Introduction
Cervical cancer is a frequent cause of
cancer-associated mortality in females worldwide (1). The current treatments for cervical
cancer vary, and the treatment of locally advanced uterine cervical
cancer, including International Federation of Gynecology and
Obstetrics (FIGO) (2) stage IIIA (in
which the tumor involves the lower third of the vagina with no
extension to the pelvic wall), IIIB (in which the tumor extends to
the pelvic wall and causes hydronephrosis or a nonfunctioning
kidney) and IVA (in which the tumor invades the mucosa of the
bladder or rectum, and extends beyond the true pelvis) is
challenging as locally advanced cervical cancer is inoperable
(3,4).
Concurrent chemoradiotherapy is the standard treatment for locally
advanced uterine cervical cancer (3,4); however,
the prognosis of patients is typically poor, with 5-year survival
rates of ~60% (5,6). A previous study reported that prognosis
may improve if neoadjuvant chemotherapy (NAC) is successful in
treating locally advanced uterine cervical cancer (7). However, the prognosis worsens if NAC is
not effective, as hysterectomy cannot be performed if downstaging
by NAC is not achievable. Subsequently changing the treatment plan
from surgery to radiotherapy may result in a fatal delay for the
patient (8,9).
The availability of specific predictive markers for
the effectiveness of NAC would facilitate the identification of
patients with positive predictive markers and allow them to be
effectively treated with NAC. However, no significant predictive
markers of NAC efficacy are currently available for locally
advanced uterine cervical cancer. Thus, it is essential to identify
such markers (10–14).
Epidermal growth factor-like domain 7 (EGFL7) is a
secreted signaling factor derived from endothelial cells (15). The EGFL7 gene encodes a 30-kDa protein
that includes a signal peptide and two epidermal growth factor-like
domains, and is located on chromosomes 2 and 9 in mice and humans,
respectively (16). EGFL7 is
upregulated during angiogenesis and is involved in the regulation
of endothelial cell migration during the sprouting process, blood
vessel lumen formation and maintenance of vascular integrity
(17–19). In certain types of human cancer cells,
EGFL7 inhibits the activity of endothelial cell adhesion molecules
[such as vascular cell adhesion molecule 1 (VCAM1) and
intercellular adhesion molecule 1 (ICAM1)], which leads to
decreased vascular tightness (increased vascular permeability),
increased immune evasion and increased tumor growth (20). Decreased blood vessel integrity is
frequently observed in malignant tumors (21). Furthermore, poor perfusion of tissues
inhibits drug delivery in malignant tumors; abnormal vasculature of
tumors and the resulting abnormal microenvironment together pose a
formidable barrier to the delivery and efficacy of cancer therapy.
A previous study revealed that EGFL7 is highly expressed in human
epithelial tumor tissues, including hepatocellular carcinoma, lung
cancer, breast cancer, gastric cancer, colorectal cancer, prostate
cancer, esophageal cancer, malignant glioma, ovarian cancer and
renal cancer (22). Certain studies
have reported that high expression levels of EGFL7 are associated
with lymph node metastasis, poor prognosis and advanced disease
stage in numerous types of human cancer, including colon cancer,
pancreatic cancer, epithelial ovarian cancer and laryngeal squamous
cell cancer (23–26). Luo et al (27) reported that the expression of EGFL7 in
gastric cancer cells promotes the epithelial-to-mesenchymal
transition (EMT) and tumor metastasis by epidermal growth factor
receptor (EGFR)-mediated protein kinase B (AKT) phosphorylation,
which subsequently activates Snail and suppresses the transcription
of E-cadherin. Numerous studies have demonstrated that increased
EMT may lead to chemoresistance in bladder, lung, breast and
ovarian cancers (28–32). In addition, Vega et al
(33) reported that Snail inhibits
the cell cycle and confers resistance to TGF-β-induced cell death,
consistent with Snail activating the Mek/Frk and PI3K/Akt survival
pathways.
To the best of our knowledge, the present study is
the first to examine EGFL7 expression in uterine cervical cancer
cells. It was hypothesized that EGFL7 may have a role in the
development of acquired chemoresistance through lowered drug
delivery, increased EMT and increased cell resistance to apoptosis
due to the downstream activation of Snail. EGFL7 may also be a
prognostic factor for the efficacy of NAC in locally advanced
cervical cancer. Therefore, the current study investigated whether
EGFL7 expression levels are associated with the efficacy of NAC for
locally advanced uterine cervical cancer.
Materials and methods
Patients and samples
Eligible patients were aged <70 years, had
histologically confirmed primary stage IIIA and IIIB cancer of the
uterine cervix, and underwent NAC; the patients were treated at the
Osaka City University Medical School Hospital (Osaka, Japan)
between 1995 and 2010. Exclusion criteria included the lack of
informed consent, the unavailability of the tumor samples, and the
patient not undergoing NAC. The tumor samples were obtained through
a punch biopsy prior to the administration of NAC. Patients were
divided into groups 1 and 2 depending on the effectiveness of NAC.
Group 1 consisted of those patients who effectively responded to
NAC, resulting in downstaging to an operable condition, and who
subsequently underwent surgery and radiotherapy (n=36). Group 2
consisted of those patients who were unsuccessfully treated with
NAC, resulting in ineligibility for hysterectomy, and who
subsequently received radiotherapy only (n=27). Written informed
consent was obtained from all patients prior to the tumor biopsy.
The Ethics Committee of Osaka City University approved the current
study protocol (IRB no. 2859, 3078).
Balloon-occluded arterial infusion
chemotherapy (BOAI) for NAC
A pelvic angiography was performed under local
anesthesia (34) in order to locate
the tumor and the feeding vessels. A balloon-wedge single-pressure
catheter was inserted (5F, 80 cm in length; Dispomedica, Hamburg,
Germany) from each femoral artery into the internal iliac artery;
the balloon catheters were advanced until in proximity to the
feeding vessel, which was usually the uterine artery, and then
inflated in order to occlude the local blood flow.
Cis-diamminedichloro-platinum (CDDP; Bristol-Myers Squibb Company,
New York, NY, USA) was intra-arterially infused through the two
catheters over 30 min (34). In the
current study, the two ovarian arteries were blocked following the
initial BOAI procedure in order to increase the concentration of
CDDP near the tumor. BOAI was performed three times to reduce the
size of the tumor. Abundant hydration, for the preservation of
renal function, was performed prior to and following CDDP
administration, and antiemetics and diuretics were used
appropriately. CDDP was administered at doses of 50, 75 or 100
mg/m2, according to the patient's age and renal
function. Magnetic resonance imaging (MRI) was used to assess the
rate of tumor reduction by evaluating its maximum dimensions
(35,36).
Immunohistochemical analysis
The expression patterns of EGFL7 and Snail were
examined in paraffin-embedded tissue sections using a monoclonal
mouse anti-human EGFL7 antibody (#sc-101349; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; dilution, 1:500), a
polyclonal rabbit anti-human Snail antibody (#13099-1-AP;
ProteinTech Group, Inc., Chicago, IL, USA; dilution, 1:100) and a
Dako LSAB2 peroxidase kit (#K0675; Dako Japan Co., Ltd., Tokyo,
Japan), according to the manufacturer's instructions.
Paraffin-embedded tissue sections (4 µm) were deparaffinized,
rehydrated and immersed in 3% hydrogen peroxide for 10 min at room
temperature to block endogenous peroxidase activity. An antigen
retrieval procedure was performed by immersing the tissue sections
in 10 mM citrate buffer (pH 6.0) and heating in an autoclave at
110°C for 20 min. The tissue sections were then washed in
phosphate-buffered saline (PBS). The tissue sections were incubated
overnight with anti-EGFL7 or anti-Snail primary antibodies in a
humidity chamber at 4°C, washed with PBS for 15 min, and then
incubated for 10 min with biotinylated goat anti-mouse and
anti-rabbit immunoglobulin G secondary antibodies (Dako Japan Co.,
Ltd). The tissue sections were then incubated with a
streptavidin-peroxidase complex and 3,3′-diaminobenzidine was used
as the chromogen. The sections were counterstained with Mayer's
hematoxylin and the specificity of the immunohistochemical
reactions was determined by omitting the primary antibody. The
expression levels of EGFL7 or Snail were quantitatively analyzed
using the scoring method of Sinicrope et al (37). Five individual fields were imaged for
each tissue specimen (magnification, ×400). The tissues were
classified into five categories according to the mean proportion of
positive tumor cells, as follows: 0, <5%; 1, 5–25%; 2, 25–50%;
3, 50–75%; 4, >75%. The intensity of the immunostaining was
classified into three categories, as follows: 1+, weak; 2+,
moderate; 3+, intense. The weighted score was calculated by
multiplying the percentage of positive tumor cells by the staining
intensity for each tissue specimen.
Statistical analysis
The data are presented as the mean ± standard
deviation. Kaplan-Meier and log-rank tests were performed for
prognostic analysis, based on overall survival rates. The weighted
scores were compared using the Mann-Whitney U test. The Student's
t-test and the χ2 test were performed as appropriate for
intergroup comparisons. SPSS software version 21.0 (IBM SPSS,
Armonk, NY, USA) was used for all statistical analyses. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient clinical characteristics
A total of 35 patients were assigned to group 1, and
27 to group 2, with mean ages of 49.3 years (range, 24–69 years)
and 52.3 years (range, 36–68 years), respectively. Of the patients
in group 1, 1 patient was diagnosed with FIGO stage IIIA uterine
cervical cancer and 34 patients were diagnosed with stage IIIB
disease. Of the patients in group 2, all 27 were diagnosed with
stage IIIB uterine cervical cancer. Histologically, the 35 patients
of group 1 comprised 30 cases of squamous cell carcinoma and 5 of
adenocarcinoma. In the patients of group 2, 22 had squamous cell
carcinomas, 3 had adenocarcinomas, 1 had adenosquamous carcinoma
and 1 had glassy cell carcinoma. There were no significant
differences observed between the two groups with regard to any of
the recorded clinical characteristics (Table I).
 | Table I.Clinical characteristics of patients
in group 1 (NAC+OP+R) and group 2 (NAC+R). |
Table I.
Clinical characteristics of patients
in group 1 (NAC+OP+R) and group 2 (NAC+R).
| Characteristic | NAC+OP+R | NAC+R | P-value |
|---|
| No. of patients | 35 | 27 |
|
| Age (years) |
|
| 0.322a |
| Mean ±
SD | 49.3±12.7 | 52.3±11.1 |
|
|
Range | 24–69 | 36–68 |
|
| FIGO stage |
|
| 0.376b |
| IIIA | 1 | 0 |
|
| IIIB | 34 | 27 |
|
| Histology |
|
| 0.433b |
|
SCC | 30 | 22 |
|
| A | 5 | 3 |
|
| AS | 0 | 1 |
|
|
Other | 0 | 1c |
|
| Tumor size (mm;
mean ± SD) | 46.9±17.2 | 53.7±14.9 | 0.144a |
Expression profile of EGFL7 in uterine
cervical cancer tissues
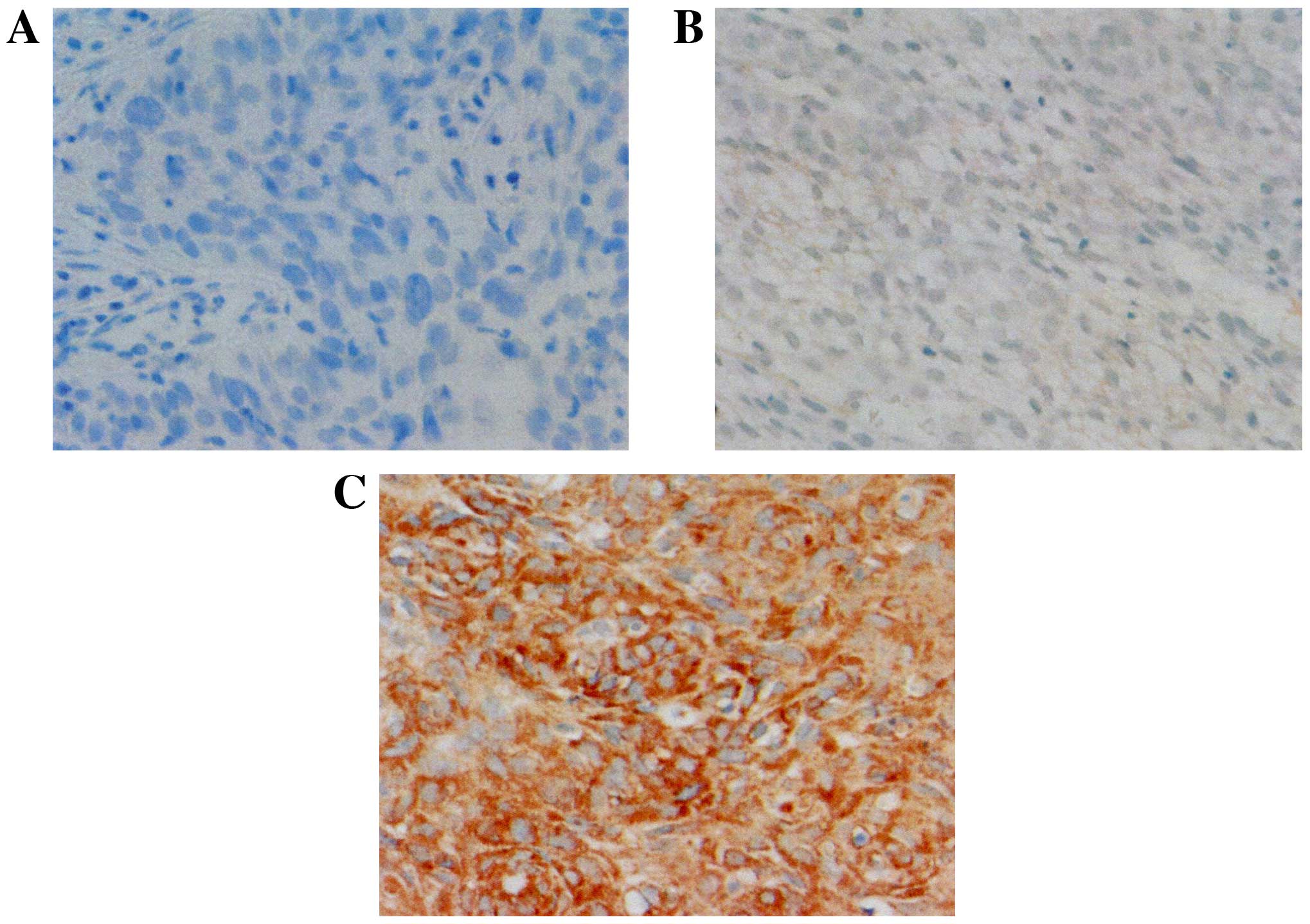
The expression of EGFL7 was observed in the
cytoplasm of the tumor cells (Fig.
1). Table II presents the
weighted scores in the tissues of patients from group 1 and group
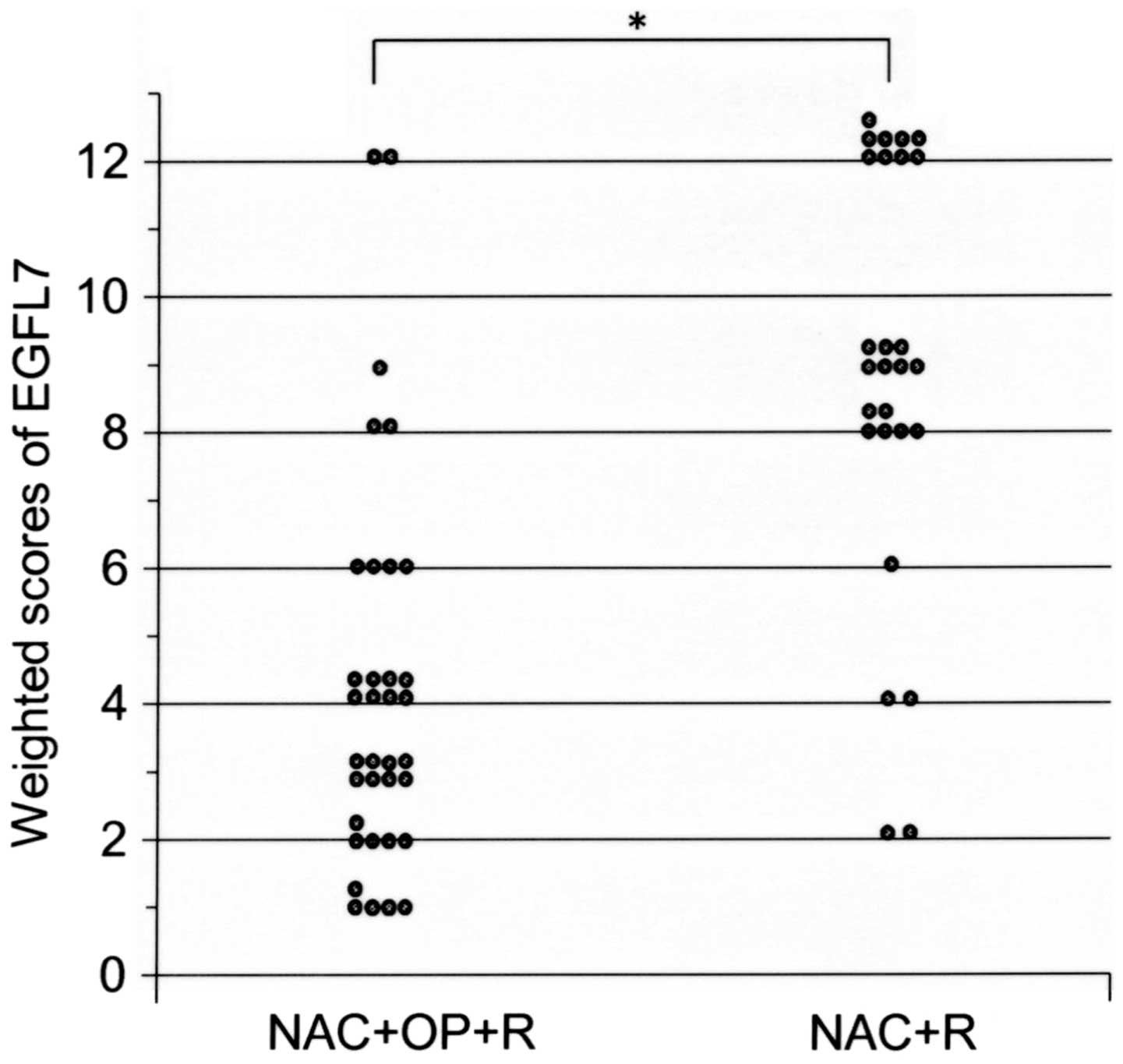
2. The mean weighted score of EGFL7 expression was significantly
lower in group 1, as compared with in group 2 (4.1 vs. 8.8;
P<0.001; Fig. 2). Of all the
patients involved, 35 exhibited low expression levels of EGFL7,
with a weighted score of ≤6, whereas 27 patients exhibited high
expression levels of EGFL7, with a weighted score of 8–12. There
were no significant differences in clinical characteristics
observed between the low EGFL7 expression group and the high EGFL7
expression group (Table III).
 | Table II.Weighted scores for epidermal growth
factor-like domain 7 expression levels in group 1 (NAC+OP+R) and
group 2 (NAC+R). |
Table II.
Weighted scores for epidermal growth
factor-like domain 7 expression levels in group 1 (NAC+OP+R) and
group 2 (NAC+R).
|
| No. of
patients |
|---|
|
|
|
|---|
| Weighted score | NAC+OP+R
(n=35) | NAC+R (n=27) |
|---|
| 0 | 0 | 0 |
| 1 | 5 | 0 |
| 2 | 5 | 2 |
| 3 | 8 | 0 |
| 4 | 8 | 2 |
| 6 | 4 | 1 |
| 8 | 2 | 6 |
| 9 | 1 | 7 |
| 12 | 2 | 9 |
| Mean score | 4.11 | 8.78 |
 | Table III.Characteristics of the patients in
the low and high EGFL7 expression groups. |
Table III.
Characteristics of the patients in
the low and high EGFL7 expression groups.
| Characteristic | Low EGFL7
expression (≤6) | High EGFL7
expression (≥8) | P-value |
|---|
| No. of
patients | 35 | 27 |
|
| Age (years) |
|
| 0.144a |
| Mean ±
SD | 48.6±12.7 | 53.1±10.8 |
|
|
Range | 24–69 | 37–68 |
|
| FIGO stage |
|
| 0.251b |
|
IIIA | 0 | 1 |
|
|
IIIB | 35 | 26 |
|
| Histology |
|
| 0.585b |
|
SCC | 28 | 24 |
|
| A | 6 | 2 |
|
| AS | 1 | 0 |
|
|
Other | 0 | 1c |
|
| Tumor size (mm;
mean ± SD) | 47.0±15.9 | 54.0±17.3 | 0.144a |
Association between the expression of
EGFL7 and the efficacy of NAC
Among the 35 patients with low EGFL7 expression, 30
patients (86%) were in group 1 and 5 patients (14%) were in group
2. With regard to high EGFL7 expression, 5/27 patients (19%) were
in group 1, while 22/27 (81%) patients were in group 2. This
indicates that NAC was significantly more effective in the low
EGFL7 expression group as compared with the high EGFL7 expression
group (P<0.001; Table IV).
 | Table IV.Number of patients with low and high
EGFL7 expression levels in group 1 (NAC+OP+R) and group 2
(NAC+R). |
Table IV.
Number of patients with low and high
EGFL7 expression levels in group 1 (NAC+OP+R) and group 2
(NAC+R).
| EGFL7
expression | NAC+OP+R | NAC+R | P-value |
|---|
| Low (≤6) | 30 (86%) | 5
(14%) |
<0.001a |
| High (≥8) | 5
(19%) | 22 (81%) |
|
Expression profile of Snail in uterine
cervical cancer tissues
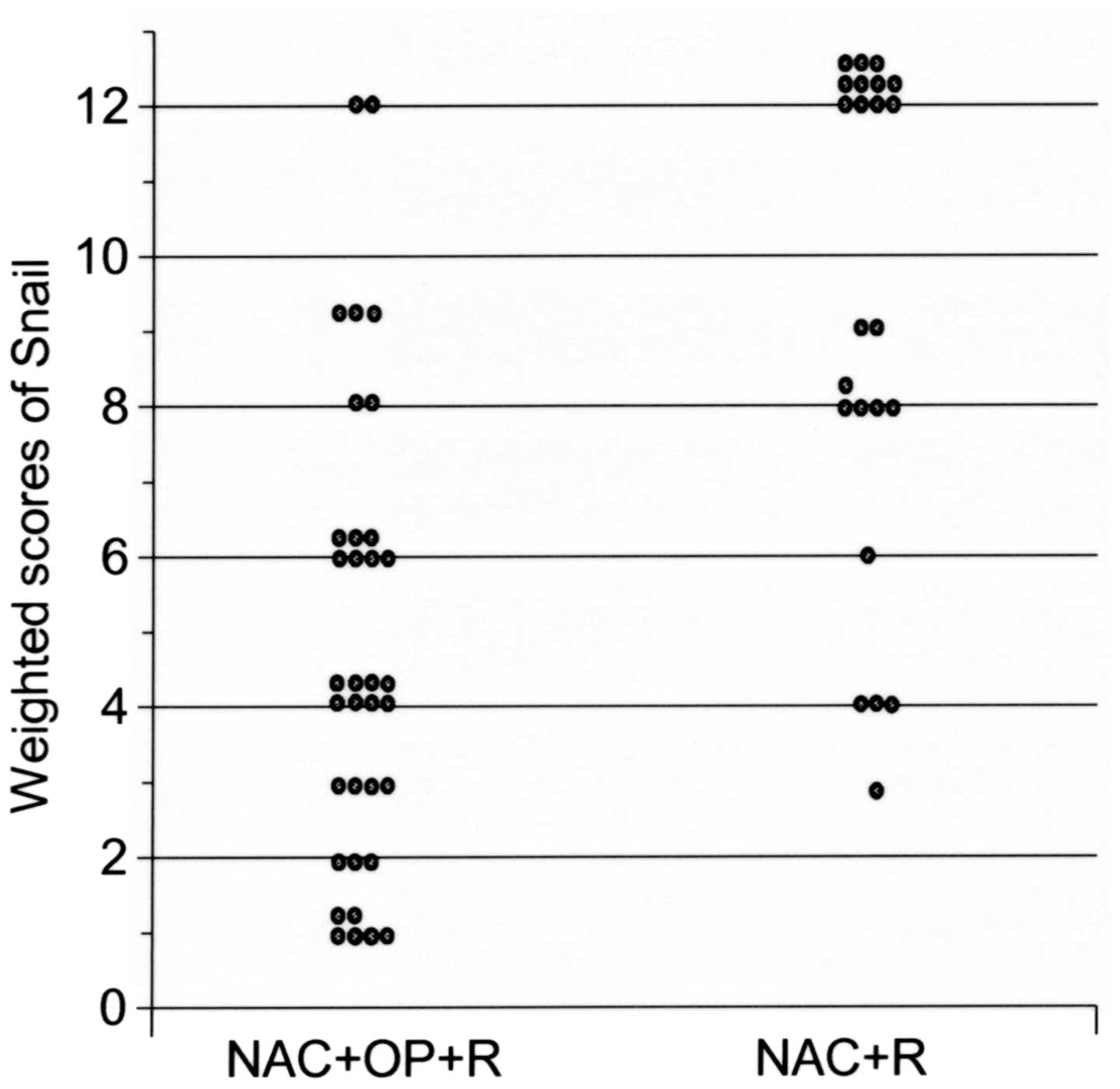
The expression of Snail was observed in the nuclei
of the tumor cells. The weighted expression scores are presented in
Table V. The mean weighted score for
Snail expression levels was significantly lower in group 1, as
compared with in group 2 (4.7 vs. 9.4; P<0.001; Fig. 3).
 | Table V.Weighted scores for Snail expression
levels in group 1 (NAC+OP+R) and group 2 (NAC+R). |
Table V.
Weighted scores for Snail expression
levels in group 1 (NAC+OP+R) and group 2 (NAC+R).
|
| No. of
patients |
|---|
|
|
|
|---|
| Weighted score | NAC+OP+R
(n=35) | NAC+R (n=27) |
|---|
| 0 | 0 | 1 |
| 1 | 6 | 0 |
| 2 | 3 | 0 |
| 3 | 4 | 1 |
| 4 | 8 | 3 |
| 6 | 7 | 0 |
| 8 | 2 | 5 |
| 9 | 3 | 2 |
| 12 | 2 | 15 |
| Mean score | 4.71 | 9.37 |
Correlation between the expression of
EGFL7 and Snail
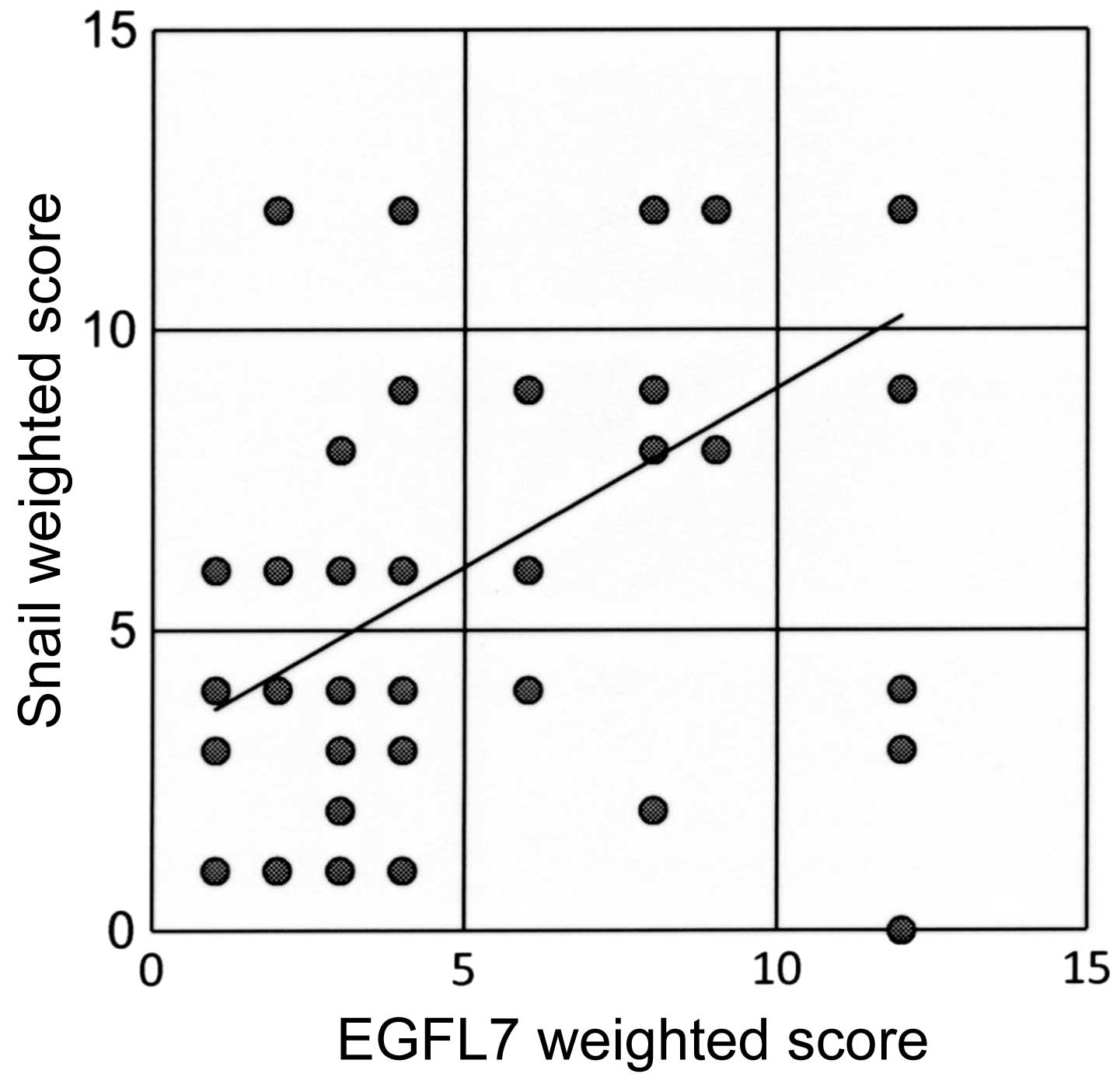
Out of all the patients involved, 29 exhibited low
expression levels of EGFL7 and of Snail, and 23 patients had high
expression levels of EGFL7 and of Snail. A significant positive
association was observed between the expression levels of EGFL7 and
Snail (r=0.56; P<0.001; Fig.
4).
Overall survival analysis
Kaplan-Meier and log-rank analyses indicated that
the overall survival time was significantly longer in group 1
(patients who responded to NAC; mean, 3,257 days), as compared with
group 2 (mean, 1,239 days; P=0.001; Fig.
5). The overall survival time was also significantly longer in
the low EGFL7 expression level group, as compared with the high
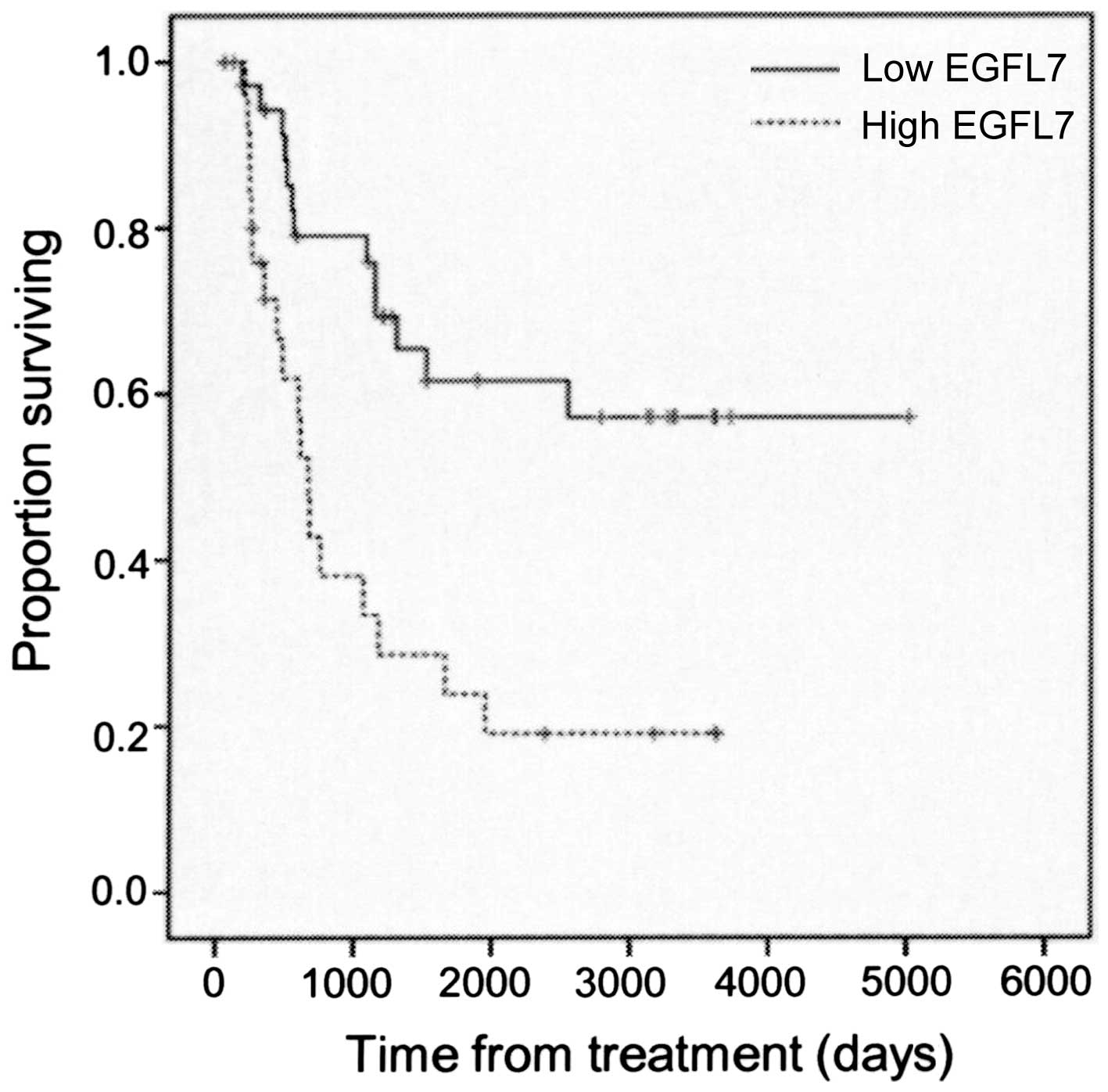
EGFL7 expression level group (P=0.001; Fig. 6).
Discussion
The results of the present study demonstrated a
significant association between the expression levels of EGFL7 and
the effectiveness of NAC in patients with locally advanced uterine
cervical cancer at FIGO stage IIIA and IIIB; NAC was determined to
be unsuccessful in the majority of patients with high EGFL7
expression levels, who were thus unable to undergo surgery and
received only radiotherapy. Overall survival was significantly
longer in group 1 patients (those for whom NAC had been
successful), as compared with group 2. These results are concordant
with previous studies, which demonstrated that patient prognosis
worsens when NAC is unsuccessful (8,9).
Similarly, overall survival time was significantly longer in the
low EGFL7 expression level group, as compared with the high EGFL7
expression level group. In addition, 30 patients (86%) in group 1
exhibited low EGFL7 expression levels. These results suggest that
patients with low EGFL7 expression levels were more likely to
respond well to NAC, compared with patients with high EGFL7
expression levels, indicating that EGFL7 may be a predictive marker
for determining whether NAC is likely to be effective in individual
patients with advanced uterine cervical cancer.
EGFL7 is a secreted signaling factor derived from
endothelial cells that controls blood vessel formation (15); this protein is upregulated during
angiogenesis and is associated with blood vessel lumen formation,
vascular integrity and the regulation of the collective migration
of endothelial cells during the blood vessel sprouting process
through the restriction of their spatial distribution (17–19). In
certain types of human cancer cells, EGFL7 suppresses the
activation of endothelial cells and inhibits the expression of
specific endothelial adhesion molecules (VCAM1 and ICAM1); it also
reduces the adhesion of lymphocytes on the endothelium and
consequently decreases vascular tightness (20).
The present study was conducted based on three
hypotheses regarding the association between EGFL7 expression and
tumor cell chemoresistance. The first hypothesis was that high
expression levels of EGFL7 may have a role in the development of
chemoresistance due to decreased drug delivery caused by reduced
vascular integrity in tumors tissues. The second hypothesis was
that the promotion of EMT by EGFL7 may induce the development of
tumor cell resistance to chemotherapy; EMT is regulated by
EGFR-mediated AKT phosphorylation induced by EGFL7, which then
activates Snail and the subsequent suppression of E-cadherin
transcription (27). Numerous studies
have previously reported that EMT may have a function in acquired
chemoresistance (28–32). The third hypothesis considered that
EGFL7 may have a role in the development of tumor chemoresistance
through an increased resistance to cell apoptosis induced by the
activation of Snail. It has previously been reported that Snail is
able to inhibit the cell cycle and confer resistance to
TGF-β-induced cell death, consistent with Snail activating the
Mek/Frk and PI3K/Akt survival pathways (33). Thus, EGFL7 and Snail expression
profiles were examined in the current study and a positive
correlation was identified between them.
To the best of our knowledge, the present study is
the first to report a significant association between EGFL7
expression levels and the response of locally advanced uterine
cervical cancer to NAC. The results indicate that NAC may be more
effective in patients with low EGFL7 expression levels, compared
with those patients with high EGFL7 expression levels; therefore,
EGFL7 expression levels may be a potential predictive marker of NAC
efficacy in patients with locally advanced uterine cervical cancer.
As high EGFL7 expression is associated with acquired
chemoresistance, tumor cells that exhibit lower EGFL7 expression
may have a greater sensitivity to NAC treatment. In addition, a
correlation between EGFL7 and Snail expression levels was observed
These results were concordant with a previous study that reported
that EGFL7 is able to positively regulate EMT by EGFR-mediated AKT
phosphorylation through the activation of Snail expression
(27). The results of the current
study support the hypotheses that the overexpression of EGFL7 may
have a role in the development of tumor chemoresistance through
EMT, and increasing resistance to cell apoptosis by activating
Snail expression; therefore, EGFL7 may be an effective prognostic
factor regarding the efficacy of NAC treatment for locally advanced
uterine cervical cancer.
Although a previous study demonstrated that surgery
following NAC in locally advanced uterine cervical cancer is an
effective treatment and improves the patient prognosis (7), NAC is not currently recommended as a
standard treatment as, if NAC is not effective, subsequent surgery
is difficult to perform and radiotherapy is required (3,4);
radiotherapy following the administration of chemotherapy may lead
to a poorer prognosis than radiotherapy alone (8,9).
Therefore, it is crucial to identify novel predictive markers of
NAC efficacy in patients with locally advanced uterine cervical
cancer.
In summary, the results of the current study suggest
that EGFL7 expression levels may be a predictive marker of NAC
effectiveness for the treatment of patients with locally advanced
uterine cervical cancer. Our previous study reported that the
protein expression levels of B-cell lymphoma (Bcl)-extra large,
Bcl-associated X-protein, mitotic arrest deficiency 2 and sirtuin 1
may be useful predictive markers of the effectiveness of NAC for
patients with locally advanced uterine cervical cancer (12,38,39). Using
a combination of these signaling proteins, the efficacy of NAC may
potentially be predicted for patients with locally advanced uterine
cervical cancer.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Japan Society of Gynecologic Oncology
(eds), . Formulation Committee of the Treatment Guidelines for
Cervical Cancer. Kanehara & Co.; Tokyo: 2011, (In
Japanese).
|
|
4
|
National Comprehensive Cancer Network
(NCCN), . NCCN Clinical Practice Guidelines in Oncology: Cervical
Cancer. Version II. NCCN; Fort Washington, PA: 2013
|
|
5
|
Morris M, Eifel PJ, Lu J, Grigsby PW,
Levenback C, Stevens RE, Rotman M, Gershenson DM and Mutch DG:
Pelvic radiation with concurrent chemotherapy compared with pelvic
and para-aortic radiation for high-risk cervical cancer. N Engl J
Med. 340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eifel PJ, Winter K, Morris M, Levenback C,
Grigsby PW, Cooper J, Rotman M, Gershenson D and Mutch DG: Pelvic
irradiation with concurrent chemotherapy versus pelvic and
para-aortic irradiation for high-risk cervical cancer: An update of
radiation therapy oncology group trial (RTOG) 90–01. J Clin Oncol.
22:872–880. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishiko O, Sumi T, Yasui T, Matsumoto Y,
Kawamura N, Ogita S, Kamino T, Nakamura K and Yamada R:
Balloon-occluded arterial infusion chemotherapy, simple total
hysterectomy and radiotherapy as a useful combination-therapy for
advanced cancer of the uterine cervix. Oncol Rep. 7:141–144.
2000.PubMed/NCBI
|
|
8
|
Souhami L, Gil RA, Allan SE, Canary PC,
Araújo CM, Pinto LH and Silveira TR: A randomized trial of
chemotherapy followed by pelvic radiation therapy in stage IIIB
carcinoma of the cervix. J Clin Oncol. 9:970–977. 1991.PubMed/NCBI
|
|
9
|
Tattersall MH, Lorvidhaya V, Vootiprux V,
Cheirsilpa A, Wong F, Azhar T, Lee HP, Kang SB, Manalo A, Yen MS,
et al: Randomized trial of epirubicin and cisplatin chemotherapy
followed by pelvic radiation in locally advanced cervical cancer.
Cervical cancer study group of the Asian Oceanian clinical oncology
association. J Clin Oncol. 13:444–451. 1995.PubMed/NCBI
|
|
10
|
Ishiko O, Sumi T, Yasui T, Matsumoto Y,
Ogita S, Kaminou T, Nakamura K and Yamada R: Tumor marker and MR
imaging criteria for evaluating the efficacy of cyclic
balloon-occluded arterial infusion for advanced cancer of the
uterine cervix. Oncol Rep. 7:827–830. 2000.PubMed/NCBI
|
|
11
|
Ishiko O, Sumi T, Yoshida H, Ogita S and
Yamada R: Expression of apoptosis regulatory proteins in advanced
cancer of the uterine cervix after cyclic balloon-occluded arterial
infusion chemotherapy. Int J Oncol. 18:1151–1155. 2001.PubMed/NCBI
|
|
12
|
Okamoto E, Sumi T, Misugi F, Nobeyama H,
Hattori K, Yoshida H, Matsumoto Y, Yasui T, Honda K and Ishiko O:
Expression of apoptosis-related proteins in advanced uterine
cervical cancer after balloon-occluded arterial infusion
chemotherapy as an indicator of the efficiency of this therapy. Int
J Mol Med. 15:41–47. 2005.PubMed/NCBI
|
|
13
|
Nobeyama H, Sumi T, Misugi F, Okamoto E,
Hattori K, Matsumoto Y, Yasui T, Honda K, Iwai K and Ishiko O:
Association of HPV infection with prognosis after neoadjuvant
chemotherapy in advanced uterine cervical cancer. Int J Mol Med.
14:101–105. 2004.PubMed/NCBI
|
|
14
|
Panici P Benedetti, Bellati F, Manci N,
Pernice M, Plotti F, Di Donato V, Calcagno M, Zullo MA, Muzii L and
Angioli R: Neoadjuvant chemotherapy followed by radical surgery in
patients affected by FIGO stage IVA cervical cancer. Ann Surg
Oncol. 14:2643–2648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parker LH, Schmidt M, Jin SW, Gray AM,
Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, et al:
The endothelial-cell-derived secreted factor Egfl7 regulates
vascular tube formation. Nature. 428:754–758. 2014. View Article : Google Scholar
|
|
16
|
Soncin F, Mattot V, Lionneton F, Spruyt N,
Lepretre F, Begue A and Stehelin D: VE-statin, an endothelial
repressor of smooth muscle cell migration. EMBO J. 22:5700–5711.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Campagnolo L, Leahy A, Chitnis S,
Koschnick S, Fitch MJ, Fallon JT, Loskutoff D, Taubman MB and
Stuhlmann H: EGFL7 is a chemoattractant for endothelial cells and
is up-regulated in angiogenesis and arterial injury. Am J Pathol.
167:275–284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmidt M, Paes K, De Mazière A, Smyczek
T, Yang S, Gray A, French D, Kasman I, Klumperman J, Rice DS and Ye
W: EGFL7 regulates the collective migration of endothelial cells by
restricting their spatial distribution. Development. 134:2913–2923.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Charpentier MS and Conlon FL: Cellular and
molecular mechanisms underlying blood vessel lumen formation.
Bioessays. 36:251–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Delfortrie S, Pinte S, Mattot V, Samson C,
Villain G, Caetano B, Lauridant-Philippin G, Baranzelli MC,
Bonneterre J, Trottein F, et al: Egfl7 promotes tumor escape from
immunity by repressing endothelial cell activation. Cancer Res.
71:7176–7186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan C, Yang LY, Wu F, Tao YM, Liu LS,
Zhang JF, He YN, Tang LL, Chen GD and Guo L: The expression of
Egfl7 in human normal tissues and epithelial tumors. Int J Biol
Markers. 28:71–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Díaz R, Silva J, García JM, Lorenzo Y,
García V, Peña C, Rodríguez R, Muñoz C, García F, Bonilla F and
Domínguez G: Deregulated expression of miR-106a predicts survival
in human colon cancer patients. Genes Chromosomes Cancer.
47:794–802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou L, Li J, Zhao YP, Guo JC, Cui QC,
Zhou WX, Zhang TP, Wu WM, You L and Shu H: Prognostic significance
of epidermal growth factor-like domain 7 in pancreatic cancer.
Hepatobiliary Pancreat Dis Int. 13:523–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh J, Park SH, Lee TS, Oh HK, Choi JH and
Choi YS: High expression of epidermal growth factor-like domain 7
is correlated with poor differentiation and poor prognosis in
patients with epithelial ovarian cancer. J Gynecol Oncol.
25:334–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li JJ, Yang XM, Wang SH and Tang QL:
Prognostic role of epidermal growth factor-like domain 7 protein
expression in laryngeal squamous cell carcinoma. J Laryngol Otol.
125:1152–1157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo BH, Xiong F, Wang JP, Li JH, Zhong M,
Liu QL, Luo GQ, Yang XJ, Xiao N and Xie B: Epidermal growth
factor-like domain-containing protein 7 (EGFL7) enhances EGF
receptor-AKT signaling, epithelial-mesenchymal transition, and
metastasis of gastric cancer cells. PLoS One. 9:e999222014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haslehurst AM, Koti M, Dharsee M, Nuin P,
Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, et al: EMT
transcription factors snail and slug directly contribute to
cisplatin resistance in ovarian cancer. BMC Cancer. 12:912012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Işeri OD, Kars MD, Arpaci F, Atalay C, Pak
I and Gündüz U: Drug resistant MCF-7 cells exhibit
epithelial-mesenchymal transition gene expression pattern. Biomed
Pharmacother. 65:40–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Helleman J, Smid M, Jansen MP, van der
Burg ME and Berns EM: Pathway analysis of gene lists associated
with platinum-based chemotherapy resistance in ovarian cancer: The
big picture. Gynecol Oncol. 117:170–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang TH, Tsai MF, Su KY, Wu SG, Huang CP,
Yu SL, Yu YL, Lan CC, Yang CH, Lin SB, et al: Slug confers
resistance to the epidermal growth factor receptor tyrosine kinase
inhibitor. Am J Respir Crit Care Med. 183:1071–1079. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McConkey DJ, Choi W, Marquis L, Martin F,
Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al: Role
of epithelial-to-mesenchymal transition (EMT) in drug sensitivity
and metastasis in bladder cancer. Cancer Metastasis Rev.
28:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vega S, Morales AV, Ocaña OH, Valdés F,
Fabregat I and Nieto MA: Snail blocks the cell cycle and confers
resistance to cell death. Genes Dev. 18:1131–1143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsuji K, Yamada R, Kawabata M, Mitsuzane
K, Sato M, Iwahashi M, Kitayama S and Nakano R: Effect of balloon
occluded arterial infusion of anticancer drugs on the prognosis of
cervical cancer treated with radiation therapy. Int J Radiat Oncol
Biol Phys. 32:1337–1345. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sironi S, Belloni C, Taccagni G and
DelMaschio A: Invasive cervical carcinoma: MR imaging after
preoperative chemotherapy. Radiology. 180:719–722. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim KH, Lee BH, Do YS, Chin SY, Park SY,
Kim BG and Jang JJ: Stage IIb cervical carcinoma: MR evaluation of
effect of intraarterial chemotherapy. Radiology. 192:61–65. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
38
|
Morishita M, Sumi T, Nakano Y, Teramae M,
Fukuda T, Nobeyama H, Yoshida H, Matsumoto Y, Yasui T and Ishiko O:
Expression of mitotic-arrest deficiency 2 predicts the efficacy of
neoadjuvant chemotherapy for locally advanced uterine cervical
cancer. Exp Ther Med. 3:341–346. 2012.PubMed/NCBI
|
|
39
|
Teramae M, Fukuda T, Wada T, Kawanishi M,
Imai K, Yamauchi M, Yasui T and Sumi T: Sirtuin1 expression
predicts the efficacy of neoadjuvant chemotherapy for locally
advanced uterine cervical cancer. Mol Clin Oncol. 3:73–78.
2015.PubMed/NCBI
|