Introduction
Conventional doxorubicin is used actively in various
malignant tumors, however, it produces a number of serious side
effects, including cardiotoxicity and myelosuppression (1). A new form of this chemotherapeutic agent
enclosed in pegylated liposomes was developed to reduce these organ
toxicities (1). Liposome
encapsulation prevents doxorubicin from penetration to compartments
with tight endothelial cells junctions and facilitates its
distribution to tissues with abnormal blood vessels (1). This results in higher drug accumulation
within the tumor when compared with normal tissues (2). Consequently, a decreased incidence of
cardiac and hematological toxicity is observed. Pegylated liposomal
doxorubicin (PLD) has the ability to deposit itself within the skin
and to induce specific mucocutaneous reactions. There are six types
of PLD-related dermal disorders, and the most common is
palmar-plantar erythrodysesthesia (PPE). Other, less frequent
manifestations are intertrigo-like dermatitis, a diffuse follicular
rash, a maculopapular rash, melanotic macules or a recall
phenomenon (3). The symptoms of PPE
develop usually within 2 to 12 days after the infusion of
chemotherapy (4). Initially,
dysesthesia, erythema or edema of the palms and plantae is noticed.
These symptoms may progress to desquamation, blistering and
ulceration. The soles are less often affected than the palms
(5).
The current study presents the case of a patient
with advanced ovarian cancer treated with PLD who developed severe
hand-foot syndrome and a diffuse maculopapular rash, which is
rarely reported in the literature. Complete resolution of the skin
lesions was observed after 4 weeks. Due to ovarian cancer
progression, the patient was disqualified from further
chemotherapy.
Case report
A 55-year-old patient without any relevant medical
history underwent suboptimal cytoreductive surgery involving a
hysterectomy, bilateral salpingo-oophorectomy, omentectomy and
appendectomy in November 7, 2011 at the Polish Mother's Memorial
Hospital Research Institute (Lodz, Poland), and was accordingly
diagnosed with stage IIIC ovarian cancer based on the
tumor-node-metastasis classification criteria (6). The patient received 6 cycles of
intravenous paclitaxel (175 mg/m2) and carboplatin [area
under the curve (AUC), 5], administered every 3 weeks, and then
follow-up surgery with cervical amputation. The disease was
considered to be in complete remission until November 2012, when
rapid progression with accompanying intestinal obstruction was
observed. The patient underwent ileostomy formation, and due to
significant loss in body weight, started parenteral nutrition.
Subsequently, from January to June 2013, 2 cycles of cisplatin (70
mg/m2 every 3 weeks) were administered, followed by 6
cycles of carboplatin (AUC, 5). Cisplatin was discontinued due to
renal insufficiency. A partial response to chemotherapy was
observed. During this time, no skin toxicity was noted. When the
ovarian cancer progressed again, therapy with 50 mg/m2
PLD administered every 4 weeks was initiated. No prevention
strategies for PPE were implemented. At 3 weeks after the second
cycle of chemotherapy, the patient developed a rash localized on
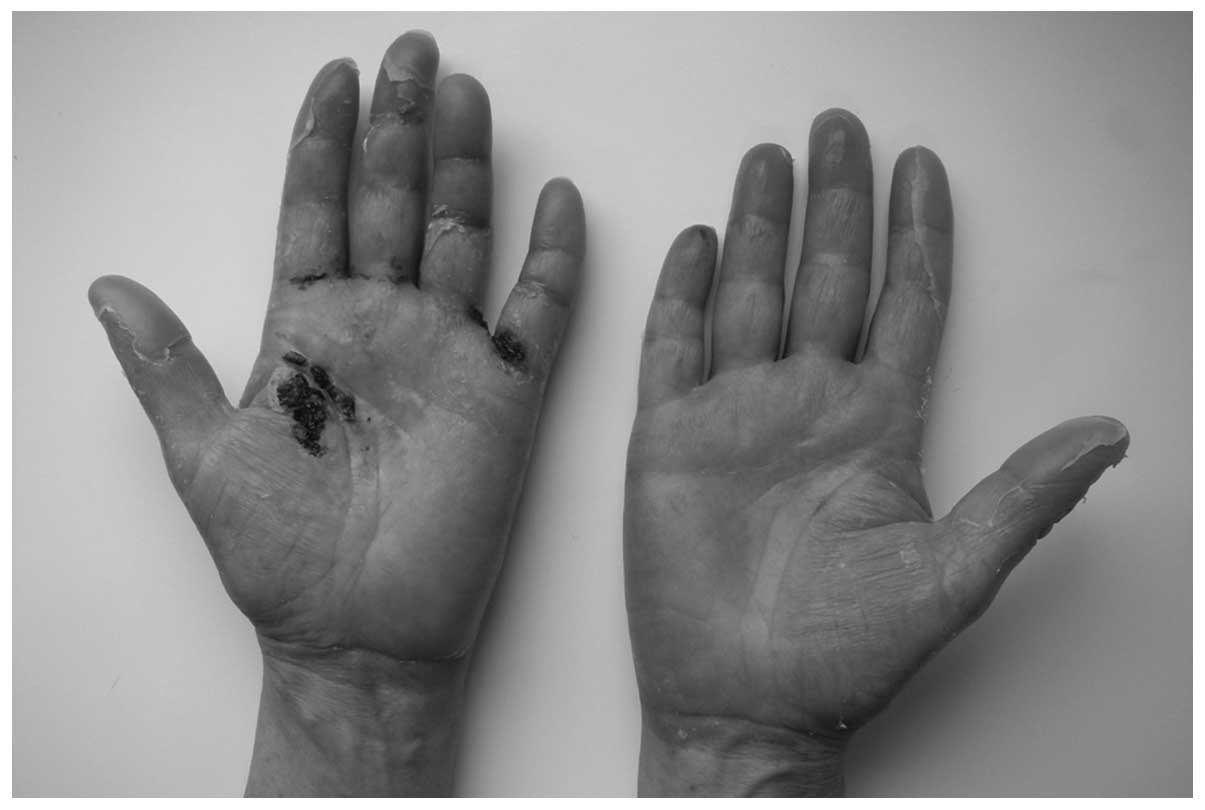
the trunk and severe skin lesions on the hands. Dermatological
evaluation revealed painful desquamative erythema with ulceration
on the palms (Fig. 1) and mild
erythema on the soles. Non-pruriginous, non-painful maculopapular
eruption accompanied by peeling was present on the trunk (Fig. 2). Oral mucous membranes and other
areas of the skin were not affected. The patient was classified
with grade 3 PPE (according to the basic scale from the Common
Terminology Criteria for Adverse Events, version 4), as
difficulties were exhibited in self-care activities, and a grade 3
maculopapular rash (7). Upon
admission on October 25, 2013, the patient was apyretic and in a
good general condition (Eastern Cooperative Oncology Group
performance status 2), with the main complaint being of pain due to
ulcerative cutaneous lesions. No previous episodes of drug
allergies were reported. The patient started 100 mg tramadol and
100 mg doxycycline, administered twice daily, and prophylactic
antifungal treatment with 50 mg fluconazole, administered daily.
Amelioration of the skin lesions was observed after 5 days of
therapy, and complete regression was apparent after 4 weeks. In
November 2013, there was a sudden deterioration in the patient's
general condition. Follow-up abdominal ultrasound and laboratory
blood tests [carbohydrate antigen-125, 4,495 U/ml (normal range,
<35 U/ml); and bilirubin, 4.3 mg/dl (normal range, 0.3–1.2
mg/dl)] revealed dynamic progression of the malignancy. The patient
was therefore disqualified from further chemotherapy and referred
to a palliative care specialist. The patient succumbed to cancer
progression in December 2013.
Discussion
Dermal toxicity is the most common adverse reaction
limiting PLD therapy. Skin lesions usually appear in regions prone
to trauma, such as the palms and soles. PPE of any grade is
observed in up to 50% of individuals treated with PLD, while grade
3 is noted in ~20% of patients (when using a PLD dose of 50
mg/m2 every 4 weeks) (8).
Less frequently intertriginous areas, such as axillary folds, are
affected. The maculopapular rash present in the current patient has
rarely been reported in the literature (9–11).
The pathophysiology of this cutaneous syndrome is
widely debated. It is presumed that drug excretion in sweat and
local microtrauma are responsible for the development of PPE
(12). Certain data have indicated
that PLD may penetrate through the damaged vessels and impair
keratinocytes, which are particularly susceptible to anticancer
drugs (13). An elevated PLD
concentration found in the skin of the palms and plantae supports
the hypothesis that the chemotherapeutic agent is excreted in the
sweat. Jacobi et al (14)
reported the appearance of PPE only in patients with hyperhidrosis
of these regions. Another hypothesis is that PPE develops due to an
excessive concentration of toxic doxorubicin within the skin and
its reaction with metal ions (particularly copper ions) (15). An underlying mechanism for the
development of other skin disorders is poorly known. Skelton et
al (9), on the basis of the late
outbreak of dermal lesions and lymphocytic inflammation affecting
keratinocytes found in the skin biopsies of 3 patients with
PLD-induced maculopapular rash, suggested the possibility of
host-vs.-altered-host reaction as a key factor responsible for the
development of cutaneous syndromes. Optimal management of
PLD-related skin reactions remains undefined. It may appear that
numerous clinical trials have been performed, but in fact, the
majority of them have limited value (5). Preventive approaches for PPE, including
administration of moisturizers, regional cooling of the skin, and
avoidance of excessive activities associated with overheating or
trauma, have been evaluated in non-randomized trials (4,16). The use
of topical antiperspirant with a beneficial effect has also been
reported in the literature (17).
Pyridoxine appeared to be a promising agent for the prevention of
PPE, but in randomized controlled trials, it proved to be
ineffective (18,19). In a meta-analysis conducted by Macedo
et al (16), celecoxib
exclusively demonstrated a 53% risk reduction (odds ratio, 0.47;
95% confidence interval, 0.29–0.78; P=0.003) of any grade PPE.
Certain studies have indicated that dimethyl sulfoxide (20) or corticosteroids (21,22) may be
beneficial in the treatment of PLD-induced dermal complications, as
they accelerate skin recovery, but in fact, the only
well-established preventive management includes dose intensity
modification or complete chemotherapy discontinuation (11).
In conclusion, apart from PPE, other skin toxicities
associated with PLD treatment are less frequent and not well known.
The aforementioned prophylactic and curative strategies for
PLD-induced dermal toxicity require further investigation, and
their usage in routine clinical practice is unsupported. As
mucocutaneous side effects are an important cause of PLD dose
modification or treatment withdrawal, it is essential to conduct
prospective randomized controlled clinical trials in order to
strictly define the preventive and curative management of this
complication.
Acknowledgements
This study was supported by a grant from the
Chemotherapy Clinic of the Medical University of Lodz (no. UM
501/1-034-02/501-91-263).
References
|
1
|
Mangana J, Zipser MC, Conrad C, Oberholzer
PA, Cozzio A, Knuth A, French LE and Dummer R: Skin problems
associated with pegylated liposomal doxorubicin - more than
palmoplantar erythrodysesthesia syndrome. Eur J Dermatol.
18:566–570. 2008.PubMed/NCBI
|
|
2
|
Green AE and Rose PG: Pegylated liposomal
doxorubicin in ovarian cancer. Int J Nanomedicine. 1:229–239.
2006.PubMed/NCBI
|
|
3
|
Cady FM, Kneuper-Hall R and Metcalf JS:
Histologic patterns of polyethylene glycol-liposomal
doxorubicin-related cutaneous eruptions. Am J Dermatopathol.
28:168–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farr KP and Safwat A: Palmar-plantar
erythrodysesthesia associated with chemotherapy and its treatment.
Case Rep Oncol. 4:229–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
von Moos R, Thuerlimann BJ, Aapro M,
Rayson D, Harrold K, Sehouli J, Scotte F, Lorusso D, Dummer R,
Lacouture ME, et al: Pegylated liposomal doxorubicin-associated
hand-foot syndrome: Recommendations of an international panel of
experts. Eur J Cancer. 44:781–790. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sobin LH, Gospodarowicz MK and Wittekind
C: Ovary cancerTNM Classification of Malignant Tumours. 7th.
Wiley-Blackwell; Hoboken, NJ: pp. 222–226. 2009
|
|
7
|
National Cancer Institute, . Common
Terminology Criteria for Adverse Events (CTCAE) version 4.03.
http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdfAccessed
June 14, 2010.
|
|
8
|
Lorusso D, Di Stefano A, Carone V, Fagotti
A, Pisconti S and Scambia G: Pegylated liposomal
doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot’
syndrome). Ann Oncol. 18:1159–1164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Skelton H, Linstrum J and Smith K:
Host-vs.-altered-host eruptions in patients on liposomal
doxorubicin. J Cutan Pathol. 29:148–153. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
English JC III, Toney R and Patterson JW:
Intertriginous epidermal dysmaturation from pegylated liposomal
doxorubicin. J Cutan Pathol. 30:591–595. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vidal C, Afonzo Y, Abal C and Rondón M: A
grade IV maculopapular skin lesion associated with pegylated
liposomal doxorubicin. MOJ. 2:57–59. 2012.
|
|
12
|
Martschick A, Sehouli J, Patzelt A,
Richter H, Jacobi U, Oskay-Ozcelik G, Sterry W and Lademann J: The
pathogenetic mechanism of anthracycline-induced palmar-plantar
erythrodysesthesia. Anticancer Res. 29:2307–2313. 2009.PubMed/NCBI
|
|
13
|
Kim RJ, Peterson G, Kulp B, Zanotti KM and
Markman M: Skin toxicity associated with pegylated liposomal
doxorubicin (40 mg/m2) in the treatment of gynecologic cancers.
Gynecol Oncol. 97:374–378. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jacobi U, Waibler E, Schulze P, Sehouli J,
Oskay-Ozcelik G, Schmook T, Sterry W and Lademann J: Release of
doxorubicin in sweat: First step to induce the palmar-plantar
erythrodysesthesia syndrome? Ann Oncol. 16:1210–1211. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yokomichi N, Nagasawa T, Coler-Reilly A,
Suzuki H, Kubota Y, Yoshioka R, Tozawa A, Suzuki N and Yamaguchi Y:
Pathogenesis of hand-foot syndrome induced by PEG-modified
liposomal doxorubicin. Hum Cell. 26:8–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Macedo LT, Lima JP, dos Santos LV and
Sasse AD: Prevention strategies for chemotherapy-induced hand-foot
syndrome: A systematic review and meta-analysis of prospective
randomised trials. Support Care Cancer. 22:1585–1593.
2014.PubMed/NCBI
|
|
17
|
Templeton AJ, Ribi K, Surber C, Sun H, Hsu
Schmitz SF, Beyeler M, Dietrich D, Borner M, Winkler A, Müller A,
et al: Prevention of palmar-plantar erythrodysesthesia with an
antiperspirant in breast cancer patients treated with pegylated
liposomal doxorubicin (SAKK 92/08). Breast. 23:244–249. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang YK, Lee SS, Yoon DH, Lee SY, Chun YJ,
Kim MS, Ryu MH, Chang HM, Lee JL and Kim TW: Pyridoxine is not
effective to prevent hand-foot syndrome associated with
capecitabine therapy: Results of a randomized, double-blind,
placebo-controlled study. J Clin Oncol. 28:3824–3829. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
von Gruenigen V, Frasure H, Fusco N,
DeBernardo R, Eldermire E, Eaton S and Waggoner S: A double-blind,
randomized trial of pyridoxine versus placebo for the prevention of
pegylated liposomal doxorubicin-related hand-foot syndrome in
gynecologic oncology patients. Cancer. 116:4735–4743. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lopez AM, Wallace L, Dorr RT, Koff M,
Hersh EM and Alberts DS: Topical DMSO treatment for pegylated
liposomal doxorubicin-induced palmar-plantar erythrodysesthesia.
Cancer Chemother Pharmacol. 44:303–306. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Drake RD, Lin WM, King M, Farrar D, Miller
DS and Coleman RL: Oral dexamethasone attenuates Doxil-induced
palmar-plantar erythrodysesthesias in patients with recurrent
gynecologic malignancies. Gynecol Oncol. 94:320–324. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Najem A, Deregnaucourt D, Ramdane S,
Dridba M, Djouba F and Vercambre-Darras S: Intertrigo-like
dermatitis with pegylated liposomal doxorubicin: Diagnosis and
management. J Clin Oncol. 32:e104–e106. 2014. View Article : Google Scholar : PubMed/NCBI
|