Introduction
Colorectal cancer (CRC) is the third most common
cancer in males and the second in females, respectively, with
>1.4 million newly diagnosed cases and 693,933 mortalities
estimated to have occurred in 2012, accounting for 8.5% of all
cancer mortalities, thus making CRC the fourth most common cause of
mortality from cancer worldwide (1).
Approximately 20% of CRC patients present with metastases at the
time of diagnosis, and ~50% of the patients without metastases at
presentation exhibit distant metastases within 3 years of diagnosis
(2). For patients with unresectable
metastatic CRC, prognosis is poor, with a 5-year survival of
<10%; however, a marked benefit in median overall survival may
be achieved with palliative systemic therapy (3). Significant advances in systemic
treatment for metastatic CRC, including targeted therapies, have
improved survival; however, even with combination of target agents
and chemotherapy, the median survival of CRC patients is only ~29
months (4). A better understanding of
the factors that lead to tumor progression and metastasis is
urgently required for the development of novel strategies for CRC
treatment.
The Notch pathway is highly conserved and functions
in numerous biological processes, including cell differentiation,
proliferation and death (5–7). Mammals have four types of membrane-bound
Notch receptors (Notch 1–4) and five types of membrane bound
ligands (Jagged 1–2, and Delta-like 1, 3 and 4) (2). Upon ligand binding, Notch receptors
undergo proteolytic cleavage to release the Notch intracellular
domain, which enters the nucleus and associates with DNA binding
proteins to act as a transcriptional factor for the regulation of
gene transcription.
Notch pathway signaling is activated in several
types of cancer, including CRC, T-cell acute lymphoblastic leukemia
(T-ALL) (8) and breast cancer
(9). Among the Notch ligands,
upregulation of JAG2 expression has been shown to be significantly
associated with vascular development, metastasis-free and overall
survival in breast cancer patients (10,11). In
ovarian carcinoma, elevated JAG2 levels are reported to be
associated with lymph node and distant metastases (12). These findings are indicative of a
pivotal role for JAG2 expression during cancer progression.
However, there is extremely limited information available with
regard to the expression pattern and functional role of JAG2
protein in human CRC. Therefore, the present study aimed to
investigate the expression and function of JAG2 in human CRC. JAG2
protein expression was assessed in 40 cases of human CRC tissues
and 7 human colon cell lines, while its functions were studied by
RNA interference in 3 CRC cell lines. The effects of JAG2 silencing
on the cellular functions of CRC cell lines were assessed by wound
healing assay, Matrigel invasion assay and cell growth assay.
Materials and methods
Cell lines and tissues
The human CRC cell lines SW480, SW620, HCT116,
DLD-1, HT29 and RKO, and a normal colon cell line, CCD-18Co, were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and cultured in RPMI-1640 medium supplemented
with 10% fetal bovine serum (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C in a humidified incubator with 5%
CO2.
Formalin-fixed, paraffin-embedded colorectal
carcinoma specimens were obtained from the Department of Pathology
of Queen Elizabeth Hospital (Hong Kong SAR, China). Approval from
an institutional ethics review board and informed consent from all
participants were obtained.
Immunohistochemical (IHC)
staining
Sections (4-µm thick) were de-waxed, rehydrated and
stained for JAG2 protein expression using a rabbit anti-human JAG2
polyclonal antibody (#06-1097; EMD Millipore, Billerica, MA, USA;
dilution, 1:500) for 92 min at room temperature in a Ventana
BenchMark XT processor (Ventana Medical Systems, Tucson, AZ, USA)
and counterstained with hematoxylin. The negative control included
sections incubated with antibody dilution buffer (#950-300; Ventana
Medical Systems) without primary antibody. Slides were visualized
using the ultraView Universal DAB Detection kit (#760-500; Ventana
Medical Systems). Two independent observers who were blinded to the
patients' clinical information assessed the scoring of positive
staining signals. In each patient tissue specimen, 5 fields at ×400
magnification were evaluated. The scoring of staining intensity was
as follows: 0, negative; 1, weak; 2, moderate; 3, strong; and 4,
very strong. An IHC score ranging from 0 to 400 was obtained by
multiplying the percentage of the positive cells (0–100%) by the
staining intensity (score 0–4) (13).
Western blot
Cells were lysed in buffer containing sodium dodecyl
sulfate, protease inhibitors and phosphatase inhibitors (Roche
Diagnostics, Basel, Switzerland). Equal amounts of protein lysates
were gel-separated and transferred onto nitrocellulose membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Blocking was
conducted in a buffer containing 5% non-fat milk and 0.05% Tween 20
in Tris-buffered saline for 1 h at room temperature. Primary
antibodies were incubated at 4°C overnight, while secondary
antibodies were incubated for 1 h at room temperature. Protein
bands were detected with SuperSignal® West Pico
Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.) and
Hyperfilm ECL film (GE Healthcare, Uppsala, Sweden). The following
antibodies were used: Rabbit anti-human JAG2 monoclonal antibody
(#2210; Cell Signaling Technology, Danvers, MA, USA; dilution,
1:1,000); mouse anti-actin monoclonal antibody (#ab3280; Abcam,
Cambridge, UK; dilution, 1:50,000); horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG polyclonal secondary antibody
(#170-6516; Bio-Rad Laboratories, Inc.; dilution, 1:100,000);
HRP-conjugated goat anti-rabbit IgG polyclonal secondary antibody
(#81-6120; Thermo Fisher Scientific, Inc.; dilution,
1:100,000).
siRNA transfection
Cells were transfected with 25 nM siRNA using
Invitrogen Lipofectamine 2000 (Thermo Fisher Scientific, Inc.). The
siRNAs used included ON-TARGETplus Human JAG2 siRNA (GE Healthcare
Dharmacon, Inc., Lafayette, CO, USA) (siRNA1), JAG2 siRNA (#s7645;
Thermo Fisher Scientific, Inc.) (siRNA2) and Silencer Select
Negative Control No. 1 siRNA (Thermo Fisher Scientific, Inc.). An
untransfected control was created by replacing siRNAs with Opti-MEM
Reduced Serum Medium (Thermo Fisher Scientific, Inc.).
Cell proliferation assay
At 72 h post transfection, 10 µl of MTS reagent
[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium;
Promega, WI, USA] was added to 100 ml of fresh culture medium in
each well of a 96-well plate and incubated for 3 h at 37°C. Optical
density at 490 nm was measured using the VICTOR3
Multilabel Plate Reader 1420 (PerkinElmer, Inc., Waltham, MA, USA).
The surviving percentage of cells was calculated by the following
formula: Cell proliferation % = (OD test
sample/ODcontrol sample) × 100%
The control sample reading was obtained from wells
containing untransfected cells. The reading was taken as the mean
of four wells, and the results were expressed as mean ± standard
error.
Monolayer scratch wound healing
assay
siRNA-transfected cells were seeded into culture
plates, allowed to form a confluent monolayer and serum starved
overnight. Wounds were created by scratching with sterile pipette
tips. Fresh culture medium was replenished every 24 h (serum-free
culture medium for DLD-1 and HT29; culture medium supplemented with
1% serum for HCT116 cells). Photographs were taken under a
microscope and the cell-free area was measured using ImageJ
analysis software version 1.43 (http://rsb.info.nih.gov/ij). Wound closure was
calculated by the following equation and expressed as a percentage
relative to the untreated control, which was taken to be 100%
(14):
Woundclosure%=[(cellfreearea)0–(cellfreearea)t]experiment[(cellfreearea)0–(cellfreearea)t]control×100%
‘Experiment’ represents experimental samples and
‘control’ represents untreated control samples. The results were
expressed as the mean ± standard error of the mean (SEM) and
plotted in bar charts. All experiments were performed in
triplicate.
Matrigel invasion assay
Cells were harvested at 48 h post transfection and
transferred to the upper chamber of BioCoat Matrigel Invasion
Chambers (BD Biosciences, San Jose, CA, USA) in serum-free medium.
Culture medium with 10% fetal bovine serum was added to the lower
compartment as a chemoattractant. Following a 48-h incubation at
37°C in a humidified incubator with 5% CO2, cells
remaining in the upper chamber were removed by cotton swabs and
cells that had invaded to the bottom surface of the membrane were
fixed in methanol and stained with 0.1% Toluidine Blue O
(Sigma-Aldrich). The numbers of invaded cells on the bottom surface
of the membrane were counted, expressed as mean ± SEM and plotted
in the bar charts. All experiments were repeated three times.
Statistical analysis
The differences in IHC scores between tumor tissues
and normal colorectal epithelia were analyzed by Wilcoxon signed
rank test. For cell proliferation, migration and invasion studies,
statistical significance was analyzed by an unpaired t-test.
P<0.05 as considered to indicate a statistically significant
difference. All calculations were performed using SPSS software
(version 16.0; SPSS Inc., Chicago, IL, USA).
Results
JAG2 is overexpressed in CRC tissues
and cell lines
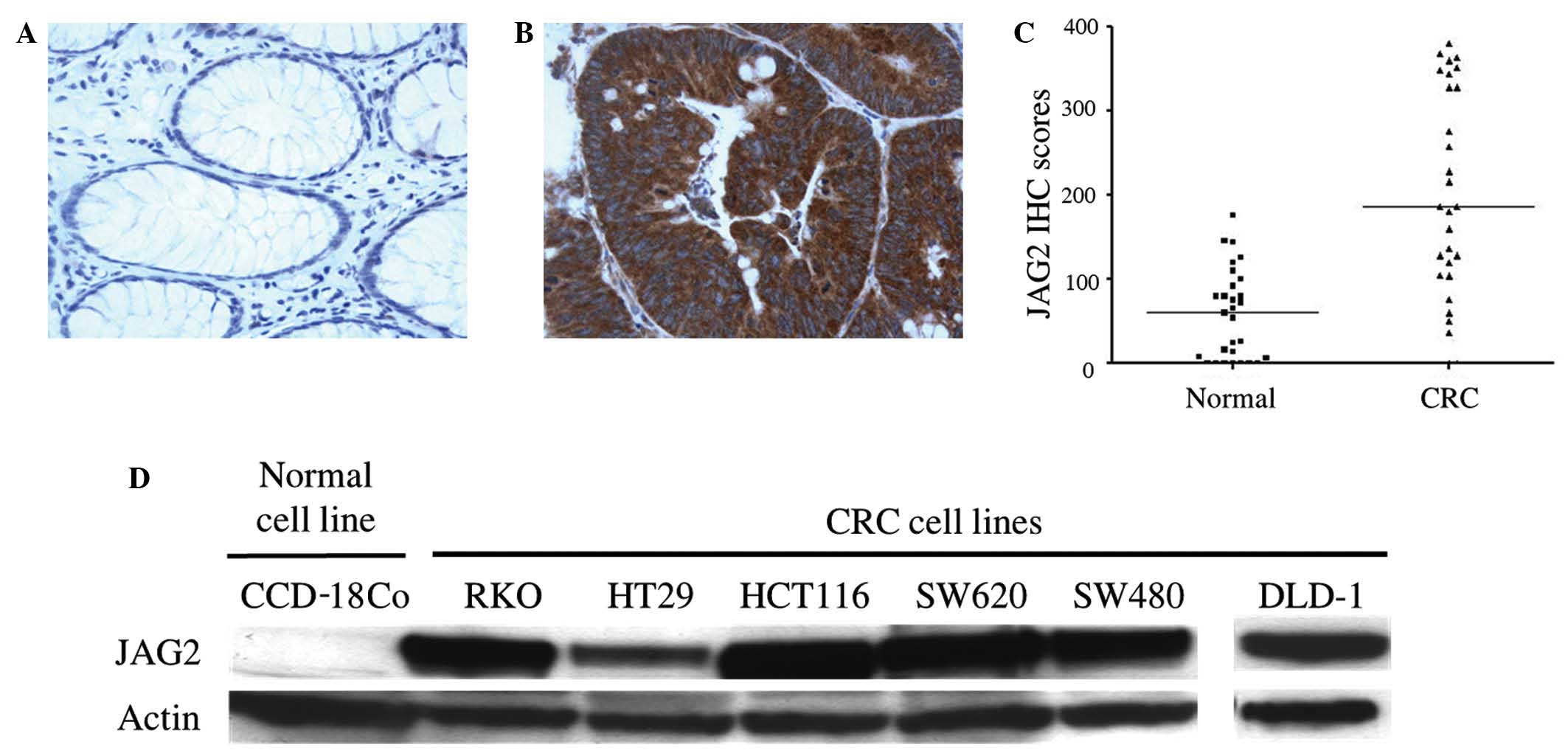
IHC staining was used to assess the level and
pattern of JAG2 protein expression in tissues from 40 cases of
human CRC. JAG2 expression was detected in 38/40 CRC cases (95.0%)
and in 5/40 surrounding normal tissues (12.5%), with a
predominantly membrane/cytoplasmic localization (Fig. 1A and B). A 3.1-fold increase in median
IHC score was observed in tumor regions compared to adjacent normal
areas (P<0.0001) (Fig. 1C),
indicating JAG2 overexpression in CRC tissues. In addition, JAG2
expression was detected by western blot in all CRC cell lines
tested, including RKO, HT29, HCT116, SW620, SW480 and DLD-1, but
not in the non-malignant colon cell line, CCD-18Co (Fig. 1D). Together, these findings indicate
that JAG2 protein is frequently overexpressed in CRC cells compared
to non-malignant colon cells.
JAG2 silencing inhibits migration of
CRC cell lines
To study the functions of JAG2 in CRC, RNA
interference was used to knockdown its expression in three CRC cell
lines: HCT116, DLD-1 and HT29. Two different siRNAs were used to
provide support to the specificity of the observed effects. The
functional consequences of JAG2 silencing on cell migration,
invasion and proliferation were then assessed by monolayer scratch
wound healing, Matrigel invasion and cell growth assays,
respectively.
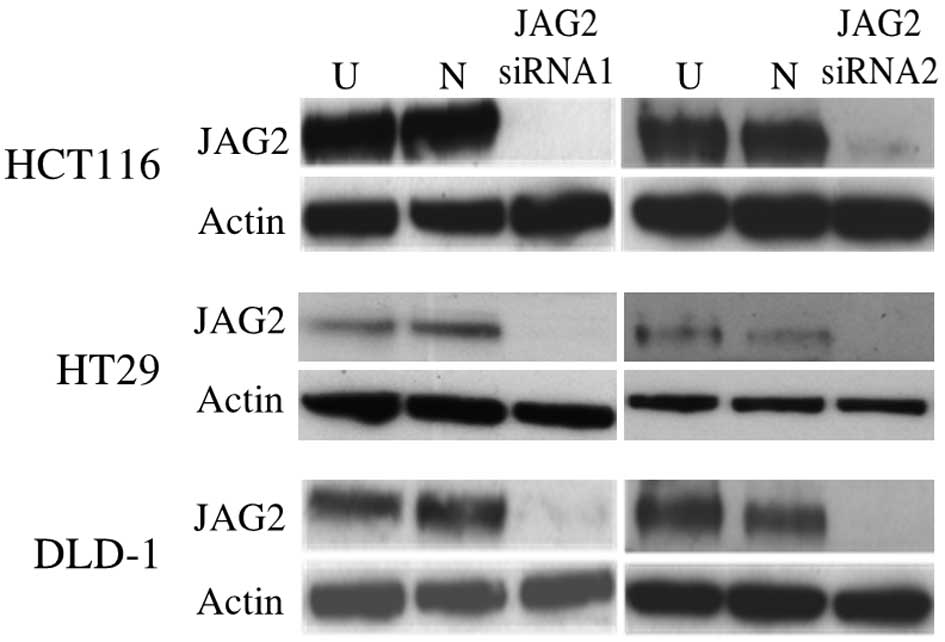
JAG2 knockdown was confirmed at the protein level
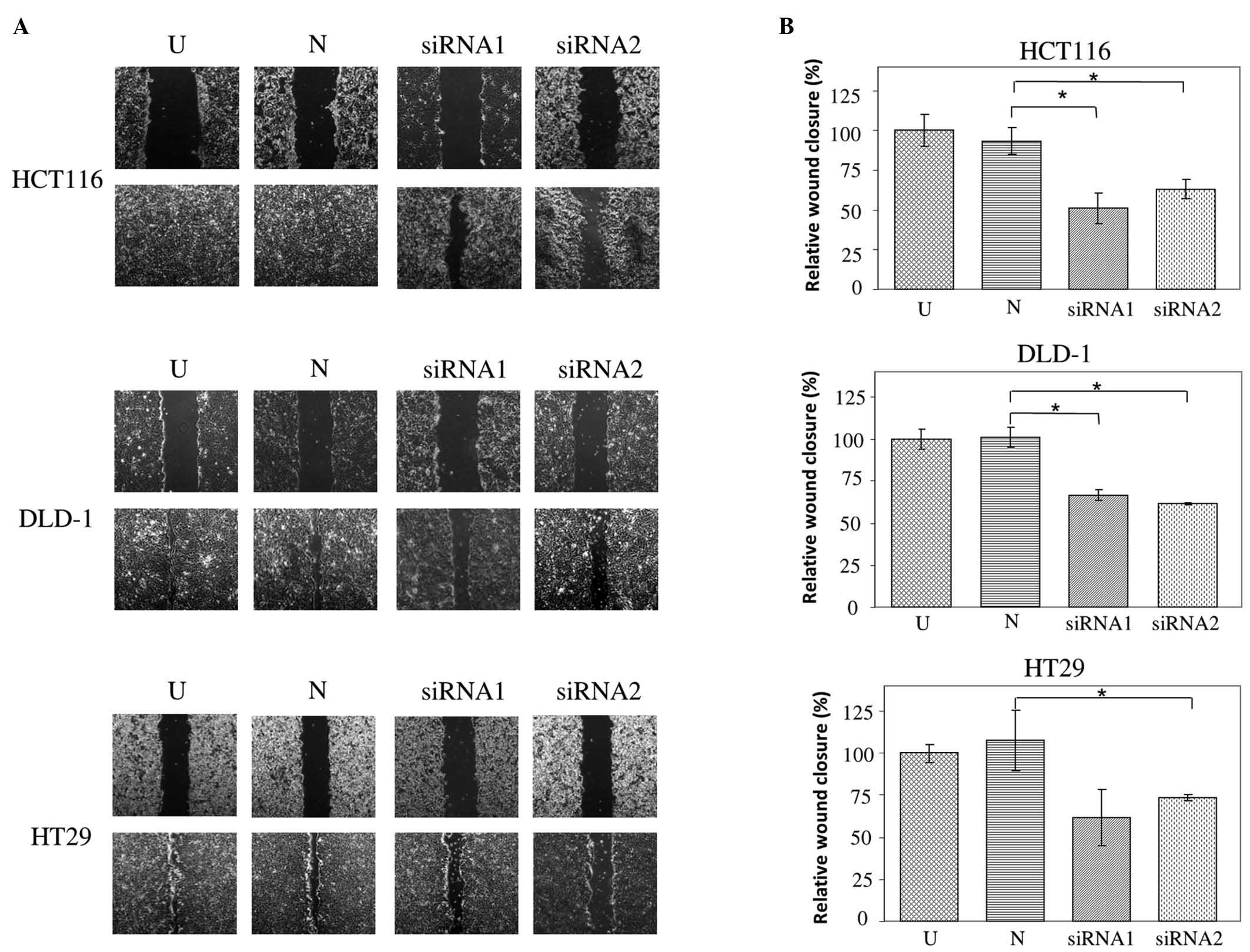
using western blot at 48 h post transfection (Fig. 2). Wound healing assays reveal impaired
wound closure of cells transfected with either of the JAG2 siRNAs
in HCT116, DLD-1 and HT29 cell lines compared to those transfected
with negative control siRNA (HCT116, P=0.015 for siRNA1 and P=0.018
for siRNA2; DLD-1, P=0.005 for siRNA1 and P=0.002 for siRNA2; and
HT29, P=0.082 for siRNA1 and P=0.019 for siRNA2) (Fig. 3A and B). This finding indicates that
JAG2 silencing can inhibit the motility of the CRC cell lines
tested.
JAG2 silencing inhibits the
invasiveness of CRC cell lines
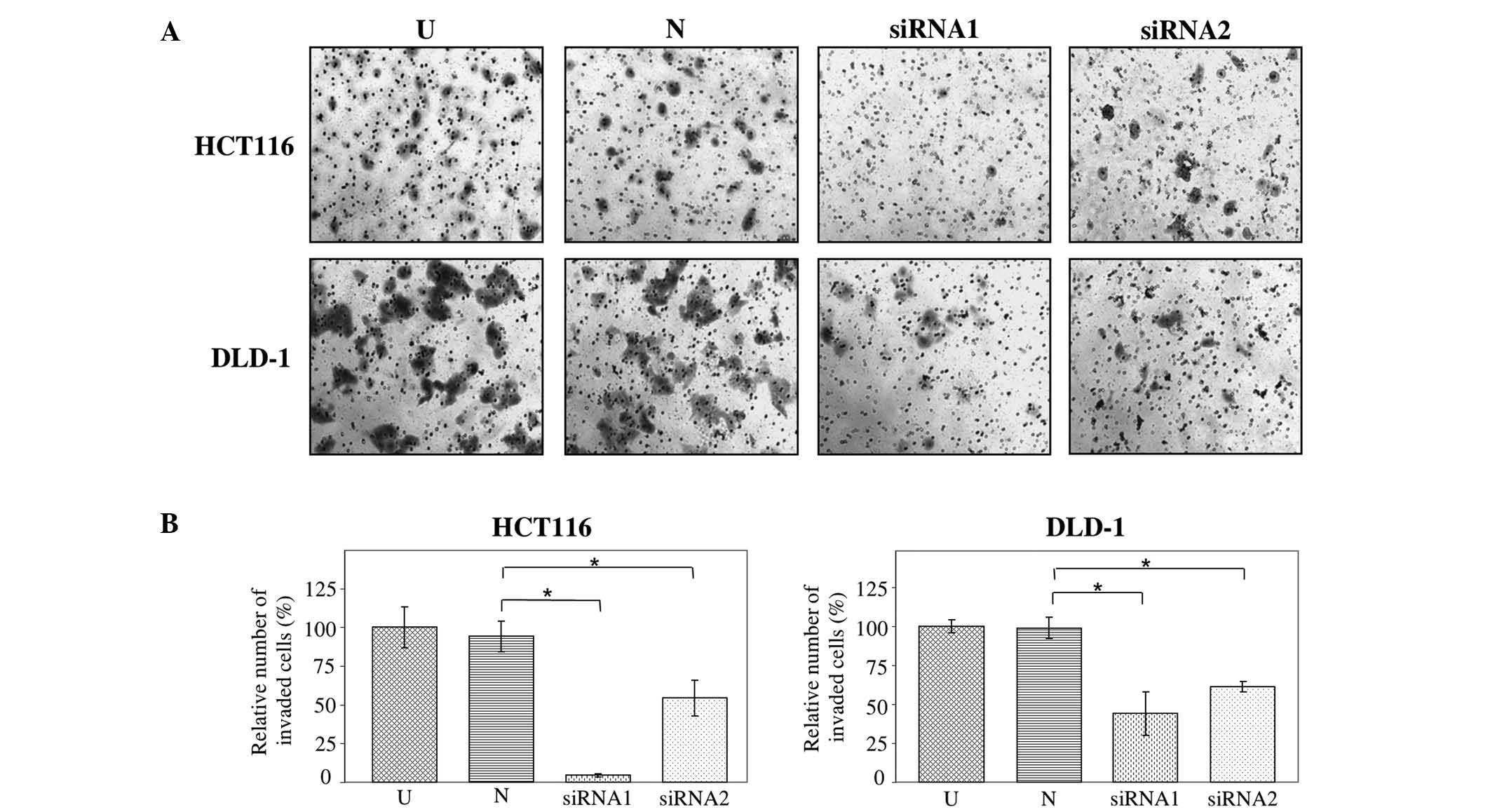
The effect of JAG2 silencing on cell invasion in
HCT116 and DLD-1 cells was assessed using Matrigel invasion assays.
HT29 cells do not invade through Matrigel and were thus excluded
from this analysis. The findings demonstrate that the invasive
capability of JAG2-silenced HCT116 and DLD-1 cells decreased
significantly relative to cells transfected with negative control
siRNA (HCT116, P=0.009 for siRNA1 and P=0.021 for siRNA2; and
DLD-1, P=0.049 for siRNA1 and P=0.013 for siRNA2) (Fig. 4A and B). This result indicates that
JAG2 silencing is inhibitory for invasiveness of CRC cell lines and
supports a pro-invasive role for JAG2 expression in CRC cells.
JAG2 silencing does not significantly
affect cell proliferation in CRC cell lines
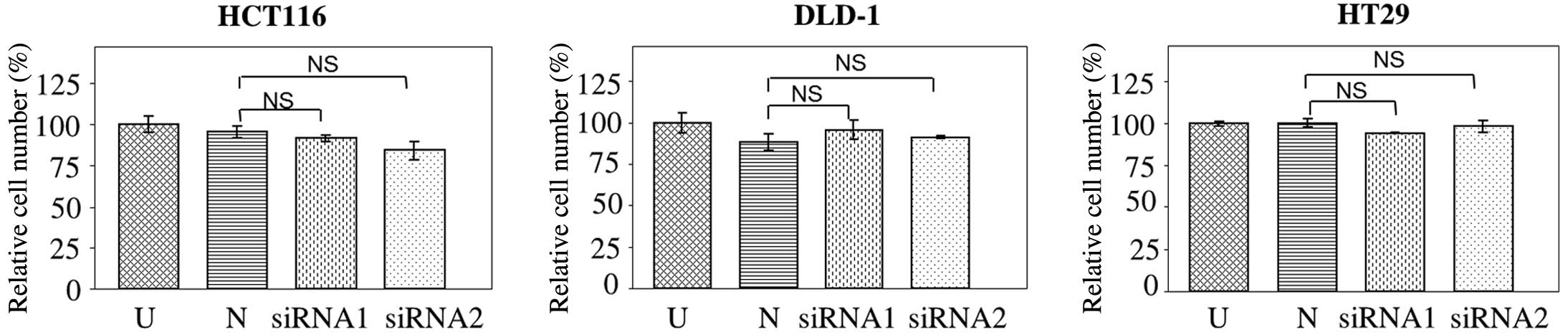
The effect of JAG2 knockdown on cell proliferation
was investigated. No significant difference in the number of
HCT116, DLD-1 or HT29 cells was detected at 72 h post JAG2
knockdown compared with negative controls (HCT116, P=0.756 for
siRNA1 and P=0.196 for siRNA2; DLD-1, P=0.265 for siRNA1 and
P=0.092 for siRNA2; and HT29, P=0.583 for siRNA1 and P=0.862 for
siRNA2) (Fig. 5), indicating that
JAG2 knockdown does not significantly affect cell proliferation in
all three CRC cell lines tested. Combined with the previously
mentioned findings, this result indicates that JAG2 knockdown
inhibits motility and invasiveness of CRC cell lines independently
of mechanisms affecting cell proliferation.
Discussion
Previous studies have indicated that Notch signaling
is upregulated in CRC (15–18). However, data regarding the expression
of JAG2 protein in CRC tissues is scarce. The current study
provides insight on this topic by demonstrating that JAG2
expression is detected in up to 95% of CRC cases (38/40 patients)
and is 3-fold overexpressed in cancerous tissues compared with
surrounding non-tumorous tissues. Furthermore, JAG2 expression was
detected in all six of the CRC cell lines tested, but not in a
normal colon epithelial cell line. These results are consistent
with a previous study that demonstrated upregulated expression of
JAG2 mRNA in all 20 CRC cases examined (15). Taken together, the finding that JAG2
is frequently overexpressed in CRC cells is suggestive of a crucial
role for this protein in CRC development.
To gain a deeper understanding of the functional
significance of JAG2 expression in CRC, the present study used RNA
interference to investigate the effects of JAG2 knockdown in three
CRC cell lines. The results indicated that JAG2 knockdown reduces
migration in HCT116, DLD-1 and HT29 cells. It can also inhibit
invasion of HCT116 and DLD-1 CRC cell lines. These effects were not
mediated through a reduction in cell proliferation, as cell numbers
were not significantly affected relative to cells transfected with
negative control siRNA at 72 h post transfection. These findings
implicate JAG2 expression in the promotion of CRC metastasis by
increasing motility and invasiveness of CRC cells, and indicate
that it may be involved in cancer progression rather than
initiation.
A similar pro-metastatic function has been reported
for JAG2 in other types of cancer. In breast cancer, JAG2
expression was found to promote metastasis and was significantly
associated with overall and metastasis-free survival of breast
cancer patients (11,19). Likewise, JAG2 was found to be capable
of inducing the metastasis of lung adenocarcinoma cells in mice
(20). These findings provide support
to the hypothesis that JAG2 functions to promote metastasis in
multiple cancer types. It would be of interest to investigate
whether JAG2 is expressed in circulating tumor cells of CRC
patients, as these cells have been demonstrated to be associated
with tumor-node-metastasis stage and lymph node status (21,22). In
addition, the clinical value of JAG2 as a novel biomarker in CRC
warrants further study (23).
In conclusion, the current study has demonstrated
that JAG2 is overexpressed in a majority of CRC tissues and is
involved in motility and invasiveness, but not proliferation, of
CRC cells. Further studies are required to gain a deeper
understanding of the precise mechanisms involved.
Acknowledgements
This work was supported by funding from the Chinese
University of Hong Kong (Direct Grants 2041697, 4054004 and
4054005).
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
IHC
|
immunohistochemical
|
|
JAG2
|
Jagged 2
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 2:87–108. 2015. View Article : Google Scholar
|
|
2
|
McArdle C: ABC of colorectal cancer:
Effectiveness of follow up. BMJ. 321:1332–1335. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Golfinopoulos V, Salanti G, Pavlidis N and
Ioannidis JP: Survival and disease-progression benefits with
treatment regimens for advanced colorectal cancer: A meta-analysis.
Lancet Oncol. 8:898–911. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 10:1065–1075. 2014. View Article : Google Scholar
|
|
5
|
Katoh M and Katoh M: Notch signaling in
gastrointestinal tract (review). Int J Oncol. 30:247–251.
2007.PubMed/NCBI
|
|
6
|
Liu J, Sato C, Cerletti M and Wagers A:
Notch signaling in the regulation of stem cell self-renewal and
differentiation. Curr Top Dev Biol. 92:367–409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weng AP and Aster JC: Multiple niches for
Notch in cancer: Context is everything. Curr Opin Genet Dev.
14:48–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weng AP, Ferrando AA, Lee W, Morris JP IV,
Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT and Aster
JC: Activating mutations of NOTCH1 in human T cell acute
lymphoblastic leukemia. Science. 306:269–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reedijk M, Odorcic S, Chang L, Zhang H,
Miller N, McCready DR, Lockwood G and Egan SE: High-level
coexpression of JAG1 and NOTCH1 is observed in human breast cancer
and is associated with poor overall survival. Cancer Res.
65:8530–8537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pietras A, von Stedingk K, Lindgren D,
Påhlman S and Axelson H: JAG2 induction in hypoxic tumor cells
alters Notch signaling and enhances endothelial cell tube
formation. Mol Cancer Res. 9:626–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xing F, Okuda H, Watabe M, Kobayashi A,
Pai SK, Liu W, Pandey PR, Fukuda K, Hirota S, Sugai T, et al:
Hypoxia-induced Jagged2 promotes breast cancer metastasis and
self-renewal of cancer stem-like cells. Oncogene. 30:4075–4086.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jung SG, Kwon YD, Song JA, Back MJ, Lee
SY, Lee C, Hwang YY and An HJ: Prognostic significance of Notch 3
gene expression in ovarian serous carcinoma. Cancer Sci.
101:1977–1983. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong SC, Lo SF, Lee KC, Yam JW, Chan JK
and Hsiao WL Wendy: Expression of frizzled-related protein and
Wnt-signalling molecules in invasive human breast tumours. J
Pathol. 196:145–153. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McLane MA, Zhang X, Tian J, Zelinskas C,
Srivastava A, Hensley B and Paquette-Straub C: Scratching below the
surface: Wound healing and alanine mutagenesis provide unique
insights into interactions between eristostatin, platelets and
melanoma cells. Pathophysiol Haemost Thromb. 34:164–168. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reedijk M, Odorcic S, Zhang H, Chetty R,
Tennert C, Dickson BC, Lockwood G, Gallinger S and Egan SE:
Activation of Notch signaling in human colon adenocarcinoma. Int J
Oncol. 33:1223–1229. 2008.PubMed/NCBI
|
|
16
|
Meng RD, Shelton CC, Li YM, Chetty R,
Tennert C, Dickson BC, Lockwood G, Gallinger S and Egan SE:
gamma-Secretase inhibitors abrogate oxaliplatin-induced activation
of the Notch-1 signaling pathway in colon cancer cells resulting in
enhanced chemosensitivity. Cancer Res. 69:573–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peignon G, Durand A, Cacheux W, Ayrault O,
Terris B, Laurent-Puig P, Shroyer NF, Van Seuningen I, Honjo T,
Perret C and Romagnolo B: Complex interplay between β-catenin
signalling and Notch effectors in intestinal tumorigenesis. Gut.
60:166–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Serafin V, Persano L, Moserle L, Esposito
G, Ghisi M, Curtarello M, Bonanno L, Masiero M, Ribatti D, Stürzl
M, et al: Notch3 signalling promotes tumour growth in colorectal
cancer. J Pathol. 224:448–460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nam DH, Jeon HM, Kim S, Kim MH, Lee YJ,
Lee MS, Kim H, Joo KM, Lee DS, Price JE, et al: Activation of notch
signaling in a xenograft model of brain metastasis. Clin Cancer
Res. 14:4059–4066. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Ahn YH, Gibbons DL, Zang Y, Lin W,
Thilaganathan N, Alvarez CA, Moreira DC, Creighton CJ, Gregory PA,
et al: The Notch ligand Jagged2 promotes lung adenocarcinoma
metastasis through a miR-200-dependent pathway in mice. J Clin
Invest. 121:1373–1385. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong SC, Chan CM, Ma BB, Hui EP, Ng SS,
Lai PB, Cheung MT, Lo ES, Chan AK, Lam MY, et al: Clinical
significance of cytokeratin 20-positive circulating tumor cells
detected by a refined immunomagnetic enrichment assay in colorectal
cancer patients. Clin Cancer Res. 15:1005–1012. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong SC, Ng SS, Cheung MT, Luk LY, Chan
CM, Cheung AH, Lee VH, Lai PB, Ma BB, Hui EP, et al: Clinical
significance of CDX2-positive circulating tumour cells in
colorectal cancer patients. Br J Cancer. 104:1000–1006. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong SC, Chan CM, Ma BB, Lam MY, Choi GC,
Au TC, Chan AS and Chan AT: Advanced proteomic technologies for
cancer biomarker discovery. Expert Rev Proteomics. 6:123–134. 2009.
View Article : Google Scholar : PubMed/NCBI
|