Introduction
Bladder cancer is ranked as the second most common
type of urinary system carcinoma; in the United States, ~74,000 new
cases and ~16,000 mortalities occurred in 2015 (1). Although there are various treatments
available, including radical cystectomy, transurethral resection of
bladder tumor (TURBT), chemotherapy and radiotherapy, the
therapeutic outcomes remain unsatisfactory. The 5-year survival
rate of invasive bladder cancers in 2014 was 70.2%, and it has a
high rate of recurrence (50–90%) (2).
Due to the absence of more effective therapies for bladder cancer,
further research into the underlying molecular mechanisms of its
tumorigenesis and the development of novel treatments aimed at
specific molecular targets are required.
Ubiquitin-conjugating enzyme E2T (UBE2T; also known
as HSPC150) is a member of E2 the family in the
ubiquitin-proteasome pathway, a complex protein degradation system
that serves crucial roles in extensive biological processes,
including cell cycle control, signal transduction and tumorigenesis
(3). Certain ubiquitin-conjugating
enzymes (E2s), such as UBC2/Rad6, UBC9 and UBE2C, have been
reported to be closely associated with tumorigenesis (4–7). The E2s
accept ubiquitin from the E1 complex and catalyze its covalent
attachment to other proteins. UBE2T has been shown to bind the E3
ubiquitin-protein ligase Fanconi anemia complementation group L
(FANCL), which is the Fanconi anaemia core complex, and to catalyze
the monoubiquitination of Fanconi anemia complementation group D2,
a key step in the DNA damage pathway (8,9). In
addition, upregulation of UBE2T has been reported in certain cancer
types (10,11). However little is known with regard to
the association between UBE2T and bladder cancer.
In the present study, the expression of UBE2T was
evaluated in human bladder cancer tissues and cell lines. In
addition, the effects of lentivirus-mediated specific small
interfering RNA (siRNA) knockdown of UBE2T on the proliferation,
colony formation, apoptosis and cell cycle of bladder cancer cells
were assessed. The findings revealed a potential oncogenic role for
UBE2T in bladder cancer, and suggest that it may serve as a
biomarker or therapeutic target for bladder cancer.
Materials and methods
Tissue samples
Tissue samples were obtained from 25 bladder cancer
patients who underwent radical cystectomy or transurethral
resection at the Department of Urology of Peking University First
Hospital (Beijing, China). Tumor-adjacent normal bladder tissues
were obtained from an area >2.0 cm away from the margin of the
cancer tissue. The histological characteristics of the samples were
evaluated by hematoxylin and eosin staining and confirmed by
experienced urological pathologists. Fresh samples were fixed in 4%
paraformaldehyde for 12–24 h and then paraffin-embedded for
immunohistochemistry, or snapfrozen immediately following the
resection and stored in liquid nitrogen for western blotting.
Informed consent was obtained in all cases and protocols were
approved by the Medical Ethics Committee of Peking University First
Hospital.
Cell culture
SV-HUC-1 normal human urinary tract epithelial cells
were obtained from ATCC (Manassas, VA, USA) and cultured in Hyclone
F-12K medium (GE Healthcare Life Sciences, Logan, UT, USA). The
bladder cancer cell lines T24, 5637, BIU-87 and EJ were cultured in
RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences), and J82
cells were cultured in high-glucose Dulbecco's modified Eagle's
medium (Hyclone; GE Healthcare Life Sciences). All bladder cancer
cell lines were obtained from the Institute of Urology of Peking
University (Beijing, China). All media contained 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
penicillin G (100 U/ml) and streptomycin (100 µg/ml)
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). Cells were
maintained as a monolayer culture at 37°C in a humidified
atmosphere containing 5% CO2.
Immunohistochemistry
After fixing with 4% formalin and embedding in
paraffin wax, tissues were cut into 5-µm sections using a
microtome. The sections were deparaffinized in xylene and
rehydrated with graded concentrations of alcohol. Subsequently, the
slides were treated with 3% H2O2 to block the
endogenous peroxidase activity and heated (95°C) for 2.5 min in
citrate buffer (10 mmol/l; pH 6.0) for antigen retrieval. To reduce
nonspecific binding, 10% normal goat serum was applied.
Subsequently, the slides were incubated with primary rabbit
anti-human UBE2T polyclonal antibody (#bs-18089R; Bioss, Inc.,
Woburn, MA, USA; dilution, 1:500) at 4°C overnight, and a
PowerVision™ two-step histostaining reagent and
3,3-diaminobenzidine tetrahydrochloride substrate kit (ZSGB-Bio,
Bejing, China) were used to visualize the localization of the
antigen, according to the manufacturer's instructions.
Western blotting
Total protein from bladder cancer samples and cell
lines were prepared with ice-cold radioimmunoprecipitation assay
buffer (Sigma-Aldrich; Merck Millipore), quantified using BCA
protein assay reagent (Pierce Chemical Co., Rockford, IL, USA), and
separated by SDS-PAGE. Following electrophoresis, proteins in the
gel were transferred to polyvinylidene difluoride membranes (Merck
Millipore). After blocking for 1 h with 5% non-fat milk, the
membranes were incubated overnight at 4°C with a rabbit monoclonal
antibody against UBE2T (#ab179802; Abcam, Cambridge, MA, USA;
dilution, 1:500) and rabbit polyclonal antibody against GAPDH
(#sc-25778; Santa Criz Biotechnology, Inc., Santa Cruz, CA, USA;
dilution, 1:1,000), followed by incubation with a horseradish
peroxidase-labeled goat anti-rabbit IgG secondary antibody
(#sc-2004/sc-2005; Santa Cruz Biotechnology, Inc.; dilution,
1:5,000) at room temperature for 1 h. Signals were detected by
application of ECL Western Blotting Detection Reagent (GE
Healthcare Life Sciences) and visualized using a G:BOX Chemi Gel
Documentation System (Syngene, Frederick, MD, USA).
Lentivirus vector construction for RNA
interference
The sequence encoding the human UBE2T gene
(NM_014176) was obtained from GenBank (http://www.ncbi.nlm.nih.gov/genbank/). The lentivirus
vector system was composed of the vector pGCSIL-GFP, which stably
expresses the siRNA and a marker (GFP-RFP fusion protein),
pHelper1.0 (gag/pol element) and Helper2.0 (VSV-G element);
pHelper1.0 and pHelper2.0 contain essential virus packaging
elements. The most effective double-stranded UBE2T-targeted siRNA
sequence, PscSI14026 (5′-GTACACAACTCAACACAGAAA-3′), was synthesized
and cloned into the pGCSIL-GFP vector by GeneChem Corporation
(Shanghai, China). Psc-NC (5′-TTCTCCGAACGTGTCACGT-3′), which shows
no homology to any known human genes, was used to generate the
negative control (NC) lentiviral vectors. Cancer cells were plated
and cultured in six-well plates (5×104 cells/well) until
60% confluent, and then appropriate volumes of the lentiviruses
were added to the cells, according to the multiplicity of infection
value (number of lentiviruses per number of cells) recommended by
the manufacturer. The interference efficiency of the vectors was
determined by western blot analysis.
Quantitative reverse transcription
(RT)-polymerase chain reaction (PCR)
Total RNA was isolated from cell lines using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). A total of 2
µg RNA was reverse-transcribed into cDNA using M-MLV reverse
transcriptase (Promega Cororation, Madison, WI, USA) and oligo(dT)
15 (Promega Corporation) as a primer. RT was performed under the
following conditions: 42°C for 1 h and 75°C for 15 min.
Quantitative PCR was performed using SYBR Green PCR Master Mix
(Toyobo Co., Ltd., Osaka, Japan) in a final volume of 10 µl in the
7500 Fast Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primer sequences were as follows: Forward,
5′-ATCCCTCAACATCGCAACTGT-3′ and reverse,
5′-CAGCCTCTGGTAGATTATCAAGC-3′ for UBE2T; forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′ for GAPDH. GAPDH served as the
quantitative internal control. PCR was performed under the
following conditions: 94°C for 30 sec, followed by 45 cycles of
94°C for 5 sec and 60°C for 30 sec. The expression of UBE2T mRNA
was normalized to GAPDH according to the ΔΔCq method
(12).
Cell growth assay
Cell growth was measured via multiparametric
high-content screening (HCS). Briefly, human bladder cancer 5637
cells that had been infected with NC lentivirus or UBE2T-siRNA
lentivirus were seeded at a density of 2,000 cells per well in
96-well plates, and then incubated at 37°C with 5% CO2
for 5 days. Plates were processed with the ArrayScan™ HCS software
(Cellomics Inc.) for analysis each day. The system is a
computerized, automated fluorescence-imaging microscope that
automatically identifies stained cells and reports the intensity
and distribution of fluorescence in each individual cell. Images
were acquired for each fluorescence channel, using suitable filters
and a 20x objective lens. In each well, >800 cells were
analyzed.
BrdU incorporation assay
DNA synthesis in proliferating cells was determined
by a BrdU incorporation assay, using a BrdU kit (#11647229001;
Roche Diagnostics, Basel, Switzerland) following the manufacturer's
instructions. The assay was performed in triplicate and repeated
three times.
Colony formation assay
Cells infected with UBE2T-siRNA lentivirus or NC
lentivirus were seeded in 6-well plates at a density of 800
cells/well and cultured at 37°C for 14 days. Medium was replaced
every 2–3 days. Cells were then washed twice with PBS, fixed with
4% paraformaldehyde, stained with Giemsa for 10 min and washed
three times with double distilled H2O. Colonies were
photographed and counted under a microscope (Leica DM IL; Leica
Microsystems, Wetzlar, Germany).
Cell cycle analysis
Cells infected with UBE2T-siRNA lentivirus or NC
lentivirus were collected, washed twice with ice-cold PBS, and
fixed with 70% ice-cold ethanol. Following fixation overnight and
subsequent rehydration in PBS for 30 min at 4°C, the samples were
stained for 30 min in darkness with 50 µg/ml propidium iodide
(#P4170; Sigma-Aldrich; Merck Millipore) containing 125 U/ml
protease-free RNase, and then analyzed using a FACSCalibur flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Cell cycle
analysis was conducted using ModFit 2.0 software (BD Biosciences).
The assay was performed in triplicate.
Cell apoptosis analysis
Cell apoptosis was assayed by staining with Annexin
V-allophycocyanin (eBioscience Annexin V Apoptosis Detection Kit
APC; #88-8007; Affymetrix, Inc., Santa Clara, CA, USA) and detected
using a FACSCalibur flow cytometer. The assay was performed in
triplicate.
Statistical analysis
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) was
employed for statistical analyses. All values in the text and
figures are expressed as the mean ± standard deviation of these
observations. P<0.05 was considered to indicate statistical
significance.
Results
UBE2T is highly expressed in bladder
cancer tissues and cell lines
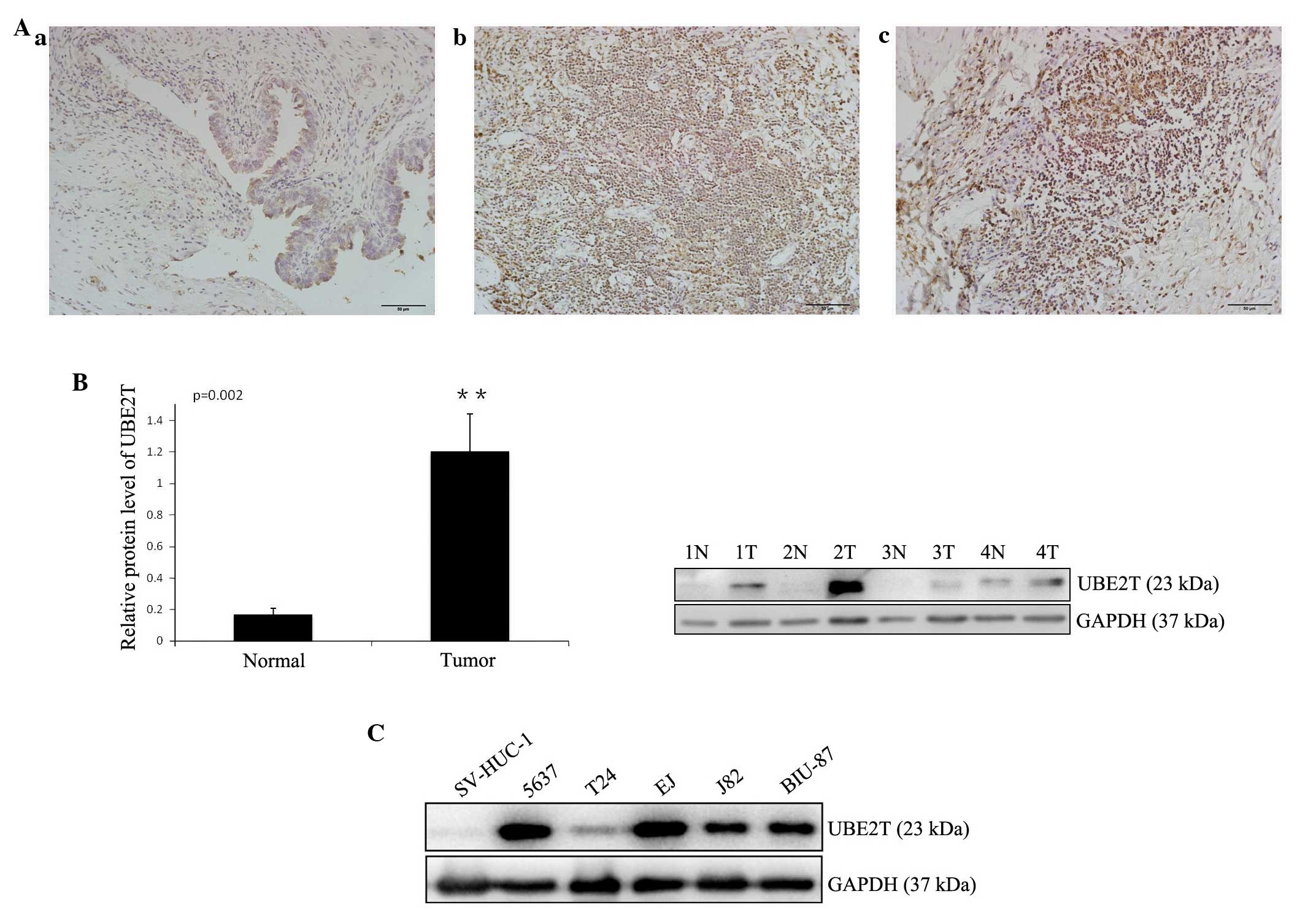
In order to study the expression of UBE2T in bladder
cancer tissues, immunohistochemistry was performed on 25 pairs of
bladder cancer and adjacent normal tissues. Weak staining was
detected in normal bladder tissue, while strong staining of UBE2T
was observed in almost all cancer specimens (Fig. 1A). UBE2T protein could be detected in
the nuclei and cytoplasm of cancer cells, but was stronger in the
nuclei, in concordance with a previous report (11). A western blot analysus was performed
to assess UBE2T protein levels in 12 bladder cancer samples and 5
cancer cell lines (5637, T24, J82, EJ and BIU-87). The results
revealed that UBE2T expression was higher in bladder cancer tissues
(Fig. 1B) and cell lines (Fig. 1C) compared with tumor-adjacent normal
bladder tissues and SV-HUC-1 normal human urinary tract epithelial
cells. Of the bladder cancer cell lines, 5637 exhibited the highest
UBE2T expression, and was therefore selected for further functional
characterization.
Lentivirus-mediated knock-down of
UBE2T in 5637 cells
In order to investigate the biological function of
UBE2T in bladder cancer, lentivirus-mediated knockdown of UBE2T was
performed in cells of the 5637 bladder cancer cell line. UBE2T mRNA
and protein levels were assessed by RT-qPCR and western blot
analyses. As shown in Fig. 2, the
mRNA and protein levels of UBE2T were reduced in cells infected
with UBE2T-siRNA compared with the cells infected with NC siRNA
(P<0.01), indicating effective knockdown of the target
sequence.
Knockdown of UBE2T inhibits the
proliferation of 5637 cells
Following infection with lentiviral-mediated siRNA,
GFP-expressing 5637 cells were monitored every day for 5 days by
Cellomics and 5-day cell proliferation curves were drawn. Cell
growth rate was defined as the cell count of Nth day/cell count of
1st day, where N = 2, 3, 4 or 5. The results revealed that
downregulation of UBE2T decreased the total number of cells and
slowed the cell growth rate (Table I
and Fig. 3A). Furthermore, DNA
synthesis was analyzed by BrdU incorporation assay on the 1st and
4th days in the 5637 cells. The results demonstrated decreased DNA
synthesis in the UBE2T-siRNA lentivirus-infected group, indicating
that cell proliferation was significantly reduced over the course
of 4 days (P=0.0127; Fig. 3B).
Collectively, these results indicate that the UBE2T gene may be
closely associated with the proliferation of 5637 cells.
 | Table I.Cell numbers and growth rate as
counted by cellomics. |
Table I.
Cell numbers and growth rate as
counted by cellomics.
|
| Cell count | Fold change in cell
count |
|---|
|
|
|
|
|---|
| Time | Control | UBE2T-siRNA | Control | UBE2T-siRNA |
|---|
| Day 1 | 513.3±13.05 | 473.0±36.29 | 1.00±0.00 | 1.00±0.00 |
| Day 2 | 1,143.3±58.79 | 826.3±39.70 | 2.23±0.09 | 1.75±0.06 |
| Day 3 | 2,258.3±76.54 | 1,103.3±97.11 | 4.40±0.17 | 2.33±0.14 |
| Day 4 | 3,646.3±176.19 | 1,302.3±14.50 | 7.11±0.37 | 2.76±0.22 |
| Day 5 | 5,113.0±295.13 | 1,457.3±93.22 | 9.96±0.47 | 3.09±0.15 |
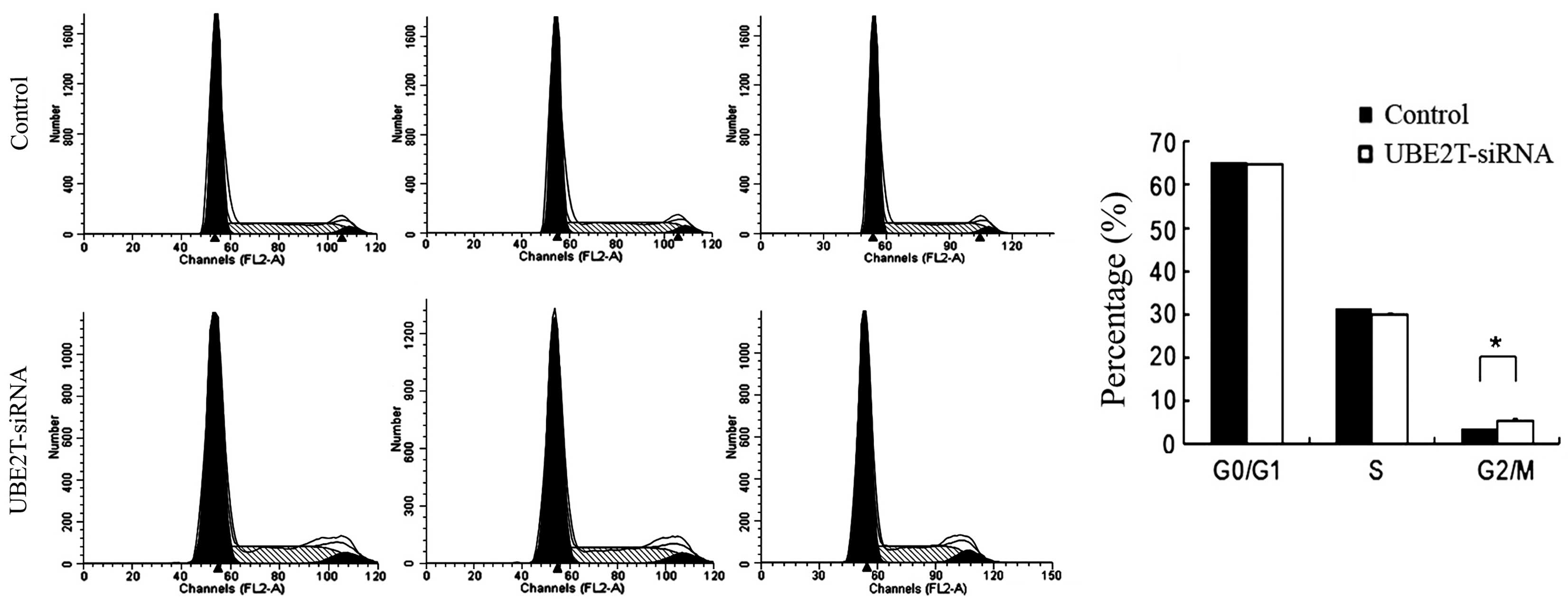
Knockdown of UBE2T induced cell cycle
arrest in 5637 cells
Alterations in cell proliferative are usually caused
by changes in the cell cycle or apoptosis. In order to investigate
whether the downregulation of UBE2T has an effect on the cell cycle
of 5637 cells, flow cytometry was employed. As shown in Fig. 4, the control group exhibited the
following distribution: G0/G1 phase, 65.5%; S phase, 31.64%; and
G2/M phase, 3.66%. By contrast, in the UBE2T-siRNA group, the
proportions were 64.58% in G1 phase, 30.83% in S phase, and 5.79%
G2/M phase. Compared with the control group, the UBE2T-siRNA group
demonstrated a significant increase in the percentage of cells in
G2/M phase (3.66±0.34% vs. 5.79±0.55%; P=0.0185), suggesting that
cells were arrested in G2/M phase following UBE2T gene silencing
and that the UBE2T gene is significantly correlated with the cell
cycle of 5637 cells.
Knockdown of UBE2T induces apoptosis
of 5637 cells
Flow cytometry was used to investigate the effect of
UBE2T on apoptosis of 5637 cells infected with NC lentivirus or
UBE2T-siRNA lentivirus. As shown in Fig.
5, the percentage of cells undergoing apoptosis was
significantly increased in the UBE2T-siRNA group compared with NC
group (24.46±0.39% vs. 9.51±0.01%; P=0.0001). This result affirmed
that UBE2T may be associated with the apoptosis of bladder cancer
cells.
Knockdown of UBE2T decreases colony
formation of 5637 cells
To determine the effect of UBE2T knockdown on
bladder cancer cell tumorigenesis in vitro, colony formation
assays were performed. The results revealed that UBE2T knockdown in
5637 cells caused a substantial reduction in colony formation
compared with the NC cells. The number of colonies in UBE2T-siRNA
lentivirus-infected cells was significantly lower than that in the
NC group (14±15 vs. 372±61; P=0.009; Fig.
6). These results suggest that the expression of UBE2T may be
associated with the progress of malignant changes.
Discussion
Although extensive information is available
regarding bladder cancer at the genetic and molecular levels,
differing clinical courses and the limited value of established
prognostic markers have compelled researchers to investigate the
mechanisms of tumorigenesis in order to identify novel molecular
components for use in bladder cancer diagnosis as well as therapy.
Tumorigenesis is considered a multistep and complex process, which
includes genomic instability and mutation, evasion of apoptosis,
resistance to cell death, and dysregulation of cellular energetic
processes (13). The
ubiquitin-proteasome pathway serves an important role in cell
proliferation, apoptosis, the cell cycle and DNA repair (14,15). For
the ubiquitin-proteasome pathway, there are 2 E1 enzymes, ~40 E2
enzymes, and >600 E3 ligases, allowing for thousands of
combinations of E2-E3 complexes (9).
UBE2T, as an important member of the E2 family, is gaining
increasing attention due to its role in the process of
tumorigenesis and its potential prognostic and therapeutic value
(9–11).
The effect of UBE2T and its interaction with the E3
ligase FANCL in the Fanconi anemia pathway, which is required for
the repair of damaged DNA, have been well studied in recent years
(9,16–19).
Furthermore, the location of the UBE2T gene, 1q32.1, is amplified
in breast cancer, liver tumors and cervical tumors (20). Ueki et al (10) demonstrated that UBE2T is overexpressed
in breast cancer and is critical in the development and/or
progression of breast cancer due to its interaction with and
regulation of the BRCA1/BARD1 complex (10). Hao et al (11) also reported that UBE2T is highly
expressed in lung cancer tissues and cell lines. It is well known
that hypoxia is one of the various conditions of tumor
microenvironment (21). Hypoxia has
been demonstrated to downregulate UBE2T expression, which
correlates with an increased sensitivity to crosslinking agents
consistent with a defective Fanconi anemia pathway (9). UBE2T has also been described as a target
gene promoted by the E2F transcription factors, which are known to
serve a key role in the timely expression of genes required for
cell cycle progression and proliferation (22). Considering these facts, UBE2T may
interact with certain key molecules that are significantly involved
in cell cycle regulation or tumorigenesis. In addition, pathways of
which UBE2T is a component, for example the Fanconi anemia pathway
and BRCA1 pathway, may also be exploited as potential targets for
tumor diagnosis and treatment.
In summary, the present study confirmed that UBE2T
is overexpressed in bladder cancers and that knockdown of UBE2T
with lentivirus-mediated specific siRNA significantly suppresses
the proliferation and colony formation of 5637 bladder cancer
cells, as well as inducing cell cycle arrest and increasing the
rate of apoptosis. Future studies should expand the number of
bladder cancer specimens to assess whether UBE2T levels are
correlated with tumor grade, pathological stage or lymph node
metastasis. In addition, the mechanisms of UBE2T in regulating
bladder cancer tumorigenesis and progression, and the potential
associations between UBE2T and downstream molecules (such as BRCA1
and Fanconi anemia pathway factors) also require further studies.
Such studies regarding the role of UBE2T in cell cycle regulation
and tumorigenesis will undoubtedly provide novel insights into
potential bladder cancer therapies.
Acknowledgements
This work was supported by grants from the National
Natural Science Foundation of China (nos. 81172419 and
81402083).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Markson G, Kiel C, Hyde R, Brown S,
Charalabous P, Bremm A, Semple J, Woodsmith J, Duley S,
Salehi-Ashtiani K, et al: Analysis of the human E2 ubiquitin
conjugating enzyme protein interaction network. Genome Res.
19:1905–1911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shekhar MP, Lyakhovich A, Visscher DW,
Heng H and Kondrat N: Rad6 overexpression induces multinucleation,
centrosome amplification, abnormal mitosis, aneuploidy, and
transformation. Cancer Res. 62:2115–2124. 2002.PubMed/NCBI
|
|
5
|
Dong M, Pang X, Xu Y, Wen F and Zhang Y:
Ubiquitin-conjugating enzyme 9 promotes epithelial ovarian cancer
cell proliferation in vitro. Int J Mol Sci. 14:11061–11071. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shuliang S, Lei C, Guangwu J and Changjie
L: Involvement of ubiquitin-conjugating enzyme E2C in proliferation
and invasion of prostate carcinoma cells. Oncol Res. 21:121–127.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie C, Powell C, Yao M, Wu J and Dong Q:
Ubiquitin-conjugating enzyme E2C: A potential cancer biomarker. Int
J Biochem Cell Biol. 47:113–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hodson C, Purkiss A, Miles JA and Walden
H: Structure of the human FANCL RING-Ube2T complex reveals
determinants of cognate E3-E2 selection. Structure. 22:337–344.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramaekers CH, van den Beucken T, Meng A,
Kassam S, Thoms J, Bristow RG and Wouters BG: Hypoxia disrupts the
Fanconi anemia pathway and sensitizes cells to chemotherapy through
regulation of UBE2T. Radiother Oncol. 101:190–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ueki T, Park JH, Nishidate T, Kijima K,
Hirata K, Nakamura Y and Katagiri T: Ubiquitination and
downregulation of BRCA1 by ubiquitin-conjugating enzyme E2T
overexpression in human breast cancer cells. Cancer Res.
69:8752–8760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hao J, Xu A, Xie X, Hao J, Tian T, Gao S,
Xiao X and He D: Elevated expression of UBE2T in lung cancer tumors
and cell lines. Tumour Biol. 29:195–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakayama KI and Nakayama K: Ubiquitin
ligases: Cell-cycle control and cancer. Nat Rev Cancer. 6:369–381.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoeller D, Hecker CM and Dikic I:
Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat
Rev Cancer. 6:776–788. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Longerich S, Kwon Y, Tsai MS, Hlaing AS,
Kupfer GM and Sung P: Regulation of FANCD2 and FANCI
monoubiquitination by their interaction and by DNA. Nucleic Acids
Res. 42:5657–5670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sato K, Toda K, Ishiai M, Takata M and
Kurumizaka H: DNA robustly stimulates FANCD2 monoubiquitylation in
the complex with FANCI. Nucleic Acids Res. 40:4553–4561. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alpi A, Langevin F, Mosedale G, Machida
YJ, Dutta A and Patel KJ: UBE2T, the Fanconi anemia core complex,
and FANCD2 are recruited independently to chromatin: A basis for
the regulation of FANCD2 monoubiquitination. Mol Cell Biol.
27:8421–8430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Machida YJ, Machida Y, Chen Y, Gurtan AM,
Kupfer GM, D'Andrea AD and Dutta A: UBE2T is the E2 in the Fanconi
anemia pathway and undergoes negative autoregulation. Mol Cell.
23:589–596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Corson TW, Huang A, Tsao MS and Gallie BL:
KIF14 is a candidate oncogene in the 1q minimal region of genomic
gain in multiple cancers. Oncogene. 24:4741–4753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren B, Cam H, Takahashi Y, Volkert T,
Terragni J, Young RA and Dynlacht BD: E2F integrates cell cycle
progression with DNA repair, replication, and G(2)/M checkpoints.
Genes Dev. 16:245–256. 2002. View Article : Google Scholar : PubMed/NCBI
|