Background
Pleomorphic lobular carcinoma in situ (PLCIS)
has only recently been identified as a distinct pathological
entity. The first mention of this lesion was in an unindexed case
report by Frost et al (1) in
1996. It has been characterised as an aggressive subtype of classic
lobular carcinoma in situ (CLCIS). However there is yet to
be a consensus among clinicians regarding the optimal treatment of
this disease. As recently as 2013 a survey presented in the San
Antonio Breast Cancer Symposium demonstrated that there were
significant differences in opinion among breast surgeons regarding
the optimal excision margins required to adequately treat PLCIS to
prevent recurrences in the future (2).
In this article, the literature available with
regards to this disease were reviewed, including the evidence that
distinguishes it from other neoplasms of the breast and the results
of the significant clinical studies which have looked into this
disease were collated and summarised. Specifically, the risk of
concomitant invasive disease or ductal carcinoma in situ
(DCIS) if PLCIS is found on core needle biopsy (CNB) was determined
and the evidence regarding the risk of recurrence in relation to
surgical margins and adjuvant therapy was collated.
For the purposes of this systematic review, MedLine,
PubMed, the WHO International Clinical Trial Registry Platform and
Google Scholar were searched using the following keywords:
‘pleomorphic lobular carcinoma in situ’, ‘pleomorphic
lobular carcinoma in situ’ and ‘PLCIS’. Further articles
were identified by manual search through the references in previous
reviews and studies (3,4).
CLCIS
CLCIS has long been associated with an increased
risk of carcinoma. However, in a seminal study in 1978, it was
observed that the disease was frequently bilateral and too
extensive for surgical excision. Also, it was noted that the
invasive disease associated with CLCIS was often invasive ductal
carcinoma (IDC) rather than lobular carcinoma (5). On the basis of these observations, CLCIS
is regarded to be a marker of increased risk of invasive disease
rather than a precursor of invasive disease (6). It is associated with a risk of invasive
breast cancer over 10 years of at least 7.1%. Currently, patients
with CLCIS are offered surveillance with regular physical
examination and imaging rather than surgical excision (7).
Notably, a recent study suggests that in some
instances, CLCIS cells may be clonally related to subsequent
invasive lobular carcinoma (ILC), which may suggest that CLCIS may
serve as a precursor to invasive disease (8).
PLCIS
PLCIS was initially described by Frost et al
(1), who described the pathology in
the context of a 78 years old woman with an ill-defined mass in the
right breast and corresponding calcification on a recent mammogram.
The lesion was identified by needle localisation and a biopsy was
performed followed by wide local excision and radiotherapy. The
lesion was grossly characterised as ‘ill defined, multifocal and
difficult to measure’. Frost et al (1) described the microscopic appearance of
the cells as large cells with abundant eosinophilic cytoplasm with
irregular nuclei with prominent single or multiple nucleoli. The
cells extended into the ducts in a pagetoid fashion and filled the
duct lumen with tightly packed cells that lacked cohesion. On
immunohistochemistry the cells tested positive for gross cystic
disease fluid protein 15 (GCDFP 15) and the c-erB-2 oncogene
(1). PLCIS has also been referred to
as ‘florid’ lobular carcinoma in situ, and is clinically and
radiologically similar to ductal carcinoma in situ (9).
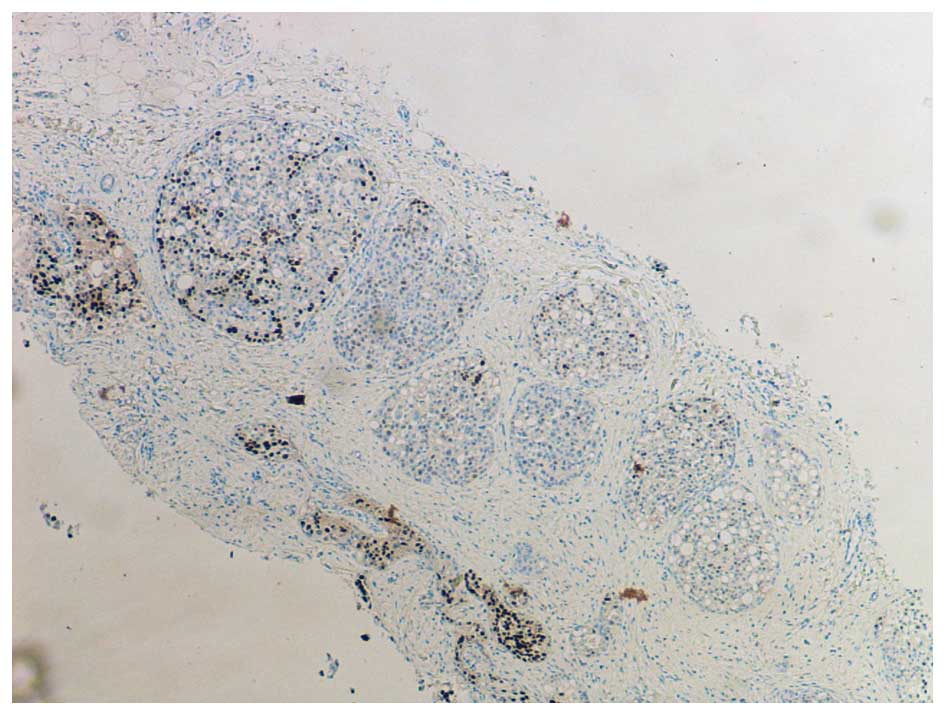
In the original description by Frost et al,
PLCIS was characterised as a hormone receptor negative lesion, in
which oestrogen and progesterone receptors were not detected
(ER-/PR-) (1), as illustrated in
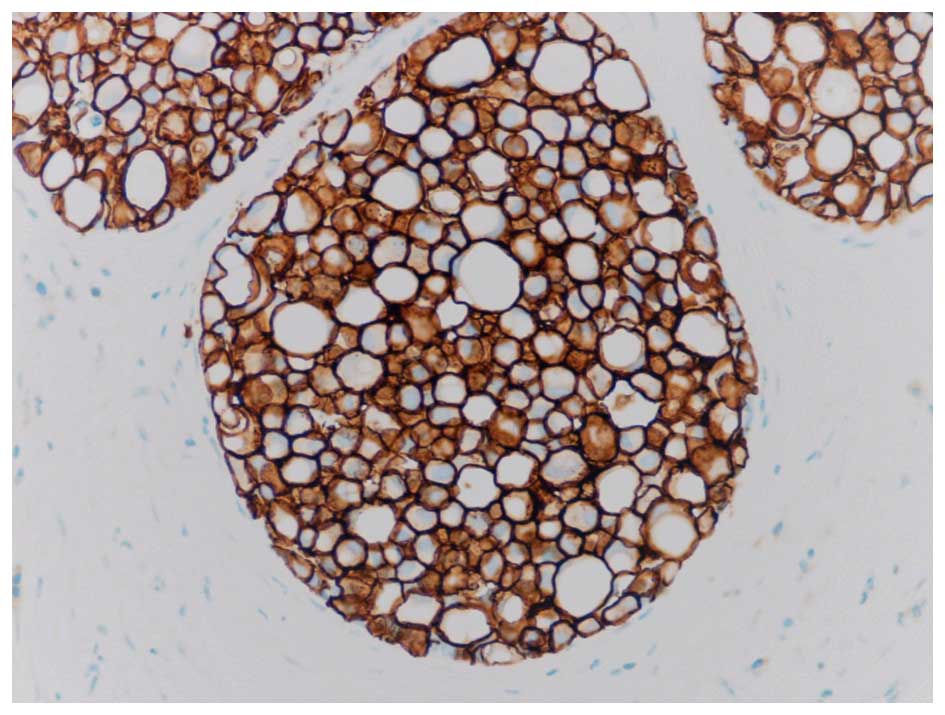
Fig. 1. Since then it has been
determined that PLCIS is often a strongly Her2 positive lesion
(Her2+), as illustrated in Fig. 2. An
additional recent study noted significant Her2 overexpression and
amplification and speculated that this may be involved in the
pathogenesis of PLCIS (10). This is
in contrast to CLCIS, which is commonly a hormone receptor positive
and Her2 negative lesion (ER+/PR+/Her2-) (11,12).
Androgen receptor expression is similar in both PLCIS and CLCIS
(13). These distinctions have
profound implications in terms of prognosis and significantly limit
the potential options available for the adjuvant therapy of
PLCIS.
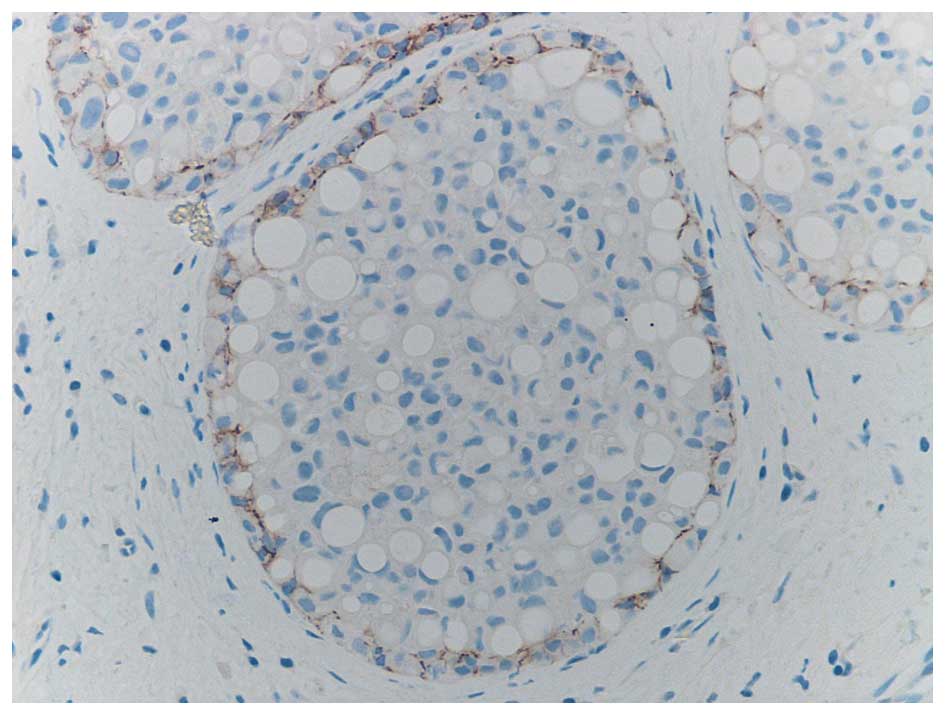
It is well recognised that the differentiation of
PLCIS from DCIS, on cytological findings alone may be difficult. It
has been found that in common with CLCIS and in contrast to DCIS,
PLCIS is characterised by a loss of heterozygosity for the
E-Cadherin gene, as illustrated in Fig.
3. This is a characteristic it shares with CLCIS which may
indicate a clonal relationship (14).
However, it has long been noted that unlike CLCIS,
PLCIS may be frequently associated with invasive disease and has
more aggressive course (4,9). PLCIS is considered to be a precursor of
invasive disease rather than a marker of increased risk like CLCIS.
This is borne out by similarities in biomarkers of synchronous
PLCIS and pleomorphic invasive lobular carcinoma (PILC). PLCIS is
characterised by increased expression of Ki67 compared to CLCIS,
which indicates a high cell turnover. These observations suggest
that this may be a radiosensitive lesion unlike LCIS (12).
This early characterisation of PLCIS as a precursor
to invasion more akin to DCIS, however, was based on impressions
gleaned from several histological series earlier in the previous
decade (15,16). Data, albeit retrospective, regarding
actual recurrence rates after excision and the presence of
concomitant invasive disease have only recently become available.
This evidence is reviewed in the following sections.
The incidence of concomitant invasive
disease with an initial diagnosis of PLCIS
Following the search of the aforementioned medical
databases and perusing the citations used in previous articles, 10
case series were identified in which the diagnosis of PLCIS was
made on CNB. A total of 121 such patients were recorded in these 10
studies (Table I) (17–25).
 | Table I.Collation of retrospective studies
reporting the incidence of concomitant invasive disease or DCIS in
the patients with a CNB positive for PLCIS. |
Table I.
Collation of retrospective studies
reporting the incidence of concomitant invasive disease or DCIS in
the patients with a CNB positive for PLCIS.
| Study | N | Diagnosis on CNB | Surgical
procedure | PLCIS alone on
surgical specimen | Concurrent DCIS or
invasion on surgical specimen | Concurrent DCIS
(%) | Concurrent invasive
cancer (%) | Ref |
|---|
| Carder et
ala | 10 | PLCIS: 5 | DB: 2 WLE: | 1 | 1 micro ILC, | 0 | 30 | (17) |
|
|
| PLCIS |
|
|
|
|
|
|
|
|
| with microinvasion:
2 | 6 Mx:2 |
| 2 ILC |
|
|
|
|
|
| PLCIS with CLCIS:
3 |
|
|
|
|
|
|
| Chivikula et
alb | 12 | PLCIS: 12 | DB: 1 WLE: | 7 | 1 DCIS+ | 8.30 | 25 | (18) |
|
|
|
| 9 Mx: 2 |
| ILC, 2 ILC |
|
|
|
| Fasola et
al | 34 | PLCIS: 13 | WLE: 20 | 4 | 9 PLCIS+DCIS, | 26.47 | 61.76 | (19) |
|
|
| PLCIS ‘with | Mx: 142 |
| 6 PLCIS+IDC, |
|
|
|
|
|
| invasion’: 21 |
|
| 15 PLCIS+ILC |
|
|
|
| Morris et
al | 17 | PLCIS: 3 PLCIS | WLE:17 | 3 | 3 PLCIS+DCIS, | 17.65 | 17.65 | (20) |
|
|
| +DCIS: 7 |
|
| 11 PLCIS+ILC |
|
|
|
|
|
| PLCIS+IC: 7 |
|
|
|
|
|
|
| Niell et
al | 5 | PLCIS: 5 | WLE: 5 | 1 | 1 PLCIS+ILC, | 40 | 60 | (21) |
|
|
|
|
|
| 1 LCIS+ILC, 1 |
|
|
|
|
|
|
|
|
| DCIS+IDC, 1
DCIS |
|
|
|
| Lavoure et
alc | 10 | PLCIS: 10 | WLE: 10 | 7 | 3 PLCIS+ILC | 0 | 30 | (22) |
| Georgian-Smith
et al | 5 | PLCIS: 5 | WLE: 5 | 3 | 2 PLCIS+ILC | – | 40 | (23) |
| Mahoney et
al | 2 | PLCIS: 2 | WLE: 2 | 1 | 1 PLCIS+ILC | – | 50 | (24) |
| Purdie et
ale | 3 | PLCIS: 3 | WLE: 3 | 1 | 1 ILC, 1
PLCIS+ILC | – | 66 | (25) |
| Flanagan et
ald | 23 | PLCIS: 23 | WLE: 16 | 12 | 5 ILC, 2 ILC+ | 17 | 30 | (28) |
|
|
|
| Mx: 5 |
| IDC, 1 DCIS, |
|
|
|
|
|
|
|
|
| 3 PLCIS+DCIS |
|
|
|
| Total | 121 |
|
|
|
| 15.70 | 40.5 |
|
While case reports were excluded, any patients who
formed a part of a larger series in view of the rarity of this
condition were included. In all these cases, the patients underwent
a core needle biopsy that returned a diagnosis of PLCIS, either
alone or in conjunction with another finding such as DCIS, CLCIS or
invasion. Furthermore, these patients underwent surgical excision
and the authors of these studies reported the final pathological
diagnosis.
Of these 121 patients, 81 were recorded to have
PLCIS alone on CNB. Of the remainder, 30 exhibited invasion or
microinvasion and 7 possessed DCIS. A total of 93 of the patients
had wide local excisions (WLE), 3 had diagnostic excisional
biopsies and 23 patients had mastectomies.
After final histopathology of the surgical specimen
only 33% (n=40) of the total were determined to not have concurrent
disease. Of the remainder, 40.5% of the cases reported some form of
invasive disease, predominantly invasive lobular carcinoma. Other
invasive carcinomas reported were invasive ductal carcinoma and
additional instances of lobular microinvasion. In addition, 16%
(n=19) of the cases were reported to have concurrent DCIS. These
results are in keeping with previous results from case series and
systematic reviews. However, it must be noted that all the data in
question was collected retrospectively and would be prone to the
biases and confounding factors inherent to such data.
The effect of surgical margins and adjuvant
therapy on recurrence rates
The question of the risk of recurrence following
excision of PLCIS is understandably a question of great interest to
clinicians. It has long been understood that the clinical history
of PLCIS is distinct from that of CLCIS, with behaviour more
consistent with a precursor of invasive disease rather than a
marker of increased risk. The present review of the literature
since the identification of PLCIS as a distinct pathological
diagnosis identified four studies that comment upon excision
margins and recurrence of the disease (Table II).
 | Table II.Summary of retrospective studies
reporting local recurrence rates after excision of PLCIS. |
Table II.
Summary of retrospective studies
reporting local recurrence rates after excision of PLCIS.
| Study | Median follow-up
(months) | Median age | N | Surgical
margins | Adjuvant
therapy | Histology of
recurrences | Time to recurrence
(months) | LRR (%) | Ref |
|---|
| Fasola et
alb | 57 | 55 | 34 | ≤1 mm: 34 | RT+/− | Not | Not | 8.82 | (19) |
|
| (12–163) | (41–84) |
|
| CT: 16 | stated | stated |
|
|
|
|
|
|
|
| HT: 9c |
|
|
|
|
| Downs-Kelly et
al | 32.5 | 59.5 | 26 | At margin: 6 | No Rx: 10 | PLCIS: 1 | 18 | 3.84 | (26) |
|
| (4–108) | (35–76) |
| <1
mm: 7 | CRT: 6 |
|
|
|
|
| 1.1–2 mm: 4 | CT: 6 |
|
|
|
|
|
|
|
|
| >2.1
mm: 9 | RT: 4 |
|
|
|
|
| Khoury et
ala | 55.6 | 59.5 | 31 | Not stated | RT: 3 | PLCIS: 2 | Not | 12.70 | (27) |
|
| (1.6–112) | (40–88) |
|
| HT: 11 | Invasion: 4 | stated |
|
|
| Flanagan et
ald | 49.2e | 55 | 18 | At margin: 14 | RT: 1 | None | – | 0 | (28) |
|
| (5.7–115) | (36–70) |
| 1.1–2 mm: 4 | HT: 3 |
|
|
|
|
The first study reported in the literature is that
of Downs-Kelly et al (26) in
2011, which reported on 26 patients who underwent resection for
PLCIS and also received adjuvant chemo and radiotherapy. These
patients were identified retrospectively from the hospital
database. Of these, 20 had PLCIS on pathology, a further 6 patients
with an invasive component were included if they were classified as
suspicious for microinvasion or invasion measuring between 1–5 cm
and >1 cm from the resection margins. The basis for these
criteria is unclear. Furthermore, the patients were stratified
according to their resection margins. Of these patients, 10
patients did not receive any adjuvant therapy, 7 received
chemotherapy only and 3 received radiotherapy only. The remainder
received both chemo- and radiotherapy. Again, the basis for this
was not clarified in the text by the authors. This group of
patients was followed up for a mean of 46 months. In this time,
only one recurrence was reported at 19 months, which was PLCIS
(3.8%). The authors remark that the patient in question had disease
at the margin and had only received chemotherapy. On the basis of
this data the authors recommend treating PLCIS as one would treat
DCIS with excision margins of at least 2 mm and to consider
adjuvant therapy (26).
The second study in question is that of Fasola et
al (19) of which only an
abstract is publically available. Out of 34 patients, all of whom
underwent wide local excision, the authors report three local
recurrences over a 5-year period. In their cohort, the authors
noted PLCIS alone had a higher local recurrence rate compared to
PLCIS associated with invasion. It was also noted that the cases
which developed local recurrences following excision of PLCIS alone
did not receive surgical re-excision or adjuvant therapy and the
surgical margins were within 1 mm of the lesion (19).
Khoury et al (27) report on a case series of 47 patients
diagnosed with PLCIS followed up over a 12-year period. Within this
group, the authors selected 31 patients with PCLIS in order to
study the risk of local recurrence following excision. The median
follow-up period was 55.6 months, 6 local recurrences were reported
of which 4 were invasive carcinoma and 2 were PLCIS only. None of
these patients received radiotherapy. Also of note is that of the
11 patients who received hormonal therapy, 3 developed local
recurrences. Khoury et al (27) identified a statistically significant
correlation between tumour recurrence and patient age in which the
mean age for recurrence was 52.5 years compared to 60.6 years for
those who did not develop recurrences (27).
The most recent study to comment on recurrence rates
was that of Flanagan et al (28) who reported on a case series of 23
patients who were diagnosed with PLCIS. Of these 23 patients, 2 had
adequate margins on the excisional biopsy and required no further
intervention, while 16 underwent wide local excision and 5 had
mastectomies. However even among the 16 who had WLE there was
difficulty in getting clear margins and were subjected to
re-excisions. Eventually another 7 patients required mastectomies
as well. Over the 4 years of follow-up, the authors reported no
instances of local recurrence. However, they also emphasize that
the pursuit of clear margins in the case of PLCIS precluded
successful breast conservation. Therefore, the authors urge caution
in the aggressive treatment of PLCIS with regards to margins
(28).
The retrospective nature of the data and the
inconsistency in reporting of surgical margins preclude the
formulation of an objective recommendation concerning the same. All
of the authors appear to agree that clear margins will lead to
lower recurrence rates. However, Flanagan et al (28) report that this may not always be
possible or feasible.
Similarly, there is a lack of consistency in the
adjuvant therapy given to patients, with little clarity in the
literature concerning the criteria used to guide such decisions. It
would be desirable to form a consensus with regards to the place of
adjuvant therapy with regards to the treatment of PLCIS. As
observed above, the numbers of patients in these studies are not
sufficient to lead to any solid conclusions. However, Downs-Kelley
et al (26) and Khoury et
al (27) and indicated that
radiotherapy led to a lower recurrence rate in their case series.
Furthermore, Khoury et al (27) noted that patients on hormone therapy
contributed to half of the recurrence cases. This issue may need to
be explored in larger well-designed prospective studies.
Conclusion
The literature regarding PLCIS is relatively novel.
In view of the rarity of this condition, usable evidence is
accumulating slowly. Consequently, it may not always be possible to
formulate recommendations based on the appropriate evidence.
However, on the basis of the observed behaviour of the disease in
retrospective studies, it would not be unreasonable to characterise
PLCIS as significant risk factors for concomitant carcinoma with a
significant risk of recurrence after excision. This is the position
of the National Cancer Comprehensive Network (NCCN) and NHS breast
screening program (NHSBSP) who recommend treating PLCIS similar to
DCIS (29,30). However, as evident in more recent
studies, such an approach may not be consistent with breast
conservation (28). This clinical
issue warrants investigation in appropriately powered,
well-designed, prospective studies for a satisfactory resolution of
all concerns.
Acknowledgements
The present study was funded by the Breast Cancer
Hope Foundation (London, UK).
References
|
1
|
Frost AR, Tsangaris TN and Silverberg SG:
Pleomorphic lobular carcinoma in situ. Pathology Case Reviews.
1:27–31. 1996. View Article : Google Scholar
|
|
2
|
Blair SL, Emerson DK, Kulkarni S, Hwang
ES, Malcarne V and Ollila DW: Breast surgeon's survey: No consensus
for surgical treatment of pleomorphic lobular carcinoma in situ.
Breast J. 19:116–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hussain M and Cunnick GH: Management of
lobular carcinoma in-situ and atypical lobular hyperplasia of the
breast-a review. Eur J Surg Oncol. 37:279–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pieri A, Harvey J and Bundred N:
Pleomorphic lobular carcinoma in situ of the breast: Can the
evidence guide practice? World J Clin Oncol. 5:546–553. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haagensen CD, Lane N, Lattes R and Bodian
C: Lobular neoplasia (so-called lobular carcinoma in situ) of the
breast. Cancer. 42:737–769. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ciocca RM, Li T, Freedman GM and Morrow M:
Presence of lobular carcinoma in situ does not increase local
recurrence in patients treated with breast-conserving therapy. Ann
Surg Oncol. 15:2263–2271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chuba PJ, Hamre MR, Yap J, Severson RK,
Lucas D, Shamsa F and Aref A: Bilateral risk for subsequent breast
cancer after lobular carcinoma-in-situ: Analysis of surveillance,
epidemiology, and end results data. J Clin Oncol. 23:5534–5541.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aulmann S, Penzel R, Longerich T, Funke B,
Schirmacher P and Sinn HP: Clonality of lobular carcinoma in situ
(LCIS) and metachronous invasive breast cancer. Breast Cancer Res
Treat. 107:331–335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bagaria SP, Shamonki J, Kinnaird M, Ray PS
and Giuliano AE: The florid subtype of lobular carcinoma in situ:
Marker or precursor for invasive lobular carcinoma? Ann Surg Oncol.
18:1845–1851. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lien HC, Chen YL, Juang YL and Jeng YM:
Frequent alterations of HER2 through mutation, amplification, or
overexpression in pleomorphic lobular carcinoma of the breast.
Breast Cancer Res Treat. 150:447–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Green AR, Young P, Krivinskas S, Rakha EA,
Paish E Claire, Powe DG and Ellis IO: The expression of ERalpha,
ERbeta and PR in lobular carcinoma in situ of the breast determined
using laser microdissection and real-time PCR. Histopathology.
54:419–427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sneige N, Wang J, Baker BA, Krishnamurthy
S and Middleton LP: Clinical, histopathologic, and biologic
features of pleomorphic lobular (ductal-lobular) carcinoma in situ
of the breast: A report of 24 cases. Mod Pathol. 15:1044–1050.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YY, Hwang ES, Roy R, DeVries S,
Anderson J, Wa C, Fitzgibbons PL, Jacobs TW, MacGrogan G, Peterse
H, et al: Genetic and phenotypic characteristics of pleomorphic
lobular carcinoma in situ of the breast. Am J Surg Pathol.
33:1683–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Palacios J, Sarrio D, Garcia-Macias MC,
Bryant B, Sobel ME and Merino MJ: Frequent E-cadherin gene
inactivation by loss of heterozygosity in pleomorphic lobular
carcinoma of the breast. Mod Pathol. 16:674–678. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sapino A, Frigerio A, Peterse JL, Arisio
R, Coluccia C and Bussolati G: Mammographically detected in situ
lobular carcinomas of the breast. Virchows Arch. 436:421–430. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fadare O, Dadmanesh F, Alvarado-Cabrero I,
Snyder R, Mitchell J Stephen, Tot T, Wang SA, Ghofrani M, Eusebi V,
Martel M and Tavassoli FA: Lobular intraepithelial neoplasia
[lobular carcinoma in situ] with comedo-type necrosis: A
clinicopathologic study of 18 cases. Am J Surg Pathol.
30:1445–1453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carder PJ, Shaaban A, Alizadeh Y,
Kumarasuwamy V, Liston JC and Sharma N: Screen-detected pleomorphic
lobular carcinoma in situ (PLCIS): Risk of concurrent invasive
malignancy following a core biopsy diagnosis. Histopathology.
57:472–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chivukula M, Haynik DM, Brufsky A, Carter
G and Dabbs DJ: Pleomorphic lobular carcinoma in situ (PLCIS) on
breast core needle biopsies: Clinical significance and
immunoprofile. Am J Surg Pathol. 32:1721–1726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fasola CE, Jensen KC and Horst KC: Local
regional recurrence among patients with pleomorphic lobular
carcinoma in situ: Is there a role for radiation therapy?
International Journal of Radiation Oncology*Biology*Physics.
84:S2382012. View Article : Google Scholar
|
|
20
|
Morris K, Howe M, Kirwan C and Harvey J:
Clinical and phenotypic characteristics of core biopsy diagnosed
pleomorphic lobular carcinoma-in-situ in a UK population (PLCIS).
European Journal of Surgical Oncology (EJSO). 39:4842013.
View Article : Google Scholar
|
|
21
|
Niell B, Specht M, Gerade B and Rafferty
E: Is excisional biopsy required after a breast core biopsy yields
lobular neoplasia? AJR Am J Roentgenol. 199:929–935. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lavoué V, Graesslin O, Classe JM,
Fondrinier E, Angibeau H and Levêque J: Management of lobular
neoplasia diagnosed by core needle biopsy: Study of 52 biopsies
with follow-up surgical excision. Breast. 16:533–539. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Georgian-Smith D and Lawton TJ:
Calcifications of lobular carcinoma in situ of the breast:
Radiologic-pathologic correlation. AJR Am J Roentgenol.
176:1255–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mahoney MC, Robinson-Smith TM and
Shaughnessy EA: Lobular neoplasia at 11-gauge vacuum-assisted
stereotactic biopsy: Correlation with surgical excisional biopsy
and mammographic follow-up. AJR Am J Roentgenol. 187:949–954. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Purdie CA, McLean D, Stormonth E,
Macaskill EJ, McCullough JB, Edwards SL, Brown DC and Jordan LB:
Management of in situ lobular neoplasia detected on needle core
biopsy of breast. J Clin Pathol. 63:987–993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Downs-Kelly E, Bell D, Perkins GH, Sneige
N and Middleton LP: Clinical implications of margin involvement by
pleomorphic lobular carcinoma in situ. Arch Pathol Lab Med.
135:737–743. 2011.PubMed/NCBI
|
|
27
|
Khoury T, Karabakhtsian RG, Mattson D, Yan
L, Syriac S, Habib F, Liu S and Desouki MM: Pleomorphic lobular
carcinoma in situ of the breast: Clinicopathological review of 47
cases. Histopathology. 64:981–993. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Flanagan MR, Rendi MH, Calhoun KE,
Anderson BO and Javid SH: Pleomorphic lobular carcinoma in situ:
Radiologic-pathologic features and clinical management. Ann Surg
Oncol. 22:4263–4269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
N.C.C.N. guidelines, . Breast cancer.
National Comprehensive Cancer Network; Fort Washington PA, USA:
2015, http://www.nccn.org/patients/guidelines/stage_0_breast/index.htmlAccessed,
July 2015.
|
|
30
|
Guidelines for pathology reporting in
breast disease, . NHS Cancer Screening Programmes & Royal
College of Pathologists. South Sheffield, UK: 2005, http://www.cancerscreening.nhs.uk/breastscreen/publications/nhsbsp58.htmlAccessed,
July 2015.
|