Introduction
Chronic lymphocytic leukemia (CLL) is a
hematological neoplasm, which is characterized by clonal
proliferation and accumulation of small round B lymphocytes within
the bone marrow, peripheral blood, lymph nodes and spleen (1). As the most common type of adult leukemia
in western countries, the age-adjusted incidence of CLL is
4.1/100,000 in the USA (1). Annually,
there are >15,000 newly diagnosed cases of CLL, and ~4,500
deaths (1). Only patients with
indications for therapy should be treated. For otherwise healthy
patients, immunochemotherapy consisting of rituximab, fludarabine
and cyclophosphamide remains the current standard therapy (1). Conversely, for unfit patients, rituximab
plus chlorambucil represents the mainstay of treatment (1). Patients with aberrations (deletions or
mutations) in the tumor protein p53 (TP53) gene typically have a
poor prognosis (1). The
immunophenotype of neoplastic CLL cells is characterized by the
coexpression of cluster of differentiation (CD)5 and CD23, weak
expression of CD20, CD79b and surface immunoglobulin (Ig), as well
as negative CD10 and FMC7 expression (2).
Chromosomal abnormalities are identified in ~80% of
CLL patients, the most common of which are deletions in chromosomes
13q14, 11q22, 17p13 and 6q21 and trisomy 12 (3). The t(14;18)(q32;q21) translocation,
which involves the immunoglobulin heavy chain (IGH) locus and the
B-cell CLL/B-cell lymphoma 2 (BCL2) gene, is considered a genetic
hallmark of germinal center (GC)-derived B-cell lymphomas and
follicular lymphoma (FL) in particular (4–6). However,
t(14;18)(q32;q21) is rare in CLL and its prognostic significance
remains unclear (4).
In this report, the clinical, morphological,
immunophenotypical, cytogenetic and molecular genetic findings of
two cases of CLL with t(14;18) (q32;q21) are presented. Written
informed consent was obtained from the patient or the patient's
family for the publication of this study.
Case report
Case 1
A 46-year-old man was admitted to The Affiliated
Jiangyin Hospital of Southeast University Medical College
(Jiangyin, China) in August 2011 with a recurrent mild fever, which
had lasted for approximately 2 years and was associated with night
sweats and weight loss. Routine blood tests in September 2009 had
revealed a white blood cell count (WBC) of 13,460/µl (normal range,
3,500–9,500/µl) with 69.3% lymphocytes (normal range, 20.0–50.0%);
however, no further examinations were performed and no treatment
was administered.
Computed tomography (CT) scans in December 2012
revealed extensive enlarged lymph nodes in the neck and moderate
hepatosplenomegaly. A complete blood examination revealed the
following: WBC, 22,310/µl [neutrophils, 22.3% (normal range,
40.0–75.0%); lymphocytes, 74.7%]; hemoglobin, 168 g/l (normal
range, 115–150 g/l); platelet count, 181,000/µl (normal range,
125,000-350,000/µl). Peripheral blood and bone marrow aspiration
revealed small mature lymphocytes without indented or cleft nuclei
(Fig. 1A and B). Flow cytometry (FCM)
using bone marrow aspirate identified a clonal B lymphocyte
population that expressed positivity for CD5, CD19, CD20, CD23, λ
light chain, CD10, and CD38, dim expression of CD22 and negativity
for FMC7 and κ light chain, which indicated a diagnosis of CLL
(Fig. 2). Conventional cytogenetic
analysis of the bone marrow aspirate revealed t(14;18)(q32;q21) in
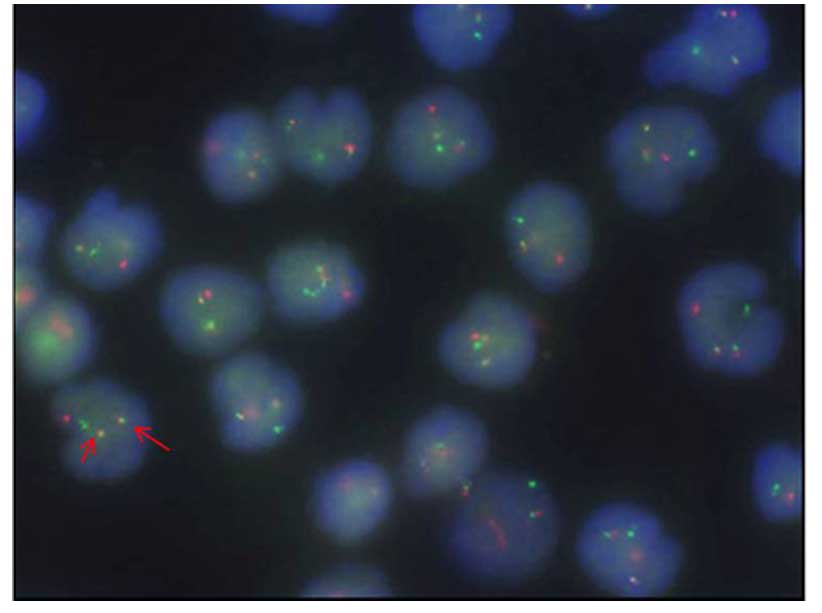
6 of 18 metaphases analyzed. Fluorescence in situ
hybridization (FISH) confirmed the presence of IGH-BCL2 fusion in
47% of analyzed nuclei (Fig. 3).
Analysis of IGH variable (IGHV) gene rearrangements revealed that
the tumor cells exhibited a mutated IGHV gene with heavy-chain
variable region gene (VH) 4–39 usage.
 | Figure 2.Case 1. Immunophenotyping by flow
cytometry. Neoplastic cells were positive for CD19, CD5, CD23, with
dim CD20 expression, and negative for FMC7, CD10 and CD38. CD,
cluster of differentiation; APC, allophycocyanin; PE,
phycoerythrin; FITC, fluorescein isothiocyanate; FSC, forward
scatter; SSC, side scatter; PerCP, Peridinin Chlorophyll Protein
Complex. |
In April 2013, the patient experienced abdominal
distention and physical examination revealed an enlarged spleen.
The patient's WBC count had increased to 52,270/µl, with 65.0%
lymphocytes. Due to persistent abdominal discomfort, the patient
received 12 cycles of oral chlorambucil (0.4 mg/kg body weight on
days 1 and 15 of every 28-day cycle). The patient is currently in
remission and undergoing follow-up.
Case 2
A 65-year-old woman presented with syncope at the
First Affiliated Hospital of Nanjing Medical University (Nanjing,
China) in October 2013. Physical examination revealed enlarged
cervical, axillary and inguinal lymph nodes, measuring 2–3 cm in
diameter. Cranial CT scans revealed no abnormalities, however, CT
scans of the chest and abdomen identified extensive enlarged
bilateral axillary, mediastinal and inguinal lymph nodes. Routine
blood examination revealed a WBC of 58,680 µl with 91.3%
lymphocytes, a hemoglobin level of 111 g/l and a platelet count of
110,000 µl. Peripheral blood smear demonstrated lymphocytosis with
16% smudge cells (Fig. 4A). Bone
marrow aspiration smear revealed numerous small mature lymphocytes
without cleaved nuclei or plasmacytoid differentiation (Fig. 4B). FCM revealed that the neoplastic
cells were positive for CD19, CD5, CD23 and CD20 with dim CD22
expression and restricted light chain expression, and negative for
CD10, CD38 and FMC7 (Fig. 5).
Immuphenotyping of bone marrow biopsy specimen demonstrated that
the neoplastic cells were negative for CD10 and positive for BCL2
(Fig. 6). Conventional cytogenetic
study revealed trisomy 12 and t(14;18)(q32;q21) in 14 of 20
metaphases analyzed (Fig. 7).
Subsequent FISH analysis confirmed these abnormalities (Fig. 8). Immunophenotyping of a cervical
lymph node biopsy specimen revealed that the tumor cells were
positive for CD20, PAX-5, BCL2 and CD79a, and negative for CD5,
CD10, BCL6 and cyclin D1. Analysis of IGHV rearrangements
demonstrated mutational IGHV status using VH3-62. Direct Sanger
sequencing of exons 4–9 revealed that the patient harbored the TP53
mutation c.829T>G, without any myeloid differentiation primary
response gene 88, splicing factor 3B subunit 1, NOTCH1 or BRIC3
mutations. Due to persistent night sweating that had lasted for
>6 months, the patient received six cycles of bendamustine (100
mg/m2/day on days 1 and 2 of a 28-day cycle). However,
an enhanced CT scan revealed that the size of the lymph nodes was
increased by 180%, which indicated disease progression. The patient
subsequently received three 14-day cycles of intravenous rituximab
(375 mg/m2 for the first cycle and 500 mg/m2
for the second and third cycles). However, the patient's disease
progressed rapidly and she succumbed to the disease in May
2015.
 | Figure 5.Case 2. Immunophenotyping by flow
cytometry. Neoplastic cells were positive for CD19, CD5, CD23, with
dim CD20 expression, and negative for FMC7, CD10 and CD38. CD,
cluster of differentiation; APC, allophycocyanin; PE,
phycoerythrin; FITC, fluorescein isothiocyanate; FSC, forward
scatter; SSC, side scatter; PerCP, Peridinin Chlorophyll Protein
Complex. |
Discussion
The t(14;18)(q32;q21) chromosomal abnormality
involves the immunoglobulin heavy chain (IGH) gene on chromosome
14q32 and the B-cell CLL/BCL2 gene on chromosome 18q21, and results
in BCL2 being placed under the regulatory control of the IgH
promoter leading to overexpression of the BCL2 protein (7). It is considered the genetic hallmark of
FL and is identified in ≤90% of FL cases (7). Although present in the majority of FL
patients, using a standardized, highly sensitive quantitative
polymerase chain reaction technique, t(14;18)(q32;q21) may be
identified at low frequencies in ≤70% healthy individuals,
suggesting that BCL2 overexpression is required but not sufficient
for FL development (8). It is also
identified in 20–30% of diffuse large B-cell lymphoma cases that
presumably originate from follicle center cells, however, it is
extremely rare in CLL (4–6). Less than 2% of CLL patients harbor
t(14;18) (q32;q21) (5,9–14). In the
present report, two rare cases of CLL patients that exhibited the
t(14;18)(q32;q21) chromosomal abnormality were presented. In these
two patients, t(14;18)(q32;q21) was identified by conventional
cytogenetics and FISH analysis. Case 1 exhibited an indolent
clinical course, however, case 2 exhibited aggressive disease that
was refractory to treatment, possibly due to TP53 mutation, which
is predictive of worse outcome in CLL (15). CLL is characterized by clonal
proliferation of mature B lymphocytes in the peripheral blood, bone
marrow, spleen and lymph nodes. Diagnosis of CLL is based on the
typical morphology and characteristic immunophenotype of
lymphocytes. Patients with CLL that harbor t(14;18)(q32;q21) and
exhibit an atypical immune phenotype may present CD5-positive FL
(6,7).
However, CD5-positive FL is extremely rare and <40 cases have
been reported in the literature to date (16). In this study, the lymphocyte count of
the two cases was >5×109/l and bone marrow
examination revealed the presence of small mature lymphocytes. Both
cases exhibited a CD5+ phenotype, which is typical of
CLL. In addition, further histopathological examination of an
enlarged lymph node in case 2 confirmed the diagnosis of CLL/small
lymphocyte lymphoma. Furthermore, both cases were negative for CD10
expression. Based on these results, the two patients were diagnosed
with CLL with t(14;18)(q32;q21). However, in cases that exhibit an
atypical immune phenotype (Matutes-Catovsky score, <4) (17), if t(14;18)(q32;q21) is present, the
histopathological examination of lymph nodes, spleen and bone
marrow is required to exclude a diagnosis of FL (17).
Trisomy 12, which is one of the most common
chromosomal abnormalities observed in CLL, is identified in 10–20%
of cases by conventional cytogenetic analysis (18). The incidence of trisomy 12 in CLL with
t(14;18)(q32;q21) ranges from 35–50%, which is markedly higher than
that in CLL patients without t(14;18)(q32;q21) (4,6,11,13,19).
Generally, CLL cases with trisomy 12 exhibit an atypical morphology
and immunophenotype (20). Consistent
with these findings, previous studies have reported that CLL cases
with t(14;18)(q32;q21) and trisomy 12 also tend to be
morphologically and/or immunophenotypically atypical (11,21). The
mechanisms underlying the frequent occurrence of trisomy 12 in CLL
with t(14;18)(q32;q21) remains to be determined; however, we
postulate that these two aberrations may cooperate with each other
in the initiation or evolution of CLL.
IGHV somatic mutation status is one of the most
important independent prognostic factors in patients with CLL
(9). Genetic analysis of the IGHV
somatic mutation status in CLL patients has identified two
prognostic subtypes: Patients with unmutated IGHV genes exhibit a
poorer prognosis than those with mutated IGHV genes, with a median
survival time of 8 and 24 years, respectively (22). The most common VH subtype used is the
VH3 family (40–50%), followed by VH4 family (25–33%) and VH1 family
(10–17%). The VH3-21 is an independent poor prognostic factor of
CLL (23). The majority of CLL cases
with the t(14;18) (q32;q21) translocation (87.5–90%) harbor mutated
IGHV genes (4,19). Approximately 75% of them used the VH3
family, with none of them using VH3-21, an independent predictor of
poor prognosis in CLL (23). Based on
these findings, the mutation rate of IGHV in CLL patients with
t(14;18)(q32;q21) appears to be higher than in CLL without the
chromosomal abnormality (~60%) (24).
Consistent with these findings, the two patients in this report
exhibited IGHV gene mutations. These findings indicate that CLL
patients with t(14;18)(q32;q21) are more likely to exhibit IGHV
somatic mutations and the most common VH usage is VH3 family
(except VH3-21).
The t(14;18)(q32;q21) translocation is a marker of
follicular center cell origin. Thus, when it occurs in the cases of
CLL, it may represent differentiation toward follicular center
cells (4,6). A number of previous studies have
investigated the function of t(14;18)(q32;q21) in the pathogenesis
of CLL (4,6,25). In two
patients, the t(14;18)(q32;q21) or its variant was identified as a
subclonal aberration with trisomy 12 as the primary change
(4). These results indicated that in
certain patients, t(14;18) may represent a secondary aberration,
which may not be responsible for the onset of disease (4). Numerous CLL patients acquire novel
abnormalities during the course of disease, which further supports
this hypothesis (25). However, Tang
et al (19), identified
t(14;18)(q32;q21) in the stemline of 10 CLL cases and identified as
the only karyotypic abnormality in 2 cases. As a result, Tang et
al proposed that t(14;18)(q32;q21) was an early pathogenetic
event in this small subset of CLL cases. Baseggio et al
(6) revealed that the BCL6 mutation
load in t(14;18)-positive CLL was lower than GC normal B-cells or
GC-derived B-cell lymphoma cells. However, the involvement of
t(14;18)(q32;q21) in the initiation and evolution of CLL remains
controversial.
At present, the prognostic significance of
t(14;18)(q32;q21) in CLL remains controversial. Certain studies
have demonstrated that the CLL patients with translocations
involving IGH exhibit a poorer prognosis (21,26).
However, in CLL, IGH translocation partners include BCL2, BCL3,
BCL11A and c-Myc, with BCL2 accounting for only a small subset of
these genes (27). Nowakowski et
al (28) observed a relatively
short median progression free survival time of 20.6 months for 8
CLL patients with IGH/BCL2 fusion. Furthermore, Tang et al
(19) revealed that t(14;18)(q32;q21)
was associated with requirement for chemotherapy and possibly
poorer survival (19). By contrast to
these findings, Put et al (4)
revealed that t(14;18)(q32;q21) in CLL was not associated with an
unfavorable clinical outcome in a large patient cohort with a
median treatment free survival time of 48 months. In addition, all
treated patients responded well to therapy (4).
In conclusion, the diagnosis of CLL is mainly based
on the typical morphology and immunophenotype of neoplastic cells.
The presence of t(14;18) should not be used to exclude a diagnosis
of CLL. Trisomy 12 and somatically mutated IGHV genes (except
VH3-21) are more common in CLL patients with t(14;18)(q32;q21)
chromosomal abnormalities. Furthermore, the exact prognostic value
of t(14;18) in CLL remains to be determined.
Acknowledgements
This study was supported by The National Natural
Science Foundation of China (grant no. 81200360), The Natural
Science Foundation of Jiangsu Province (grant no. BK2012484), The
National Public Health Grand Research Foundation (grant no.
201202017), The Program for Development of Innovative Research
Teams in the First Affiliated Hospital of Nanjing Medical
University and Project of National Key Clinical Specialty, The
National Science & Technology Pillar Program (grant no.
2014BAI09B12) The Jiangsu Provincial Special Program of Medical
Science (grant no. BL2014086).
References
|
1
|
Hallek M: Chronic lymphocytic leukemia:
2013 update on diagnosis, risk stratification and treatment. Am J
Hematol. 88:803–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jaffe ES, Harris NL, Stein H, Campo E,
Pileri SA and Swerdlow SH: Introduction and overview of the
classification of the lymphoid neoplasmsWHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. Swerdlow SH, Campo
E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thilele J and Vardiman
JW: 2. 4th. IARC Press; Lyon: pp. 158–166. 2008
|
|
3
|
Xu W, Li JY, Pan JL, Qiu HR, Shen YF, Li
L, Wu YF and Xue YQ: Interphase fluorescence in situ
hybridization detection of cytogenetic abnormalities in B-cell
chronic lymphocytic leukemia. Int J Hematol. 85:430–436. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Put N, Meeus P, Chatelain B, Rack K,
Boeckx N, Nollet F, Graux C, Van Den Neste E, Janssens A, et al:
Translocation t(14;18) is not associated with inferior outcome in
chronic lymphocytic leukemia. Leukemia. 23:1201–1204. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu G, Kong Y and Yue C: Genetic and
immunophenotypic profile of IGH@ rearrangement detected by
fluorescence in situ hybridization in 149 cases of B-cell
chronic lymphocytic leukemia. Cancer Genet Cytogenet. 196:56–63.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baseggio L, Geay MO, Gazzo S, Berger F,
Traverse-Glehen A, Ffrench M, Hayette S, Callet-Bauchu E, Verney A,
Morel D, et al: In non-follicular lymphoproliferative disorders,
IGH/BCL2-fusion is not restricted to chronic lymphocytic leukaemia.
Br J Haematol. 158:489–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang F, Yan LX, Lin SX, Ye ZY, Zhuang HG,
Yun JP, Lin HL, Luo DL, Xu FP, Luo XL, et al: Immunophenotypic
features and t(14;18) (q32;q21) translocation of Chinese follicular
lymphomas helps to distinguish subgroups. Diagn Pathol. 8:1542013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schüler F, Dölken L, Hirt C, Kiefer T,
Berg T, Fusch G, Weitmann K, Hoffmann W, Fusch C, Janz S, et al:
Prevalence and frequency of circulating t(14;18)-MBR translocation
carrying cells in healthy individuals. Int J Cancer. 124:958–963.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haferlach C, Dicker F, Schnittger S, Kern
W and Haferlach T: Comprehensive genetic characterization of CLL: A
study on 506 cases analysed with chromosome banding analysis,
interphase FISH, IgV(H) status and immunophenotyping. Leukemia.
21:2442–2451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amare PS, Gadage V, Jain H, Nikalje S,
Manju S, Mittal N, Gujral S and Nair R: Clinico-pathological impact
of cytogenetic subgroups in B-cell chronic lymphocytic leukemia:
Experience from India. Indian J Cancer. 50:261–267. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sen F, Lai R and Albitar M: Chronic
lymphocytic leukemia with t(14;18) and trisomy 12. Arch Pathol Lab
Med. 126:1543–1546. 2002.PubMed/NCBI
|
|
12
|
Kern W, Haferlach T, Schnittger S and
Schoch C: Detection of t(14;18)(q32;q21) in B-cell chronic
lymphocytic leukemia. Arch Pathol Lab Med. 129:410–411.
2005.PubMed/NCBI
|
|
13
|
Kojima K, Taniwaki M, Yoshino T, Katayama
Y, Sunami K, Fukuda S, Omoto E, Harada M and Sezaki T: Trisomy 12
and t(14;18) in B-cell chronic lymphocytic leukemia. Int J Hematol.
67:199–203. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jonveaux P, Hillion J, Bennaceur AL,
d'Agay MF, Brice P, Daniel MT, Sigaux F and Berger R: t(14;18) and
bcl-2 gene rearrangement in a B-chronic lymphocytic leukaemia. Br J
Haematol. 81:620–621. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong HJ, Zhou LT, Zhu DX, Wang DM, Fang C,
Zhu HY, Zhuang Y, Miao KR, Xu W and Li JY: The prognostic
significance of TP53 mutations in Chinese patients with chronic
lymphocytic leukemia is independent of del(17p13). Ann Hematol.
90:709–717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sekiguchi Y, Imai H, Wakabayashi M, Sawada
T, Ichikawa K, Komatsu N and Noguchi M: CD5-positive follicular
lymphoma: A case report and literature review. Intern Med.
50:899–904. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oscier D, Dearden C, Eren E, Fegan C,
Follows G, Hillmen P, Illidge T, Matutes E, Milligan DW, Pettitt A,
et al: British Committee for Standards in Haematology: Guidelines
on the diagnosis, investigation and management of chronic
lymphocytic leukaemia. Br J Haematol. 159:541–564. 2012.PubMed/NCBI
|
|
18
|
Juliusson G, Oscier DG, Fitchett M, Ross
FM, Stockdill G, Mackie MJ, Parker AC, Castoldi GL, Guneo A,
Knuutila S, et al: Prognostic subgroups in B-cell chronic
lymphocytic leukemia defined by specific chromosomal abnormalities.
N Engl J Med. 323:720–724. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang G, Banks HE, Sargent RL, Medeiros LJ
and Abruzzo LV: Chronic lymphocytic leukemia with
t(14;18)(q32;q21). Hum Pathol. 44:598–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matutes E, Oscier D, Garcia-Marco J, Ellis
J, Copplestone A, Gillingham R, Hamblin T, Lens D, Swansbury GJ and
Catovsky D: Trisomy 12 defines a group of CLL with atypical
morphology: Correlation between cytogenetic, clinical and
laboratory features in 544 patients. Br J Haematol. 92:382–388.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mayr C, Speicher MR, Kofler DM, Buhmann R,
Strehl J, Busch R, Hallek M and Wendtner CM: Chromosomal
translocations are associated with poor prognosis in chronic
lymphocytic leukemia. Blood. 107:742–751. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Damle RN, Wasil T, Fais F, Ghiotto F,
Valetto A, Allen SL, Buchbinder A, Budman D, Dittmar K, Kolitz J,
et al: Ig V gene mutation status and CD38 expression as novel
prognostic indicators in chronic lymphocytic leukemia. Blood.
94:1840–1847. 1999.PubMed/NCBI
|
|
23
|
Mauerer K, Zahrieh D, Gorgun G, Li A, Zhou
J, Ansén S, Rassenti LZ and Gribben JG: Immunoglobulin gene segment
usage, location and immunogenicity in mutated and unmutated chronic
lymphocytic leukaemia. Br J Haematol. 129:499–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamblin TJ, Davis Z, Gardiner A, Oscier DG
and Stevenson FK: Unmutated Ig V(H) genes are associated with a
more aggressive form of chronic lymphocytic leukemia. Blood.
94:1848–1854. 1999.PubMed/NCBI
|
|
25
|
Shanafelt TD, Witzig TE, Fink SR, Jenkins
RB, Paternoster SF, Smoley SA, Stockero KJ, Nast DM, Flynn HC,
Tschumper RC, et al: Prospective evaluation of clonal evolution
during long-term follow-up of patients with untreated early-stage
chronic lymphocytic leukemia. J Clin Oncol. 24:4634–4641. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smolewski P, Witkowska M and
Korycka-Wołowiec A: New insights into biology, prognostic factors,
and current therapeutic strategies in chronic lymphocytic leukemia.
ISRN Oncol. 2013:7406152013.PubMed/NCBI
|
|
27
|
Cavazzini F, Hernandez JA, Gozzetti A,
Russo Rossi A, De Angeli C, Tiseo R, Bardi A, Tammiso E, Crupi R,
Lenoci MP, et al: Chromosome 14q32 translocations involving the
immunoglobulin heavy chain locus in chronic lymphocytic leukaemia
identify a disease subset with poor prognosis. Br J Haematol.
142:529–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nowakowski GS, Dewald GW, Hoyer JD,
Paternoster SF, Stockero KJ, Fink SR, Smoley SA, Remstein ED,
Phyliky RL, Call TG, et al: Interphase fluorescence in situ
hybridization with an IGH probe is important in the evaluation of
patients with a clinical diagnosis of chronic lymphocytic
leukaemia. Br J Haematol. 130:36–42. 2005. View Article : Google Scholar : PubMed/NCBI
|