Introduction
Diffuse malignant pleural mesothelioma (MPM) is an
aggressive, rare tumor that originates from the surface of the
pleura. Although relatively uncommon, the incidence of MPM is
expected to increase in the next 20 years (1). Asbestos exposure is a major risk factor,
with ~70% of MPM patients presenting with a defined history of
chronic asbestos exposure (1). MPM is
regarded as an incurable disease, with a median survival of 2 years
following intensive multimodality treatment (2).
Surgery is only considered in select patients
diagnosed with epithelial mesothelioma with a good performance
status and optimal cardiopulmonary function (3). The role of exclusive chemoradiotherapy
is currently under investigation (4).
Presently, radiotherapy is confined to adjuvant settings and is
often used in association with chemotherapy, or palliation in
advanced stages (5). Therefore,
chemotherapy is the primary treatment option for unresectable
lesions. First-line treatment with cisplatin- pemetrexed is
considered the gold standard (6).
However, an alternative schedule of cisplatin-raltitrexed may also
be administered, which was previously indicated to improve survival
in comparison with cisplatin alone during a phase III study
(7). A second-line, single agent
regimen with gemcitabine or vinorelbine may also be considered in
patients with a good performance status (8,9).
Pancreatic cancer is one of most aggressive forms of
malignancy, with <2% of patients surviving for 5 years or more
(10). Surgery has a limited role in
the treatment of the disease, with >80% of patients diagnosed
with unresectable lesions. As with MPM, multimodal
chemoradiotherapy is a valid treatment option for cases of
pancreatic cancer (11).
Over the last two decades, gemcitabine has remained
the principal chemotherapeutic drug for the treatment of pancreatic
cancer, exhibiting modest clinical benefit with a median patient
survival time of 5.4 months (12). A
number of chemotherapeutics and targeted agents have been combined
with gemcitabine to produce no clinical benefit, including taxane
(13,14). Previously, gemcitabine in combination
with nab-paclitaxel demonstrated efficient clinical activity in a
phase III trial, with statistically significant improvement in
median overall survival compared with gemcitabine alone (15). Therefore, the USA Food and Drug
Administration approved paclitaxel protein-bound particles
(albumin-bound) in combination with gemcitabine for first-line
treatment of patients with metastatic adenocarcinoma of the
pancreas (16). Alternative therapies
include capecitabine, oxaliplatin plus fluorouracil plus
leucovorin/capecitabine plus oxaliplatin, and oxaliplatin plus
fluorouracil plus leucovorin plus irinotecan (17). Therefore, considering the poor
responsiveness to the treatment available to date and the
aggressiveness of both tumors, novel therapies are required for the
treatment of MPM and pancreatic cancer.
The development of synchronous MPM and other tumors
is rare. In a review of 500 patients with asbestos-related MPM, 9
(1.8%) patients presented with synchronous carcinoma (17). In 6/9 (66.7%) cases, the secondary
tumor was lung carcinoma, including three adenocarcinomas, two
squamous cell carcinomas and one small-cell carcinoma, and in 3/9
cases (33.3%) the secondary tumor was non-bronchogenic carcinoma,
including breast, colonic and pancreatic (18). In a previous study, among 215 cases of
malignant pleural mesothelioma, the occurrence of a second
malignancy was observed in 32 cases (18.9%), suggesting that these
diseases may share certain etiological factors such as asbestos and
others (19).
The onset of bowel metastasis is a rare event in
cases of MPM, and to the best of our knowledge, has not been
previously described in association with pancreatic cancer.
Case report
The present study describes the case of a
59-year-old man with a professional history of asbestos exposure,
who was diagnosed with locally-advanced MPM in 2011. In November
2011, a computed tomography (CT) scan revealed right pleural
effusion and Barety and subcarinal nodes involvement. A suspected
nodule, measuring 18 mm in diameter, at the head of the pancreas
was noted. An 18-fluorodeoxyglucose (18FDG) positron
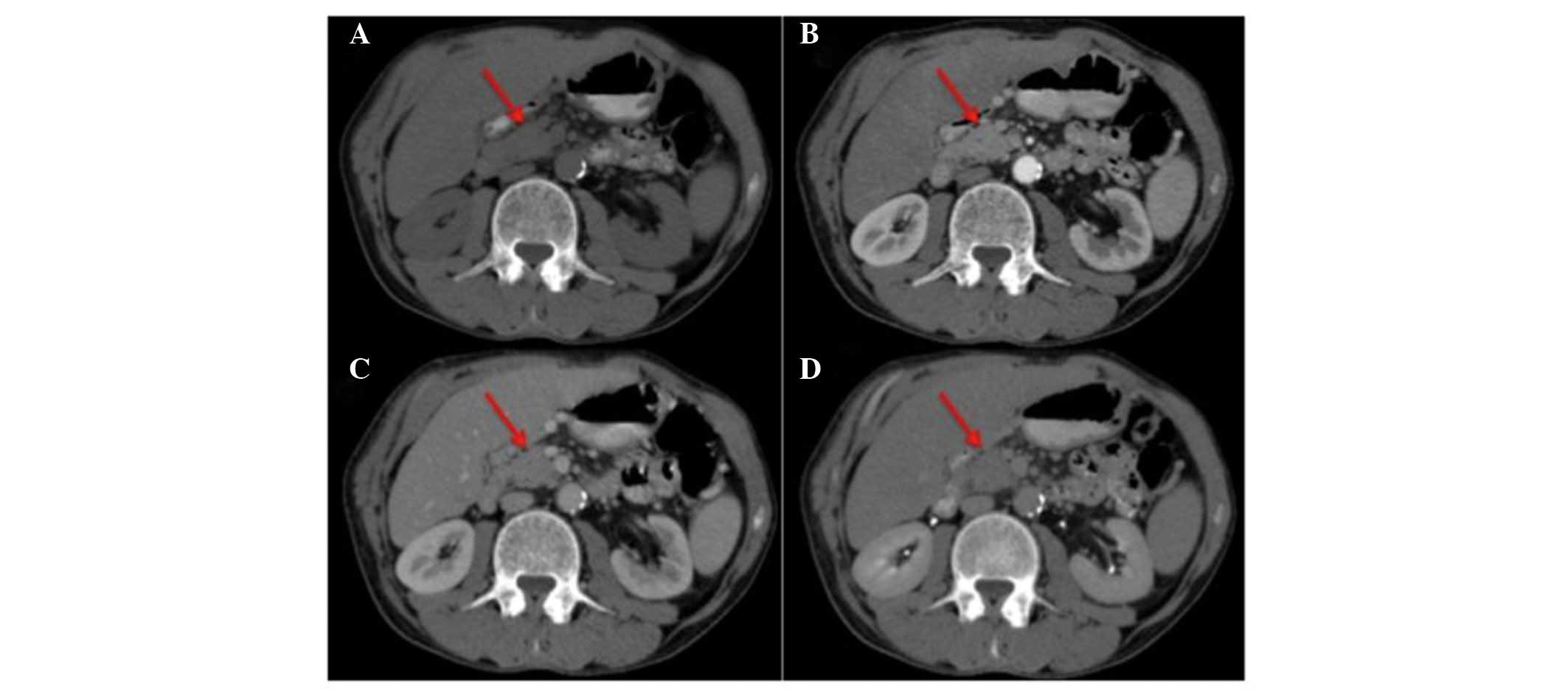
emission tomography (PET) scan was performed (Fig. 1). The scan exhibited uptake of
18FDG in the pleural region [maximum standardized uptake
(SUVmax), 2.5] with no uptake in the pancreas (Fig. 1A). Video-assisted thoracoscopy was
performed to assist a pleural biopsy, and following analysis, a
diagnosis of biphasic mesothelioma was confirmed with
immunohistochemical positivity for epithelial specific antigen
(EpCAM) and calretinin.
In accordance with the European Organization for
Research and Treatment of Cancer prognostic scoring system
(20), the patient received 2 cycles
of first-line cisplatin-pemetrexed chemotherapy: Pemetrexed, 500
mg/mq i.v. over 10 min plus cisplatin, 75 mg/mq i.v. over 30 min 60
min later, on day 1 every 3 weeks. During February 2012, a tumor
assessment reported stability of the pleural disease, an increase
in size of the pancreatic lesion (40 mm diameter vs. 18 mm diameter
at initial evaluation) and the onset of peripancreatic and axillary
node metastasis by CT scan with contrast (5-mm thickness slides)
(Fig. 2).
Axillary node surgical excision and a laparoscopic
pancreatic biopsy were performed, and histological examination of
the surgical samples diagnosed an undifferentiated pancreatic
adenocarcinoma. The case was evaluated by a multidisciplinary
board, and single-agent gemcitabine treatment (gemcitabine, 1,000
mg/mq iv over 30 min on days 1, 8 and 15 of each 28-day cycle) was
initiated in May 2012. Following 2 cycles, an 18FDG PET
scan demonstrated stability of the pleural lesions (SUVmax, 2.6)
(Fig. 1B).
Based on disease stability, the multidisciplinary
board advised that multimodal treatment with gemcitabine and
concomitant radiotherapy on the pleural lesion should be performed
during July 2012. The prescribed dose was 36 Gy, delivered over 12
consecutive fractions at 3 Gy, plus a three-dimensional conformal
boost of 3 Gy to the gross tumor volume delivered using Precise 6
MV Linac system (Elekta AB, Stockholm, Sweden).
Following radiotherapy, the patient continued with
single-agent gemcitabine treatment until October 2012, when an
18FDG PET scan confirmed pleural stability (SUVmax,
2.5). However, the pancreatic lesion had begun to indicate an
uptake of 18FDG (SUVmax, 3.1) (Fig. 1C). Considering the good performance
status of the patient, concomitant pancreatic stereotactic body
radiation therapy (SBRT) was performed on November 2012. The
prescribed dose was 21 Gy over 3 consecutive fractions using the
Precise 15 MV Linac system (Elekta AB). In February 2013, a further
18FDG PET scan revealed light pleural uptake (SUVmax,
1.9) (Fig. 1D), and single-agent
gemcitabine chemotherapy (gemcitabine, 800 mg/mq iv over 30 min on
days 1, 8 and 15 of each 28-day cycle) was continued.
In May 2013, an 18FDG PET scan documented
a pleural uptake increase (SUVmax, 3.1) (Fig. 1E). Due to the exacerbation of right
hemithorax pain, which was unresponsive to analgesic therapy, the
multidisciplinary board proposed that the patient undergo pleural
SBRT. The patient received SBRT with a prescribed dose of 25 Gy,
over 5 fractions, delivered on 5 alternate days using the Precise
15 MV Linac system. The patient subsequently experienced a
significant relief from symptoms, and monochemotherapy with
gemcitabine (gemcitabine, 1,000 mg/mq iv over 30 min on days 1, 8
and 15 of each 28-day cycle) was continued until February 2014.
In March 2014, another 18FDG PET scan
revealed pleural stability and bowel uptake. The patient was also
experiencing abdominal pain and was suspected of having ‘acute
abdomen’ (Fig. 1F). A CT scan
revealed gaseous distension of the small intestine, therefore the
patient underwent emergency intestinal resection.
Histological examination was performed at the
Department of Pathology of the Second University of Naples (Naples,
Italy), according to standard local practice (21). Immunohistochemical analysis was
performed on 5-µm thick, formalin-fixed, paraffin-embedded tissue
sections with the following antibodies: Anti-cytokeratin (CK) 7
(dilution 1:100; catalogue no. ab183344; Abcam, Cambridge, UK),
anti-CK20 (dilution 1:100; catalogue no. ab97511; Abcam),
anti-caudal type homeobox transcription factor 2 (CDX2; dilution
1:250; catalogue no. ab76541; Abcam), anti-CK5/6 (dilution 1:50;
catalogue no. ab17133; Abcam), anti-EpCAM (clone Ber-EP4; dilution
1:250; catalogue no. ab7504; Abcam) and anti-calretinin (dilution
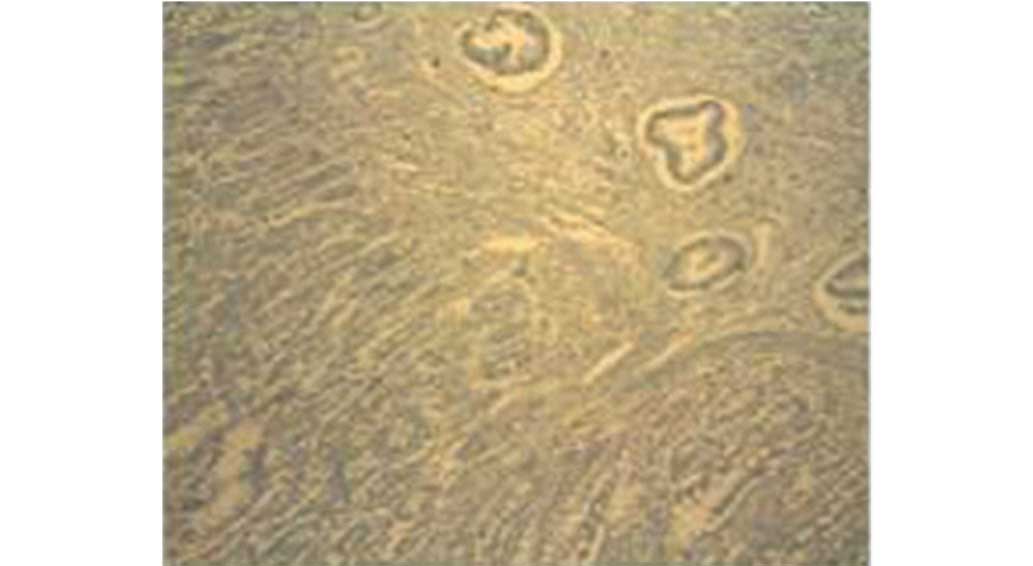
1:50; catalogue no. ab702; Abcam). Analysis of the small intestine
sample confirmed the presence of moderately-differentiated
adenocarcinoma. Immunohistochemical examination demonstrated that
the sample was positive for CK7, negative for CK20 and
focally-positive for CDX2. Based on this immunohistochemical
profile (negativity for CK20 and positivity for CK7 and CDX2),
pathologists excluded an intestinal origin.
Considering the medical history of the patient, a
revision of the small intestine sample was performed and
immunohistochemistry demonstrated that the tissue was negative for
CK5, CK6, CK20, EpCAM and calretinin; therefore, a mesothelial
origin of metastasis was also excluded, and the pathologists
determined that the pancreatic cancer was an initial form of small
bowel metastasis (Figs. 3 and
4).
The multidisciplinary board advised that the patient
should begin chemotherapy with gemcitabine and nab-paclitaxel,
since previous clinical studies had demonstrated the efficacy of
this type of chemotherapy for pancreatic cancer (15,22). The
reintroduction of gemcitabine was supported by good results
obtained on pleural lesion during previous line of treatment with
single agent gemcitabine. The patient continued to receive
gemcitabine plus nab-paclitaxel for 3 cycles. Then, for clinical
progression, the patient underwent only supportive care for ~2
months, until succumbing to the disease.
At the time of writing the present case report, the
patient was still undergoing treatment. Written informed consent
was obtained from the patient for publication of the present study
and accompanying images.
Discussion
MPM is the most common primary tumor of the pleura
(1). Exposure to asbestos is the
predominant cause of MPM development, with a latency period of at
least 35–40 years (2). The incidence
of the disease occurs most commonly during the sixth and seventh
decades of life with a median survival of 12–18 months regardless
of treatment regimen (1,3). Local invasion often affects other
contiguous organs, including the pericardium (tamponade and
pericardial effusions), the spinal cord (paralysis and back pain)
and the contralateral lung (often contralateral pleural effusion)
(23). Metastases are usually located
at the lymphatic nodes, liver, kidneys and adrenal glands, but are
extremely infrequent at the gastrointestinal tract (24). CT and PET scans are important for the
diagnosis and assessment of treatment response in cases of
mesothelioma.
Regarding pancreatic cancer, no early diagnostic
tests or effective treatment options are currently available;
therefore, pacreatic cancer remains the fourth leading cause of
cancer-associated mortality (25).
Typically, pancreatic cancer initially metastasizes to the regional
lymph nodes, later to the liver or peritoneal cavity and rarely to
the lungs, bone or brain (25).
The present study described the case of patient with
an initial diagnosis of MPM and pancreatic carcinoma, with
subsequent evidence of a lesion in the small bowel. Based on
clinical history, the disease course and immunohistochemical data,
it was extremely difficult to identify with certainty whether the
bowel lesion was a metastasis from the mesothelioma or pancreatic
cancer. Immunohistochemical positivity for CK7 and negativity for
CK20 excluded the possibility of an intestinal origin. Therefore,
to determine the origin of the bowel metastases, the pathologist
performed a revision of the small intestine sample, and further
immunohistochemical analysis demonstrated negativity for CK5, CK6,
EpCAM and calretinin. This immunohistochemical profile suggested
that the pancreatic cancer may have been an initial form of
intestinal metastases.
To the best of our knowledge, the current case is
the first to describe the development of intestinal metastasis from
pancreatic cancer. Certain previous cases of MPM have instead
described total involvement of the gastrointestinal lumen by
effusion (26–33).
Chen et al (27) reported the case of a 73-year-old man
presenting with duodenal metastases from sarcomatoid MPM.
Immunohistochemical analysis reported positivity for CK7 and
vimentin, and negativity for cluster of differentiation (CD)34,
CD117 and calretinin (27).
Liu et al (32)
described a patient that presented with MPM with metastases in the
jejunum, and the ascending and transverse colon.
Immunohistochemical examination on the intestinal sample reported
vimentin and cytokeratin positivity and calretinin, CK20 and
thyroid transcription factor-1 negativity. This profile was the
same as the pleural sample, confirming that the metastases
originated from MPM (32).
Based on immunohistochemical results of the present
case, a chemotherapy regimen of gemcitabine plus nab-paclitaxel was
initiated. The use of gemcitabine was supported by the good results
obtained on the pleural lesion during previous lines of treatment
with gemcitabine, which was started in May 2012 and exhibited a
good control of the disease until March 2014, when it was added to
nab-paclitaxel for diagnosis of small bowel metastasis.
The current case highlights the possible role of
SBRT in the treatment of oligometastatic diseases, however its
application is not yet conventional. If lesions are unable to be
surgically resected, radiation therapy may serve a crucial role in
disease control on metastatic foci and primary tumors (34). A patient with a good performance
status and a lesion <4 cm in size is an ideal candidate for SBRT
with minimal toxicity on surrounding normal tissue (34). Thus, the role of SBRT in the treatment
of metastatic cancer may change from palliative to potentially
curative.
The appropriate timing of combining SBRT with
chemotherapy remains unclear, considering high risk of recurrence
in these patients. It is currently under investigation as to which
chemotherapeutic drug should be administered in order to achieve
positive tolerability and a radiosensitizing effect (35).
Taking this into account, it is considered that the
long-term survival of the current case (33 months) and lasting
disease control occurred as a result of a combination of
chemotherapy regimens, containing active drugs targeting each
cancer, and the application of SBRT on the pancreatic cancer
lesions.
In conclusion, the present case highlights the
requirement of prompt treatment and the use of all available tools
to provide an accurate differential diagnosis, even in patients
with a presumably poor prognosis. The present case study may
implicate future research on the role of SBRT with concomitant
chemotherapy in patients with advanced stage disease.
Acknowledgements
The present study was supported by the Associazione
Italiana Per La Ricerca Sul Cancro project (Milan, Italy; grant no.
MFAG 2013-N.14392).
References
|
1
|
Teta MJ, Mink PJ, Lau E, Sceurman BK and
Foster ED: US mesothelioma patterns 1973–2002: Indicators of change
and insights into background rates. Eur J Cancer Prev. 17:525–534.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krug LM, Pass HI, Rusch VW, Kindler HL,
Sugarbaker DJ, Rosenzweig KE, Flores R, Friedberg JS, Pisters K,
Monberg M, et al: Multicenter phase II trial of neoadjuvant
pemetrexed plus cisplatin followed by extrapleural pneumonectomy
and radiation for malignant pleural mesothelioma. J Clin Oncol.
27:3007–3013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edwards JG, Abrams KR, Leverment JN, Spyt
TJ, Waller DA and O'Byrne KJ: Prognostic factors for malignant
mesothelioma in 142 patients: Validation of CALGB and EORTC
prognostic scoring systems. Thorax. 55:731–735. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Opitz I: Management of malignant pleural
mesothelioma - the European experience. J Thorac Dis. 6:(Suppl 2).
S238–S252. 2014.PubMed/NCBI
|
|
5
|
Baldini EH: Radiation therapy options for
malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg.
21:159–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Manegold C, et al: Phase III study of pemetrexed in combination
with cisplatin versus cisplatin alone in patients with malignant
pleural mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Meerbeeck JP, Gaafar R, Manegold C,
Van Klaveren RJ, Van Marck EA, Vincent M, Legrand C, Bottomley A,
Debruyne C and Giaccone G: European Organisation for Research and
Treatment of Cancer Lung Cancer Group; National Cancer Institute of
Canada: Randomized phase III study of cisplatin with or without
raltitrexed in patients with malignant pleural mesothelioma: An
intergroup study of the European Organisation for Research and
Treatment of Cancer Lung Cancer Group and the National Cancer
Institute of Canada. J Clin Oncol. 23:6881–6889. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stebbing J, Powles T, McPherson K, Shamash
J, Wells P, Sheaff MT, Slater S, Rudd RM, Fennell D and Steele JP:
The efficacy and safety of weekly vinorelbine in relapsed malignant
pleural mesothelioma. Lung Cancer. 63:94–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Meerbeeck JP, Baas P, Debruyne C,
Groen HJ, Manegold C, Ardizzoni A, Gridelli C, van Marck EA, Lentz
M and Giaccone G: European Organization for Research and Treatment
of Cancer Lung Cancer Cooperative Group: A phase II study of
gemcitabine in patients with malignant pleural mesothelioma.
European organization for research and treatment of cancer lung
cancer cooperative group. Cancer. 85:2577–2582. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brunner TB and Scott-Brown M: The role of
radiotherapy in multimodal treatment of pancreatic carcinoma.
Radiat Oncol. 5:642010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ghosn M, Kourie HR, El Karak F, Hanna C,
Antoun J and Nasr D: Optimum chemotherapy in the management of
metastatic pancreatic cancer. World J Gastroenterol. 20:2352–2357.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
National Cancer Institute of Canada Clinical Trials Group:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Belli C, Cereda S and Reni M: Role of
taxanes in pancreatic cancer. World J Gastroenterol. 18:4457–4465.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saif MW: U.S. Food and Drug Administration
approves paclitaxel protein-bound particles (Abraxane®)
in combination with gemcitabine as first-line treatment of patients
with metastatic pancreatic cancer. JOP. 14:686–688. 2013.PubMed/NCBI
|
|
17
|
Kothari N, Saif MW and Kim R: First-line
treatment for advanced pancreatic cancer. JOP. 14:129–132.
2013.PubMed/NCBI
|
|
18
|
Attanoos RL, Thomas DH and Gibbs AR:
Synchronous diffuse malignant mesothelioma and carcinomas in
asbestos-exposed individuals. Histopathology. 43:387–392. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bianchi C, Bianchi T and Ramani L:
Malignant mesothelioma of the pleura and other malignancies in the
same patient. Tumori. 93:19–22. 2007.PubMed/NCBI
|
|
20
|
Curran D, Sahmoud T, Therasse P, van
Meerbeeck J, Postmus PE and Giaccone G: Prognostic factors in
patients with pleural mesothelioma: The European Organization for
Research and Treatment of Cancer experience. J Clin Oncol.
16:145–152. 1998.PubMed/NCBI
|
|
21
|
Raucci R, Colonna G, Guerriero E, Capone
F, Accardo M, Castello G and Costantini S: Structural and
functional studies of the human selenium binding protein-1 and its
involvement in hepatocellular carcinoma. Biochim Biophys Acta.
1814:513–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Von Hoff DD, Ramanathan RK, Borad MJ,
Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias
JL, et al: Gemcitabine plus nab-paclitaxel is an active regimen in
patients with advanced pancreatic cancer: A phase I/II trial. J
Clin Oncol. 29:4548–4554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robinson BW, Musk AW and Lake RA:
Malignant mesothelioma. Lancet. 366:397–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang W, Wu X, Wu L, Zhang W and Zhao X:
Advances in the diagnosis, treatment and prognosis of malignant
pleural mesothelioma. Ann Transl Med. 3:1822015.PubMed/NCBI
|
|
25
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Agaimy A and Wünsch PH: Epithelioid and
sarcomatoid malignant pleural mesothelioma in endoscopic gastric
biopsies: A diagnostic pitfall. Pathol Res Pract. 202:617–622.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen HC, Tsai KB, Wang CS, Hsieh TJ and
Hsu JS: Duodenal metastasis of malignant pleural mesothelioma. J
Formos Med Assoc. 107:961–964. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang YJ, Wu MH and Lin MT: Multiple
small-bowel intussusceptions caused by metastatic malignant
melanoma. Am J Surg. 196:e1–e2. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eo WK, Kim GY and Choi SI: A case of
multiple intussusceptions in the small intestine caused by
metastatic renal cell carcinoma. Cancer Res Treat. 40:97–99. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Terashita S, Hirano K, Hirai T,
Narabayashi T, Hara Y, Mori H, Endo K and Hirabayashi M: A case of
malignant pleural mesothelioma with gastrointestinal metastases
which were diagnosed by endoscopic biopsy. Nihon Kokyuki Gakkai
Zasshi. 47:133–138. 2009.(In Japanese). PubMed/NCBI
|
|
31
|
Gocho K, Isobe K, Kaburaki K, Honda Y,
Mitsuda A, Akasaka Y, Shimada N, Takagi K and Homma S: Malignant
pleural mesothelioma presenting as an acute surgical abdomen due to
metastatic jejunal perforation. Intern Med. 49:597–601. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu H, Cheng YJ, Chen HP, Hwang JC and
Chang PC: Multiple bowel intussusceptions from metastatic localized
malignant pleural mesothelioma: A case report. World J
Gastroenterol. 16:3984–3986. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martínez Caselles A, Baños Madrid R, Egea
Valenzuela J, Molina Martínez J and Carballo Álvarez F:
Gastrointestinal bleeding secondary to duodenal metastases of
malignant pleural mesothelioma. Rev Esp Enferm Dig. 102:602–603.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de Vin T, Engels B, Gevaert T, Storme G
and De Ridder M: Stereotactic radiotherapy for oligometastatic
cancer: A prognostic model for survival. Ann Oncol. 25:467–471.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alongi F, Arcangeli S, Filippi AR, Ricardi
U and Scorsetti M: Review and uses of stereotactic body radiation
therapy for oligometastases. Oncologist. 17:1100–1107. 2012.
View Article : Google Scholar : PubMed/NCBI
|