positive PARP-1 staining in the human
ovarian cancer specimens was 73.3% (44/60). VEGF-A was expressed in
all 60 samples (mean score, 3.05±1.61; range, 1–6) and the mean MVD
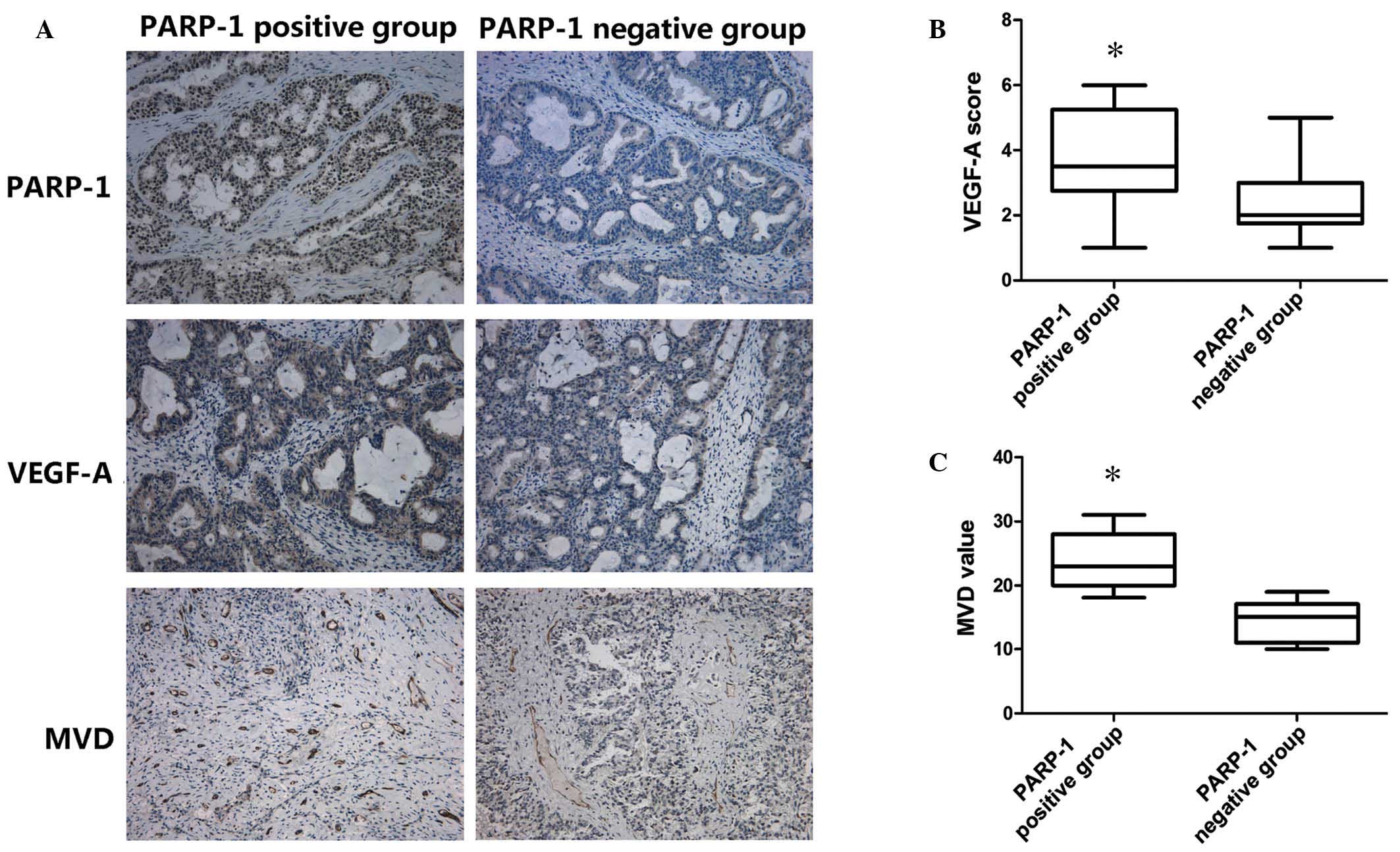
was 19.14±6.24 per field (Fig.
1A).
The mean VEGF-A staining score was significantly
higher for patients with PARP-1-positive tumors (3.80±1.69)
compared with those with PARP-1-negative tumors (2.30±1.16;
P=0.026; Fig. 1B). Additionally,
patients with PARP-1-positive tumors had a significantly higher MVD
(23.86±4.67 per field) than patients with PARP-1-negative tumors
(14.43±3.26 per field; P=0.01; Fig.
1C).
Positive expression of PARP-1 was significantly
associated with tumor size (P=0.018), histological grade (P=0.001)
and lymphatic metastasis (P=0.005), but not age (P=0.464) or FIGO
stage (P=0.302) in the 60 cases of human ovarian cancer (Table I).
 | Table I.Association between PARP-1 and the
clinicopathological features of human epithelial ovarian
cancer. |
Table I.
Association between PARP-1 and the
clinicopathological features of human epithelial ovarian
cancer.
|
| Number of
patients |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Feature | All | PARP-1-positive | PARP-1-negative | Positive rate
(%) | χ2 | P-value |
|---|
| Age (years) |
|
|
|
|
0.536 | 0.464 |
|
<50 | 13 | 8 | 5 | 61.5 |
|
|
| ≥50 | 47 | 36 | 11 | 76.6 |
|
|
| Tumor size (cm) |
|
|
|
|
5.556 | 0.018 |
|
<2 | 18 | 9 | 9 | 50.0 |
|
|
| ≥2 | 42 | 35 | 7 | 83.3 |
|
|
| Histological
grade |
|
|
|
| 10.484 | 0.001 |
|
G1+G2 | 28 | 15 | 13 | 53.6 |
|
|
| G3 | 32 | 29 | 3 | 90.6 |
|
|
| FIGO stage |
|
|
|
|
1.065 | 0.302 |
| I+II | 20 | 13 | 7 | 65.0 |
|
|
|
III+IV | 40 | 31 | 9 | 77.5 |
|
|
| Lymphatic
metastasis |
|
|
|
|
7.934 | 0.005 |
| No | 27 | 15 | 12 | 55.6 |
|
|
|
Yes | 33 | 29 | 4 | 87.9 |
|
|
Silencing of PARP-1 reduces the
angiogenic capacity of SKOV3 cells and downregulates VEGF-A
RT-qPCR and western blot analyses confirmed that
PARP1-siRNA-transfected SKOV3 cells expressed significantly
lower levels of PARP-1 than cells transfected with the NC-siRNA
(Fig. 2).
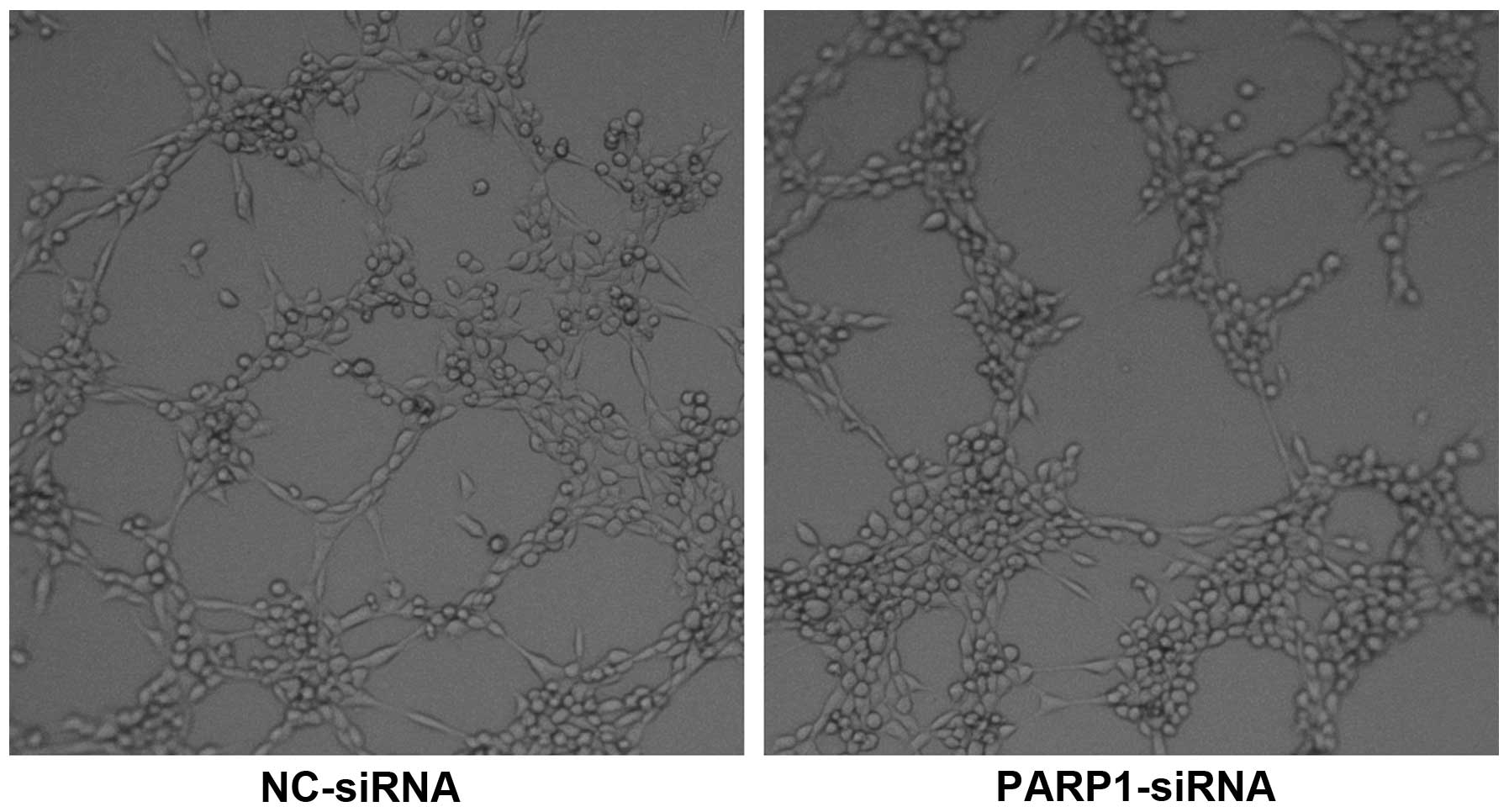
HUVECs cultured on Matrigel in conditioned media
from SKOV3 cells transfected with PARP-1-siRNA formed
significantly fewer tubules than HUVECs cultured in conditioned
media from cells transfected with NC-siRNA (14.67±1.21 vs.
8.83±1.47 per high-power field; P=0.005; Fig. 3), suggesting that PARP-1 may enhance
the ability of ovarian cancer cells to promote angiogenesis.
To investigate the mechanism by which PARP-1 affects
the ability of ovarian cancer cells to promote endothelial cell
tubule formation, the expression levels of VEGF-A in SKOV3 cells
transfected with PARP-1-siRNA or NC-siRNA were examined.
RT-qPCR demonstrated that SKOV3 cells transfected with
PARP-1-siRNA expressed lower levels of VEGF-A mRNA
compared with cells transfected with NC-siRNA. Western blotting
demonstrated that knockdown of PARP-1 significantly reduced
the relative VEGF-A protein expression in SKOV3 cells (0.41±0.08
vs. 0.90±0.18 for NC-siRNA; P=0.008; Fig.
4B and C). The level of VEGF-A in the cell supernatant was also
reduced in PARP-1-knockdown cells compared with the
NC-siRNA-transfected cells (248.12±82.74 vs. 447.22±188.52 pg/ml;
Fig. 4D), as demonstrated by ELISA.
Collectively, these results indicate PARP-1 may upregulate
VEGF-A in ovarian epithelial cancer cells.
Discussion
PARP-1 is overexpressed and serves important roles
in the progression of breast cancer (13), prostate cancer (14) and pancreatic cancer (15). Additionally, PARP-1 has been
associated with tumor invasion and lymph node metastasis in gastric
cancer (16), and its overexpression
associated with tumor stage, overall survival and prognosis in
breast cancer (17). Indeed, PARP-1
is currently being investigated as a target for cancer therapy and
a number of PARP-1 inhibitors have been tested in phase II clinical
studies (18). In the present study,
PARP-1 was found to be overexpressed in 73.3% (44/60) of the human
epithelial ovarian cancer tissues examined, and was associated with
tumor size, pathological grade and lymph node metastasis. These
data indicate that PARP-1 may also be involved in the progression
of ovarian cancer.
Angiogenesis is necessary for continued tumor growth
and is a prerequisite for tumor invasion and metastasis. A high MVD
is associated with poor prognosis in a range of tumor types,
including esophageal (19), breast
(20) and ovarian cancer (21). An association between PARP-1 and
angiogenesis has also been reported in other types of cancer. For
example, PARP-1 was found to be associated with MVD, and
overexpression of PARP-1 enhanced the angiogenic capacity of colon
cancer cells (22). Additionally, the
PARP inhibitor DPQ was demonstrated to significantly inhibit the
growth of human hepatocellular carcinoma xenografts in nude mice
and attenuate angiogenesis during tumor progression via a process
involving altered gene expression (23). In the present study, an association
between PARP-1 and the MVD in human epithelial ovarian cancer was
identified, indicating that PARP-1 exerts a pro-angiogenic effect
in epithelial ovarian cancer. Additionally, knockdown of
PARP-1 significantly suppressed the ability of conditioned
media from SKOV3 cells to promote HUVEC tubule formation in
vitro, suggesting that PARP-1 may promote angiogenesis in
ovarian cancer.
VEGF-A is a well-characterized pro-angiogenic factor
that activates multiple downstream effectors, including
extracellular signal-regulated kinases, Src and phosphoinositide
3-kinase/Akt, to stimulate endothelial cell proliferation, invasion
and basement membrane degradation (24). Based on the present observations
indicating a correlation between PARP-1 and VEGF-A in human
epithelial ovarian cancer, we hypothesized that PARP-1 promotes
angiogenesis by upregulating VEGF-A. In confirmation of this
hypothesis, knockdown of PARP-1 significantly decreased the
expression and secretion of VEGF-A in SKOV3 cells. These results
are consistent with a study by Rajesh et al (5), who demonstrated that the PARP inhibitors
3-AB and PJ34 inhibited the VEGF-induced proliferation, migration
and angiogenic capacity of HUVECs. However, the underlying
mechanism by which PARP-1 directly or indirectly regulates VEGF-A
requires further investigation.
In summary, the present study suggests that PARP-1
is overexpressed and promotes angiogenesis in epithelial ovarian
cancer by regulating VEGF-A. As it is overexpressed in ovarian
cancer and is important in tumorigenesis and angiogenesis, PARP-1
may represent a potential therapeutic target for ovarian
cancer.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81441075) and the
Natural Science Foundation of Shandong Province (grant nos.
ZR2014HM108 and ZR2013HQ030).
References
|
1
|
Desai A, Xu J, Aysola K, Qin Y, Okoli C,
Hariprasad R, Chinemerem U, Gates C, Reddy A, Danner O, et al:
Epithelial ovarian cancer: An overview. World J Transl Med. 3:1–8.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Itamochi H: Targeted therapies in
epithelial ovarian cancer: Molecular mechanisms of action. World J
Biol Chem. 1:209–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiavone MB, Bashir S and Herzog TJ:
Biologic therapies and personalized medicine in gynecologic
malignancies. Obstet Gynecol Clin North Am. 39:131–144. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ocana A, Pandiella A, Siu LL and Tannock
IF: Preclinical development of molecular-targeted agents for
cancer. Nat Rev Clin Oncol. 8:200–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rajesh M, Mukhopadhyay P, Godlewski G,
Bátkai S, Haskó G, Liaudet L and Pacher P:
Poly(ADP-ribose)polymerase inhibition decreases angiogenesis.
Biochem Biophys Res Commun. 350:1056–1062. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Langelier MF and Pascal JM: PARP-1
mechanism for coupling DNA damage detection to poly(ADP-ribose)
synthesis. Curr Opin Stru Biol. 23:134–143. 2013. View Article : Google Scholar
|
|
7
|
Horton JK, Stefanick DF and Wilson SH:
Involvement of poly(ADP-ribose) polymerase activity in regulating
Chk1-dependent apoptptic cell death. DNA Repair (Amst).
4:1111–1120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shunkwiler L, Ferris G and Kunos C:
Inhibition of poly(ADP-Ribose) polymerase enhances
radiochemosensitivity in cancers proficient in DNA double-strand
break repair. Int J Mol Sci. 14:3773–3785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krietsch J, Caron MC, Gagné JP, Ethier C,
Vignard J, Vincent M, Rouleau M, Hendzel MJ, Poirier GG and Masson
JY: PARP activation regulates the RNA-binding protein NONO in the
DNA damage response to DNA double-strand breaks. Nucleic Acids Res.
40:10287–10301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Kan Y, Tian Y, Wang Z and Zhang
J: Effects of poly (ADP-ribosyl) polymerase (PARP) inhibitor on
cisplatin resistance & proliferation of the ovarian cancer C13*
cells. Indian J Med Res. 137:527–532. 2013.PubMed/NCBI
|
|
11
|
Pyriochou A, Olah G, Deitch EA, Szabó C
and Papapetropoulos A: Inhibition of angiogenesis by the
poly(ADP-ribose) polymerase inhibitor PJ-34. Int J Mol Med.
22:113–118. 2008.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gonçalves A, Sabatier R, Charafe-Jauffret
E, Gilabert M, Provansal M, Tarpin C, Extra JM, Viens P and
Bertucci F: Triple-negative breast cancer: Histoclinical and
molecular features, therapeutic management and perspectives. Bull
Cancer. 100:453–464. 2013.(In French). PubMed/NCBI
|
|
14
|
Salemi M, Galia A, Fraggetta F, La Corte
C, Pepe P, La Vignera S, Improta G, Bosco P and Calogero AE: Poly
(ADP-ribose) polymerases 1 protein expression in normal and
neoplastic prostatic tissue. Eur J Histochem. 57:e132013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Porcelli L, Quatrale AE, Mantuano P, Leo
MG, Silvestris N, Rolland JF, Carioggia E, Lioce M, Paradiso A and
Azzariti A: Optimize radiochemotherapy in pancreatic cancer: PARP
inhibitors a new therapeutic opportunity. Mol Oncol. 7:308–322.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim J, Pyun JA, Cho SW, Lee K and Kwack K:
Lymph node metastasis of gastric cancer is associated with the
interaction between poly (ADP-ribose) polymerase 1 and matrix
metallopeptidase 2. DNA Cell Biol. 30:1011–1017. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rojo F, García-Parra J, Zazo S, Tusquets
I, Ferrer-Lozano J, Menendez S, Eroles P, Chamizo C, Servitja S,
Ramírez-Merino N, et al: Nuclear PARP-1 protein overexpression is
associated with poor overall survival in early breast cancer. Ann
Oncol. 23:1156–1164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ledermann J, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott CL, Meier W,
Shapira-Frommer R, Safra T, et al: Olaparib maintenance therapy in
patients with platinum-sensitive relapsed serous ovarian cancer: A
preplanned retrospective analysis of outcomes by BRCA status in a
randomised phase 2 trial. Lancet Oncol. 15:852–861. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi JY, Jang KT, Shim YM, Kim K, Ahn G,
Lee KH, Choi Y, Choe YS and Kim BT: Prognostic significance of
vascular endothelial growth factor expression and microvessel
density in esophageal squamous cell carcinoma: Comparison with
positron emission tomography. Ann Surg Oncol. 13:1054–1062. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rau KM, Huang CC, Chiu TJ, Chen YY, Lu CC,
Liu CT, Pei SN and Wei YC: Neovascularization evaluated by CD105
correlates well with prognostic factors in breast cancers. Exp Ther
Med. 4:231–236. 2012.PubMed/NCBI
|
|
21
|
Wang L, Liu X, Wang H and Wang S:
Correlation of the expression of vascular endothelial growth factor
and its receptors with microvessel density in ovarian cancer. Oncol
Lett. 6:175–180. 2013.PubMed/NCBI
|
|
22
|
Li Q, Li M, Wang YL, Fauzee NJ, Yang Y,
Pan J, Yang L and Lazar A: RNA interference of PARG could inhibit
the metastatic potency of colon carcinoma cells via PI3-kinase/Akt
pathway. Cell Physiol Biochem. 29:361–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quiles-Perez R, Muñoz-Gámez JA,
Ruiz-Extremera A, O'Valle F, Sanjuán-Nuñez L, Martín-Alvarez AB,
Martín-Oliva D, Caballero T, Muñoz de Rueda P, León J, et al:
Inhibition of poly adenosine diphosphate-ribose polymerase
decreases hepatocellular carcinoma growth by modulation of
tumor-related gene expression. Hepatology. 51:255–266. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Claesson-Welsh L and Welsh M: VEGFA and
tumor angiogenesis. J Intern Med. 273:114–127. 2013. View Article : Google Scholar : PubMed/NCBI
|