Introduction
Multiple myeloma (MM) is a malignancy of plasma
cells in the bone marrow. It is the second most common
hematological cancer, accounting for ~1% of all cancers and >10%
of all hematological neoplastic diseases (1,2). As a
result of its heterogeneous symptoms, the diagnosis of MM tends to
be delayed, with an average median survival time of 3 to 4 years
upon diagnosis. Newer therapeutic modalities such as autologous
stem cell transplantation and the use of drugs, including
thalidomide, lenalidomide and bortezomib, have considerably
improved the clinical outcomes and survival of patients with MM
(1,2).
However, despite these advances in the treatment of MM, it remains
an incurable disease owing to its protean clinical manifestations
and complications.
MM is characterized by uncontrolled bone remodeling
caused by the imbalance between bone resorption and bone formation
resulting from increased osteoclastic activation with concomitant
osteoblast suppression (3). Previous
studies have demonstrated that the activation of the Wnt/β-catenin
signaling pathway has a critical role in both osteoblastogenesis
and osteoclastogenesis (4,5). In addition, aberrant activation of the
Wnt/β-catenin signaling pathway has been shown to be involved in
numerous types of cancers (6,7). In the absence of Wnt ligands, the
downstream effector of the canonical Wnt signal, the transcription
factor β-catenin, is phosphorylated by glycogen synthase kinase
(GSK)-3β. Phosphorylated β-catenin is then subjected to
proteasome-mediated degradation to prevent its accumulation.
Conversely, upon binding of Wnt ligands, the frizzled receptor and
low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6)
form a complex and inhibit β-catenin phosphorylation. β-catenin is
thus stabilized and translocated into the nucleus, where it
associates with T-cell factor to permit DNA binding and the
subsequent regulation of the expression of various target genes,
including c-Myc and cyclin D1 (8–11).
Activation of the Wnt/β-catenin signaling pathway is frequently
observed in MM and some other malignant tumors (6,7). Since
inhibition of Wnt/β-catenin is known to suppress MM progression
(11,12), blockage of the Wnt/β-catenin signaling
pathway may prove to be a novel therapeutic approach.
Apoptosis and autophagy are two types of programmed
cell death mechanisms implicated in cancer suppression (13,14).
Previous studies have shown that inhibition of the Wnt/β-catenin
signaling pathway attenuates survival signals and induces apoptosis
(15,16). Autophagy is a highly conserved pathway
that is activated under stress and plays a pivotal role in a
plethora of physiological processes including cell death (17). Previous studies have documented that
autophagy negatively regulates Wnt signaling by promoting the
degradation of dishevelled under metabolic stress (18,19).
Furthermore, β-catenin silencing induced both apoptotic and
autophagic cell death in squamous cell carcinoma (20). However, the role of β-catenin signal
in regulating autophagy has not been adequately researched.
RNA interference is a promising gene-silencing
approach in the field of cancer therapeutics. In the present study,
β-catenin silencing in MM cells was achieved by infection of the
cells with a lentivirus vector encoding β-catenin-specific small
interfering (si)RNA. The β-catenin-knockdown MM cells were then
analyzed for any changes in the patterns of apoptosis or autophagy
and the underlying mechanisms.
Materials and methods
Cell culture
The RPMI-8826 MM cell line was obtained from Fudan
University Institutes of Biomedical Sciences cell bank, Shanghai,
China. The cells were cultured in RPMI-1640 medium supplemented
with 10% (v/v) fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 1% penicillin/streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C and 5% CO2 in a
humidified incubator.
Development of a β-catenin-deficient
MM cell line
Three siRNA-targeting regions (siA,
CCGGGCTTGGAATGAGACTGCTGATCTCGAGATCAGCAGTCTCATTCCAAGCTTTTT; siB,
CCGGCGCATGGAAGAAATAGTTGAACTCGAGTTCAACTATTTCTTCCATGCGTTTTT; siC,
CCGGAGGTGCTATCTGTCTGCTCTACTCGAGTAGAGCAGACAGATAGCACCTTTTTT) of the
human CTNNB1 gene (encoding the β-catenin protein; GenBank
accession no. NM_001,904) or scramble siRNA (sc,
CCGGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTG) were
designed, synthesized and ligated with the
hU6MCS-Ubiquitin-EGFP-IRES-puromycin vector (Genechem, Shanghai,
China). Lentivirus was packaged by transfection of recombinant
constructs and package plasmids into 293T cells (Genechem) using
Lipofectamine 2000 (Genechem), and the viruses were then harvested
after 48 h of transfection; the multiplicity of infection (MOI) for
the virus was determined using the 293T cells. To calculate the
infection rate, images of 10 random fields of cells were captured
under fluorescent channel and bright field, and the number of green
fluorescent protein (GFP)-positive cells and total cells were
counted, respectively. The infection rate was calculated by
dividing the average number of GFP-positive cells by the average
number of total cells.
To optimize the dose of puromycin required to kill
RPMI-8826 cells, the cells were pretreated with different doses (1,
2.5, 5 or 10 µg/ml) of puromycin (Genechem), after which the dose
of 1 µg/ml puromycin was selected. To establish stable cells
expressing β-catenin siRNA, RPMI-8826 cells (5×103/ml)
were infected with lentiviruses expressing scramble or different
β-catenin siRNAs at a MOI of 100, and the media was replaced after
8 h of infection. Puromycin at a concentration of 1 µg/ml was added
after 72 h of infection, and maintained for 2 weeks. Cell counting
kit (CCK)-8 (Dojindo Molecular Technologies, Inc., Shanghai, China)
assays, western blotting and apoptosis assays were performed on
RPMI-8826 myeloma cells stably expressing the different siRNAs.
CCK-8 assay
RPMI-8826 scramble (8826-sc) and β-catenin siRNA
(8826-siC) cells (5×103 cells/well) 100 µl were seeded
separately in 96-well plates, which had been pre-incubated for 24 h
at 37°C in an atmosphere of CO2. A total of 10 µl CCK-8
solution was added to each well and incubated for 1–4 h. The
absorbance was then measured at a wavelength of 450 nm using a
microplate reader (Thermo Fisher Scientific, Inc.).
Transmission electron microscope (TEM)
analysis
A total of 1 million RPMI-8826 scramble (8826-sc)
and β-catenin siRNA (8826-siC) expressing cells were centrifuged at
11,500 × g for 5 min at 25°C, and fixed with 4% glutaraldehyde for
2 h at room temperature. The cells were washed twice with PBS after
fixing with osmic acid for 1 h at 4°C. The samples were dehydrated
and embedded with epoxy resin, after which 70-nm thick sections
were cut. The sections were stained with uranyl acetate and lead
citrate, and visualized under a TEM (H7650; Hitachi, Ltd., Tokyo,
Japan) at ×20,000 magnification.
Western blotting
The cells were washed thrice with PBS before being
lysed with radioimmunoprecipitation assay buffer (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). Protein
concentrations were determined using the bicinchoninic acid protein
assay kit (Beijing Solarbio Science & Technology Co., Ltd.),
according to the manufacturer's protocol. Equal amounts of protein
lysate (30 µg) were separated by 10% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes. After blocking with 5% skim
milk, the membranes were incubated with primary antibodies
(1:1,000) against β-catenin (cat. no. 9562), microtubule-associated
protein 1 light chain 3 (LC3; cat. no. 3868), B-cell lymphoma
(Bcl)-2 (cat. no. 2872), Bcl-2-associated X protein (Bax; cat. no.
2774), Beclin-1 (cat. no. 3495), phosphorylated-p53 (cat. no.
9284), phosphorylated-mechanistic target of rapamycin (mTOR; cat.
no. 5536), phosphorylated-5′-adenosine monophosphate-activated
protein kinase (AMPK; cat. no. 2535) and AMPK (cat. no. 2532) (all:
Cell Signaling Technology, Inc., Danvers, MA, USA), active
caspase-3 (cat. no. 1476-1; Epitomics, Burlingame, CA, USA) and
β-actin (1:1,000; AA128, Beyotime, Shanghai, China) overnight at
4°C. The same membrane was washed with Tris-buffered saline with
Tween 20 and reblotted with peroxidase-conjugated affinipure goat
anti-rabbit (cat. no. ZB-2301) and anti-mouse (cat. no. 2305) IgG
secondary antibodies (1:10,000; ZSGB-BIO, Beijing, China) for 1 h
at 25°C. For quantitative analysis, the bands were selected and
quantified using ImageJ 1.44 software (National Institutes of
Health, Bethesda, MD, USA), and the data were transformed and
normalized to β-actin.
Apoptosis assay by flow cytometry
The apoptosis of RPMI-8826 cells infected with
lentiviruses encoding scramble or β-catenin-specific siRNAs was
assessed by flow cytometry using the Annexin V/Propidium
iodide-fluorescein isothiocyanate Apoptosis Detection kit (BD
Biosciences, Franklin Lakes, NJ, USA), according to the
manufacturer's protocol. Cells treated with PBS were used as
negative controls.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Student's t-tests were performed using SPSS 11.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered statistically
significant.
Results
Transfection efficacy of β-catenin
siRNA lentivirus in a MM cell line
To evaluate the effect of β-catenin silencing in MM
cells, siRNAs were designed by targeting different regions of
β-catenin. RPMI-8826 MM cells were infected with lentiviruses
expressing scramble siRNA (8826-sc) or three β-catenin siRNAs
(8826-siA, siB and siC), and selected with puromycin.
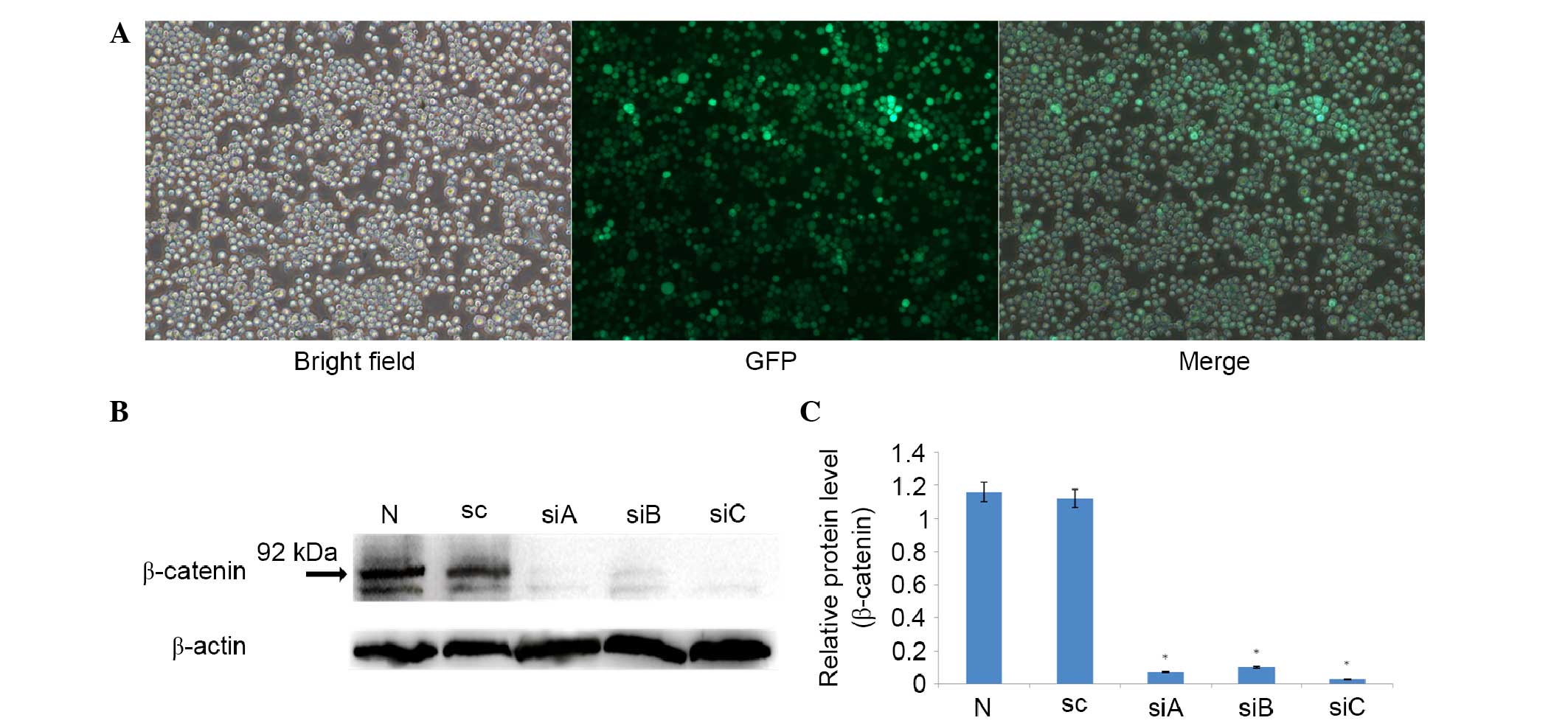
Non-transfected cells were used as a control. As shown in Fig. 1A, ~70% of cells were GFP-positive
after 72 h of infection, whereas no GFP-positive cells were
identified in the control group (data not shown), suggesting that
RPMI-8826 MM cells were successfully infected with the lentiviruses
expressing siRNAs. In addition, to further validate the targeting
of β-catenin by siRNA, stable cells expressing β-catenin siRNA were
harvested for western blot analysis. As shown in Fig. 1B, non-transfected and scramble siRNA
(8826-sc) transfected cells expressed high levels of β-catenin
protein, which is consistent with a previous study (21). Furthermore, all siRNAs had a >90%
knockdown efficacy, as compared with the scramble siRNA (8826-sc)
(P<0.05; Fig. 1B and C). However,
siC showed slightly more effective silencing than siA and siB, and
the difference was significant (P=0.017 for siA vs. siC; P=0.003
for siB vs. siC; Fig. 1C). Therefore,
siC was selected for the subsequent analyses. The slightly
different knockdown efficacies among these three siRNAs may have
occurred as a result of different target regions and infection
rates.
β-catenin silencing decreases the
viability of MM cells
CCK-8 assays were performed to evaluate the
anti-proliferative effect of β-catenin silencing on MM cells. Cell
viabilities of 8826-sc and 8826-siC cells were monitored. As shown
in Fig. 2, the cell viability was
decreased by 16.4% in 8826-siC cells compared with 8826-sc cells,
and the difference was significant (P=0.019).
Autophagy is induced in
β-catenin-deficient MM cells
After confirming the successful silencing of
β-catenin in MM cells using siRNA, the present study determined
whether autophagy was induced as a result of β-catenin silencing.
Upon TEM analysis to assess cytoplasmic changes in 8826-siC and
8826-sc cells, autophagic vacuoles (round enclosed compartments)
were observed in the former but not in the latter cells (Fig. 3A-C). To further confirm
β-catenin-deficiency-induced autophagy, the protein expression
levels of LC3 and Beclin-1, which are indicators of autophagosome
formation and activation of the autophagy pathway (22), were examined. β-catenin silencing
induced LC3-I (18 kDa) cleavage into LC3-II (16 kDa), resulting in
increased LC3-II and decreased LC-I expression (Fig. 3D and E). β-catenin silencing also
significantly increased the levels of Beclin-1 protein (P<0.05;
Fig. 3F and G). These results suggest
that β-catenin silencing in myeloma cell lines induces
autophagy.
Involvement of the AMPK/mTOR pathway
in β-catenin knockdown-induced autophagy
The present study investigated whether the AMPK/mTOR
signaling pathway is involved in the activation of autophagy by
β-catenin silencing. In 8826-siC cells, the expression levels of
both phosphorylated-AMPK and total AMPK were significantly
increased, as compared with 8826-sc cells (P<0.05; Fig. 4A and B), while the expression levels
of phosphorylated-mTOR were significantly decreased in 8826-siC
cells compared with 8826-sc cells (P<0.05; Fig. 4A and B). These results suggest that
the AMPK/mTOR pathway is activated by β-catenin silencing and may
be involved in the activation of autophagy.
β-catenin silencing increases the
apoptosis of RPMI-8826 cells
The present study determined whether apoptosis was
induced by knockdown of β-catenin in a MM cell line. To determine
the activation of apoptosis, the Annexin V/propidium iodide (PI)
assay was performed to analyze 8826-siC and 8826-sc cells by flow
cytometry. As shown in Fig. 5A,
12.2±0.93 and 6.30±1.67% of the 8826-siC cells were Annexin
V+/PI− and Annexin
V+/PI+, respectively, which was markedly
higher than 8826-sc cells (2.60±1.57% Annexin
V+/PI− and 3.50±0.48% Annexin
V+/PI+), suggesting that apoptosis was
induced by β-catenin silencing in myeloma cells. Subsequently, the
protein expression of apoptosis-related proteins, including p53,
active caspase-3, Bax and Bcl-2, was evaluated. As shown in
Fig. 4B, β-catenin silencing resulted
in increased protein expression of phosphorylated-p53, active
caspase-3 and Bax, and decreased protein expression of Bcl-2
(P<0.001; Fig. 5B). Together,
these findings suggest that β-catenin silencing promoted apoptosis
in MM cells probably via the mitochondrial apoptotic pathway.
 | Figure 5.Activation of apoptosis following
silencing of β-catenin. (A) Annexin V and PI staining followed by
flow cytometry demonstrated increased apoptosis of MM cells
following β-catenin knockdown. (B) Western blotting demonstrated
that the protein expression levels of p-p53, active caspase-3 and
Bax were increased, and those of Bcl-2 were decreased, in MM cells
following β-catenin knockdown. *P<0.001, **P<0.01 and
***P<0.05 vs. SC. MM, multiple myeloma; PI, propidium iodide;
p-p53, phosphorylated-p53; Bcl-2, B-cell lymphoma-2; Bax, B-cell
lymphoma-2-associated X protein; SC, RPMI-8826 cells infected with
lentivirus carrying scramble siRNA; siA-C, RPMI-8826 cells infected
with lentiviruses carrying β-catenin-specific siRNAs; siRNA, small
interfering RNA. |
Discussion
MM is an incurable disease characterized by
malignant proliferation of plasma cells in the bone marrow. The
canonical Wnt/β-catenin signaling pathway, which is related to MM
cell growth, survival and migration, is highly active in MM cells
(4,11). The present study aimed to investigate
the role of β-catenin in apoptosis and autophagy in MM cells by
silencing β-catenin using a lentivirus vector carrying
β-catenin-specific siRNA. The results of the present study
demonstrated that both apoptosis and autophagy were induced by
β-catenin silencing, suggesting its value as a potential
therapeutic target in the treatment of MM.
The canonical Wnt/β-catenin signaling pathway is
recurrently aberrant in several types of cancer, including MM
(22). Binding of Wnt ligands to
frizzed and LRP5/6 receptors induces the suppression of GSK-3β
activity and accumulation of β-catenin in the nucleus, leading to
activation of downstream target genes such as c-Myc and cyclin D1
(8–11). A previous study demonstrated β-catenin
expression in nearly all primary MM cells, whereas it was absent in
normal plasma cells (23). Several
studies have shown that treatment with Wnt ligand or GSK-3β
inhibitor significantly increased the accumulation of β-catenin and
MM cell proliferation, indicating highly activated Wnt/β-catenin
signaling in MM cells (23,24). In the present study, the effect of
β-catenin knockdown in human RPMI-8826 MM cells was examined. High
expression of β-catenin was detected in the MM cells prior to
silencing, which was consistent with a previous study (21). However, significantly decreased
protein expression levels of β-catenin were observed following
β-catenin silencing, suggesting that β-catenin silencing may
present a promising strategy for cancer therapy.
Autophagy is a lysosomal degradation pathway that
has a critical role in cell survival and differentiation. It serves
as an adaptive mechanism to protect organisms in response to
stresses or pathological states such as infection and cancer
(25). Previous studies have
demonstrated that the mTOR inhibitor rapamycin and nutrient
deficiencies attenuate Wnt signaling, while knockdown of the
autophagy effectors, LC3 or Beclin-1, increases Wnt-induced
transcriptional activity, suggesting that Wnt signaling
downregulates autophagy (18,26). These findings suggest an important
role for the Wnt/β-catenin signaling pathway in the process of
autophagy. To the best of our knowledge, this is the first study to
report an association between Wnt/β-catenin signaling and autophagy
regulation in MM cells. In the present study, β-catenin induced
autophagy in MM cells, as evidenced by the presence of autophagic
vacuoles and increased expression of LC3 and Beclin-1. In addition,
the expression of mTOR, a key negative regulator of autophagy
(27), was decreased. Autophagy is
promoted by AMPK, which is a key energy sensor and regulates
cellular metabolism to maintain energy homeostasis (28). The present study demonstrated that
AMPK was activated by β-catenin silencing, indicating its
involvement in the activation of autophagy.
In addition to autophagy, a pro-apoptotic effect of
silencing β-catenin in MM cells was observed. β-catenin silencing
increased the protein expression of phosphorylated-p53, the
pro-apoptotic protein Bax and active caspase-3, while it decreased
the expression of the anti-apoptotic protein, Bcl-2. These findings
suggested that β-catenin silencing promoted apoptosis in MM cells,
potentially via activation of the mitochondrial apoptotic pathway.
The role of β-catenin in the regulation of cell proliferation and
apoptosis differs in different cell types. For example, β-catenin
accumulation induces the proliferation and survival of some tumor
cells (29,30), which is similar to the findings of the
present study. Conversely, in hematopoietic progenitor cells,
activation of β-catenin induces apoptosis via the mitochondrial
pathway (31). There is a complex
relationship between autophagy and apoptosis, which may vary in
different biological contexts (25).
Both can share overlapping functions, or have modifying effects on
each other (25). In the present
study, parallel activation of autophagy and apoptosis by β-catenin
silencing in MM cells, both of which are related to cell death, was
observed.
In conclusion, the present study demonstrated that
β-catenin silencing induced autophagy as well as apoptosis in MM
cells. Therefore, inhibition of β-catenin may be considered a
promising strategy for the treatment of MM. Further studies are
required to further delineate the specific pathways underlying the
activation of autophagy and apoptosis in β-catenin-knockdown MM
cells.
Acknowledgements
This study was supported by Liaoning Bureau of
Science and Technology Science and Technology program (grant no.
2013021031).
References
|
1
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hideshima T, Mitsiades C, Tonon G,
Richardson PG and Anderson KC: Understanding multiple myeloma
pathogenesis in the bone marrow to identify new therapeutic
targets. Nat Rev Cancer. 7:585–598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roodman GD: Pathogenesis of myeloma bone
disease. Leukemia. 23:435–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qiang YW, Chen Y, Brown N, Hu B, Epstein
J, Barlogie B and Shaughnessy JD Jr: Characterization of
Wnt/beta-catenin signalling in osteoclasts in multiple myeloma. Br
J Haematol. 148:726–738. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Houben A, Kostanova-Poliakova D,
Weissenböck M, Graf J, Teufel S, von der Mark K and Hartmann C:
β-catenin activity in late hypertrophic chondrocytes locally
orchestrates osteoblastogenesis and osteoclastogenesis.
Development. Sep 12–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morin PJ: Beta-catenin signaling and
cancer. Bioessays. 21:1021–1030. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. Sep 12–2016.(Epub ahead of print).
View Article : Google Scholar
|
|
8
|
Zhang B, Abreu JG, Zhou K, Chen Y, Hu Y,
Zhou T, He X and Ma JX: Blocking the Wnt pathway, a unifying
mechanism for an angiogenic inhibitor in the serine proteinase
inhibitor family. Proc Natl Acad Sci USA. 107:6900–6905. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Macdonald BT, Semenov MV and He X:
SnapShot: Wnt/beta-catenin signaling. Cell. 131:12042007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qi W, Yang C, Dai Z, Che D, Feng J, Mao Y,
Cheng R, Wang Z, He X, Zhou T, et al: High levels of pigment
epithelium-derived factor in diabetes impair wound healing through
suppression of wnt signaling. Diabetes. 64:1407–1419. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sukhdeo K, Mani M, Zhang Y, Dutta J, Yasui
H, Rooney MD, Carrasco DE, Zheng M, He H, Tai YT, et al: Targeting
the beta-catenin/TCF transcriptional complex in the treatment of
multiple myeloma. Proc Natl Acad Sci USA. 104:7516–7521. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fulciniti M, Tassone P, Hideshima T,
Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT,
Chauhan D, et al: Anti-DKK1 mAb (BHQ880) as a potential therapeutic
Agent for multiple myeloma. Blood. 114:371–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsujimoto Y and Shimizu S: Another way to
die: Autophagic programmed cell death. Cell Death Differ. 12:(Supp
2). S1528–S1534. 2005. View Article : Google Scholar
|
|
14
|
Bursch W, Ellinger A, Gerner C, Frohwein U
and Schulte-Hermann R: Programmed cell death (PCD). apoptosis,
autophagic PCD, or others? Ann N Y Acad Sci. 926:1–12. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S, Guttridge DC, You Z, Zhang Z,
Fribley A, Mayo MW, Kitajewski J and Wang CY: Wnt-1 signaling
inhibits apoptosis by activating beta-catenin/T cell
factor-mediated transcription. J Cell Biol. 152:87–96. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hseu YC, Thiyagarajan V, Tsou HT, Lin KY,
Chen HJ, Lin CM, Liao JW and Yang HL: In vitro and in vivo
anti-tumor activity of CoQ0 against melanoma cells: inhibition of
metastasis and induction of cell-cycle arrest and apoptosis through
modulation of Wnt/β-catenin signaling pathways. Oncotarget.
7:22409–22426. 2016.PubMed/NCBI
|
|
17
|
Puissant A, Robert G and Auberger P:
Targeting autophagy to fight hematopoietic malignancies. Cell
Cycle. 9:3470–3478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao C, Cao W, Bao L, Zuo W, Xie G, Cai T,
Fu W, Zhang J, Wu W, Zhang X and Chen YG: Autophagy negatively
regulates Wnt signalling by promoting dishevelled degradation. Nat
Cell Biol. 12:781–790. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang HW, Lee YS, Nam HY, Han MW, Kim HJ,
Moon SY, Jeon H, Park JJ, Carey TE, Chang SE, et al: Knockdown of
beta-catenin controls both apoptotic and autophagic cell death
through LKB1/AMPK signaling in head and neck squamous cell
carcinoma cell lines. Cell Signal. 25:839–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ashihara E, Kawata E, Nakagawa Y,
Shimazaski C, Kuroda J, Taniguchi K, Uchiyama H, Tanaka R, Yokota
A, Takeuchi M, et al: Beta-catenin small interfering RNA
successfully suppressed progression of multiple myeloma in a mouse
model. Clin Cancer Res. 15:2731–2738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Derksen PW, Tjin E, Meijer HP, Klok MD,
MacGillavry HD, van Oers MH, Lokhorst HM, Bloem AC, Clevers H,
Nusse R, et al: Illegitimate WNT signaling promotes proliferation
of multiple myeloma cells. Proc Natl Acad Sci USA. 101:6122–6127.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takada K, Zhu D, Bird GH, Sukhdeo K, Zhao
JJ, Mani M, Lemieux M, Carrasco DE, Ryan J, Horst D, et al:
Targeted disruption of the BCL9/β-catenin complex inhibits
oncogenic Wnt signaling. Sci Transl Med. 4:148ra1172012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sukhdeo K, Mani M, Hideshima T, Takada K,
Pena-Cruz V, Mendez G, Ito S, Anderson KC and Carrasco DR:
Beta-catenin is dynamically stored and cleared in multiple myeloma
by the proteasome-aggresome-autophagosome-lysosome pathway.
Leukemia. 26:1116–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jung CH, Ro SH, Cao J, Otto NM and Kim DH:
mTOR regulation of autophagy. FEBS Lett. 584:1287–1295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tetsu O and McCormick F: Beta-catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
He TC, Sparks AB, Rago C, Hermeking H,
Zawel L, da Costa LT, Morin PJ, Vogelstein B and Kinzler KW:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ming M, Wang S, Wu W, Senyuk V, Le Beau
MM, Nucifora G and Qian Z: Activation of Wnt/beta-catenin protein
signaling induces mitochondria-mediated apoptosis in hematopoietic
progenitor cells. J Biol Chem. 287:22683–22690. 2012. View Article : Google Scholar : PubMed/NCBI
|