Introduction
Neuroblastoma (NB) is the most common malignant
solid tumor from the peripheral nervous system. The disease
originates from neural crest cells and accounts for 7–10% of
malignant tumors among children (1–3). Several
genetic abnormalities associated with NB have been characterized,
including DNA content, gain of chromosome arm 17q or deletion of
chromosome arms 1p and 11q (4–8). NB occurs
frequently in infants, with a high degree of malignancy. According
to the stage standard of the International Neuroblastoma Staging
System (INSS) (9), NB is classified
into stages 1–4, with a special stage 4s. Stage 4s cases occur in
infants who are <1 year old. These cases are sensitive to
chemotherapy, with a better prognosis than cases at stages 3 and 4.
NB infants are mainly treated with surgery in conjunction with
chemotherapy and radiotherapy. The response to the treatment also
depends on the age of the patients and their sensitivity to
chemotherapy. Previous studies have shown that the most robust
prognostic factors are age, stage, histology and amplification of
the N-Myc oncogene (10–14). N-Myc is a member of the Myc family of
oncogenic proteins, which are well known for their association with
a large proportion of human forms of cancer (15). NB patients with amplification of the
N-Myc gene tend to have poor clinical outcomes (16).
The present study retrospectively analyzed the cases
of 16 infants with NB who were <1 year old and treated in
Beijing Tongren Hospital (Beijing, China) between February 2007 and
August 2013. The analysis included the clinical characteristics at
diagnosis, the treatments used and the response to the therapy.
Patients and methods
Patient information
A total of 16 infant NB cases diagnosed by imaging
serology and/or pathological histology were admitted to Beijing
Tongren Hospital between February 2007 and August 2013. Ethical
approval was obtained from the Ethics Committee of Beijing Tongren
Hospital. Informed consent was gained from the legal guardians of
all infant patients.
Diagnosis and clinical stages
The NB patients were diagnosed based on
histopathology results after surgical excision or pathological
biopsy, with reference to the onset age, the primary site, any
visible tumor calcification on a primary site computed tomography
(CT) scan and the neuron-specific tumor marker nicotinic acid
esterase (NSE). The criteria for inclusion of the patients were as
follows: i) An onset age of <12 months. ii) A diagnosis
confirmed by biopsy and pathological examination after surgery. If
the patient presented with a large tumor (diameter >10 cm) at
the initial diagnosis, the diagnosis was based on the localization
of the primary site by CT/magnetic resonance imaging, with serum
NSE above the normal value. iii) Patients with guardians who were
collaborative in required treatments, re-diagnosis and follow-up.
The exclusion criteria were: i) Patients that did not fit the
inclusion criteria; and ii) patients whose guardians decided to
stop treatment prior to completion.
Treatments
Patients were mainly treated by chemotherapy
combined with surgery. For those infants with a large mass
(diameter >10 cm) and an age of <3 months, treatment with
chemotherapy was applied prior to surgery. For infants <6 months
old, ≤6 cycles of chemotherapy were performed, while for infants
between 6 and 12 months, ≤9 cycles of chemotherapy were performed.
For stage 4 patients who showed recurrence or exhibited no initial
response to chemotherapy, the treatment was extended to 12–18
cycles, and high-dose chemotherapy combined with autologous
peripheral blood stem cells transplantation (APBSCT) was
applied.
Selection of chemotherapy
Chemotherapies used in this study are shown in
Table I. Patients <6 months old
were treated with a cisplatin, etoposide, cyclophosphamide and
doxorubicin (PECA) regimen or a low-dose topotecan and
cyclophosphamide (TC) regimen for 2–4 cycles prior to surgery and
for 4–6 cycles after surgery. Stage 4 patients between 6 and 12
months old were treated with PECA or TC prior to surgery, and a
cyclophosphamide, cincristine and daunorubicin/etoposide and
cisplatin (CDV/CIE) regimen (alternately) following surgery.
Patients at stage 3 were treated with PECA or low-dose CDV/CIE
(alternately).
 | Table I.Chemotherapy strategies for infant NB
cases. |
Table I.
Chemotherapy strategies for infant NB
cases.
| Strategy | Drugs | Dosage | Treatment time
point |
|---|
| PECA | P: Cisplatin | 90
mg/m2 | D1 |
|
| E: Etoposide | 100
mg/m2 | D3 |
|
| C:
Cyclophosphamide | 150
mg/m2 | D7-13 |
|
| A: Doxorubicin | 30
mg/m2 | D14 |
| TC | T: tTopotecan | 1.2
mg/m2 | D1-5 |
|
| C:
Cyclophosphamide | 13.3 mg/kg/d | D1-5 |
| CDV | C:
Cyclophosphamide | 1.5
g/m2 | D1-2 |
|
|
Mesna | 420
mg/m2 | 0, 3, 6 and 9 h,
D1-2 |
|
| V: Vincristine | 0.53
mg/m2 | D1-3 |
|
| D: Daunorubicin | 20
mg/m2 | D1-3 |
| CiE | E: Etoposide | 160
mg/m2 | D1-3 |
|
| P: Cisplatin | 40
mg/m2 | D1-4 |
High-dose chemotherapy combined with
APBSCT
According to Children's Oncology Group (COG)
guidelines (17), patients received
either a carboplatin, etoposide and melphalan (CEM) regimen or
busulfan/melphalan as conditioning for APBSCT. Patients with bone
metastasis or bone marrow metastasis received
131I-metaiodobenzylguanidine (131I-MIBG)
prior to pretreatment.
Radiation treatment
Patients ≤1 year old 23re not recommended to undergo
radiotherapy. Patients could be treated with radiotherapy for
metastases when they were >2 years old.
Fluorescence in situ hybridization
(FISH)
Peripheral blood specimens from patients with the
presence of spontaneously dividing tumor cells were collected for
the FISH test. The N-myc gene probe (Invitrogen; Thermo Fisher
Scientific, Waltham, MA, USA), 2,143-bp long, was cloned and marked
as a digoxin DNA probe. A DIG DNA Labeling and Detection kit
(Boehringer Ingelheim, Mannheim, Germany) was used for the FISH
experiments, according to the manufactor's protocols. The N-myc
copy number was scored by counting and averaging the number of
fluorescence signals per interphase nucleus. Cases with a N-myc
copy number of >4 were considered positive for N-myc
amplification.
Serum NSE test
Serum NSE level was assessed with the Roche Elecsys
2010 system (Roche Diagnostics, Basel, Switzerland). The NSE
electrochemical luminescence detection kit was also purchased from
Roche. Patient serum samples were processed according to the
manufactor's protocols. The reference serum NSE value was 0–15.2
ng/dl.
Clinical responses and follow-up
The clinical responses were categorized as follows
(18): i) Complete response (CR):
Tumors totally disappeared after treatment and could not be
detected by CT. The level of α-fetoprotein in the serum stayed
normal for >4 weeks. ii) Partial response (PR): Tumors were
shrunk by >50% and there were no new lesions. CR and PR cases
were counted as cases with effective treatment. iii) Stable disease
(SD): Tumors were shrunk by <50%. The primary tumor size was not
increased and there were no new lesions. iv) No response (NR):
Tumors were shrunk by <25% and there were no new lesions. v)
Progressive disease (PD): Tumor size increased by >25% or new
lesions appeared. Toxicity was evaluated according to World Health
Organisation classification (19),
and classified into stages I–IV.
Follow-up started when the regular chemotherapy
cycles were complete and ended by recurrence, mortality or the
decision to end therapy. The last follow-up was performed on
February 2014.
Statistical analysis
Enumeration data were analyzed by χ2 test
and measurement data were analyzed by Student's t-test. P<0.05
was considered to indicate a statistically significant difference.
The survive rate was analyze by Kaplan-Meier method. All
statistical analyses were performed using SPSS software (version
17.0; SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Among the 16 cases, 10 patients were male and 6 were
female, with a median age of 9.5 months (range, 1–12 months old).
Overall, 5 cases (31.25%) were <3 months old (4 cases at stage
4s and 1 case at stage 4), 2 cases (12.5%) were 3–6 months old
(both at stage 4s) and 9 cases (56.25%) were 6–12 months old (1
case at stage 4s, 6 cases at stage 4 and 2 cases at stage 3). The
data indicated that the frequency of stage 4s cases in patients
<6 months old was significantly higher than the frequency of
stage 4s cases between 6 and 12 months old (χ2 test,
P=0.012). Primary sites were as follows: Mediastinum in 5 cases,
pelvic cavity in 1 case, and retroperitoneal and adrenal area in 10
cases. There were 6 cases with tumors exhibiting N-myc gene
amplification (>4 copies), and only case number 15 had a tumor
with >10 copies. All the clinical information is shown in
Table II.
 | Table II.Clinical features, treatments and
responses of 16 infant neuroblastoma cases. |
Table II.
Clinical features, treatments and
responses of 16 infant neuroblastoma cases.
| Case no. | Gender | Age, months | Stage | Primary site | Metastatic
site | Treatments | Response |
|---|
| 1 | Male | 3 | 4 | Right upper
mediastinum | Superior lobe of
right lung, pleura, thoracic vertebrae, spinal canal | Surgery +
chemotherapy after surgery | CR |
| 2 | Male | 9 | 4 | Breast
neoplasm | Pleural metastasis,
lymph node, thoracic vertebrae, spinal canal | Surgery +
chemotherapy prior to/after surgery | CR |
| 3 | Male | 10 | 4 | Posterior
mediastinum | Right lung,
thoracic cavity | Surgery +
chemotherapy after surgery | CR |
| 4 | Male | 3 | 4s | Right adrenal
gland | Intrahepatic
metastasis (diffuse), hemangioma, spleen | Surgery +
chemotherapy prior to/after surgery | CR |
| 5 | Male | 3 | 4s | Mainly right
adrenal gland | Intrahepatic
metastasis (diffuse) | Surgery +
chemotherapy prior to/after surgery | CR |
| 6 | Male | 2 | 4s | Left adrenal
gland | Intrahepatic
metastasis (diffuse) | Surgery +
chemotherapy after surgery | CR |
| 7 | Female | 1 | 4s | Right adrenal
gland | Intrahepatic
metastasis (diffuse) | Surgery +
chemotherapy prior to/after surgery | CR |
| 8 | Male | 11 | 4 | Left
retroperitoneal space | Liver, lymph node,
skull and left iliac fossa | Surgery +
chemotherapy prior to/after surgery + APBSCT | CR |
| 9 | Female | 11 | 4s | Left adrenal
gland | Left abdominal
subcutaneous, lymph node | Surgery +
chemotherapy after surgery | CR |
| 10 | Male | 12 | 3 | Right
retroperitoneal space | None | Surgery +
chemotherapy after surgery | CR |
| 11 | Male | 12 | 4 | Left
retroperitoneal space | Lumbar vertebrae,
spinal neoplasm canal invasion | Surgery +
chemotherapy after surgery | PR |
| 12a | Male | 5 | 4s | Left adrenal gland,
right retroperitoneal space | Liver, skin, bone,
retroperitoneal space | Surgery +
chemotherapy after surgery | CR |
| 13 | Female | 11 | 4 | Neck,
mediastinum | Neck lymph node,
thoracic vertebrae | Surgery +
chemotherapy prior to/after surgery | CR |
| 14 | Female | 11 | 3 | Front pelvic
sacrum | Spinal canal
invasion | Surgery +
chemotherapy prior to/after surgery | PR |
| 15 | Female | 11 | 4 | Upper and right
retroperitoneal space | Left subclavian
lymph node, retroperitoneal space, spinal cord | Surgery +
chemotherapy prior to/after surgery + 131I-MIBG APBSCT +
radiotherapy | PD |
| 16 | Female | 4 | 4s | Right adrenal
neoplasm | Intrahepatic
metastasis (diffuse) | Surgery +
chemotherapy prior to/after surgery | CR |
The serum NSE levels in all 16 cases (100%) were
above the normal value at the initial diagnosis, with a median of
59.95 ng/dl (range, 17.48–264 ng/dl). After treatment, the serum
NSE levels of all cases were significantly decreased. The serum NSE
levels were within the reference range (0–15.2 ng/dl) in 12 cases
(75.0%), and were slightly above the reference range in the
remaining 3 cases (18.75%).
Summary of treatments, clinical
responses and survival
In this study, 2 cases were diagnosed by biopsy
pathology, 7 cases were diagnosed by surgery and the remaining 7
cases were diagnosed by CT. The International Neuroblastoma Staging
System (INSS) is a post-surgical staging system, which was
developed to establish a consensus approach for pre-treatment risk
stratification of patients with neuroblastoma (20). According to INSS, 2 cases were stage 3
disease (favorable histopathological features), 7 cases were stage
4 (unfavorable histopathological features) and 7 cases were stage
4S (<1 year old with unfavorable biological features). According
to the INSS, 2 cases were at stage 3, 7 cases were at stage 4 and
the remaining 7 cases were at stage 4s. According to COG and
Neuroblastoma Risk Grouping (21), 7
cases were in the high-risk group, 7 cases were in the
intermediate-risk group and 2 cases were in the low-risk group.
From the initial diagnosis to the last follow-up, 7
cases (case numbers 2, 4, 5, 7, 13, 14 and 16) received surgery
plus chemotherapy prior to/after the surgery. Another 7 cases (case
numbers 1, 3, 6, 9, 10, 11 and 12) received surgery plus
chemotherapy after the surgery. A single case (case number 8)
received surgery plus chemotherapy prior to/after surgery, as well
as APBSCT. Another case (case number 15) received surgery plus
chemotherapy prior to/after surgery, as well as APBSCT with
131I-MIBG.
Until the last follow-up in September 2014, the
median follow-up time of the patients was 29.5 months. As shown in
Table II, 13 cases (81.25%)
presented with a CR, 2 cases with a PR (12.5%) and 1 case with PD
(6.25%). The patient with PD (case number 15) experienced
recurrence following transplantation and now survived with tumors.
The prognosis of the stage 4 cases was significantly worse than
that of the stage 4s and stage 3 cases (χ2 test,
P=0.045). There was no significant difference in the prognosis
between the stage 4s and stage 3 cases (P>0.05).
At the time of the last follow-up, all patients
remained alive. Kaplan-Meier analysis showed that the overall
survival rate for 2 years was 100% (16/16). The event-free survival
rate was 81.25% (13/16).
Outcome of PECA strategy for stage 4s
patients
A total of 7 cases at stage 4s were treated with
PECA prior to and after surgery; of these, 5 cases (71.4%) received
6 cycles of chemotherapy, 1 case (14.3%) received 7 cycles of
chemotherapy and another 1 case (14.3%) received 8 cycles of
chemotherapy. Until the last follow-up, 6 cases presented with a CR
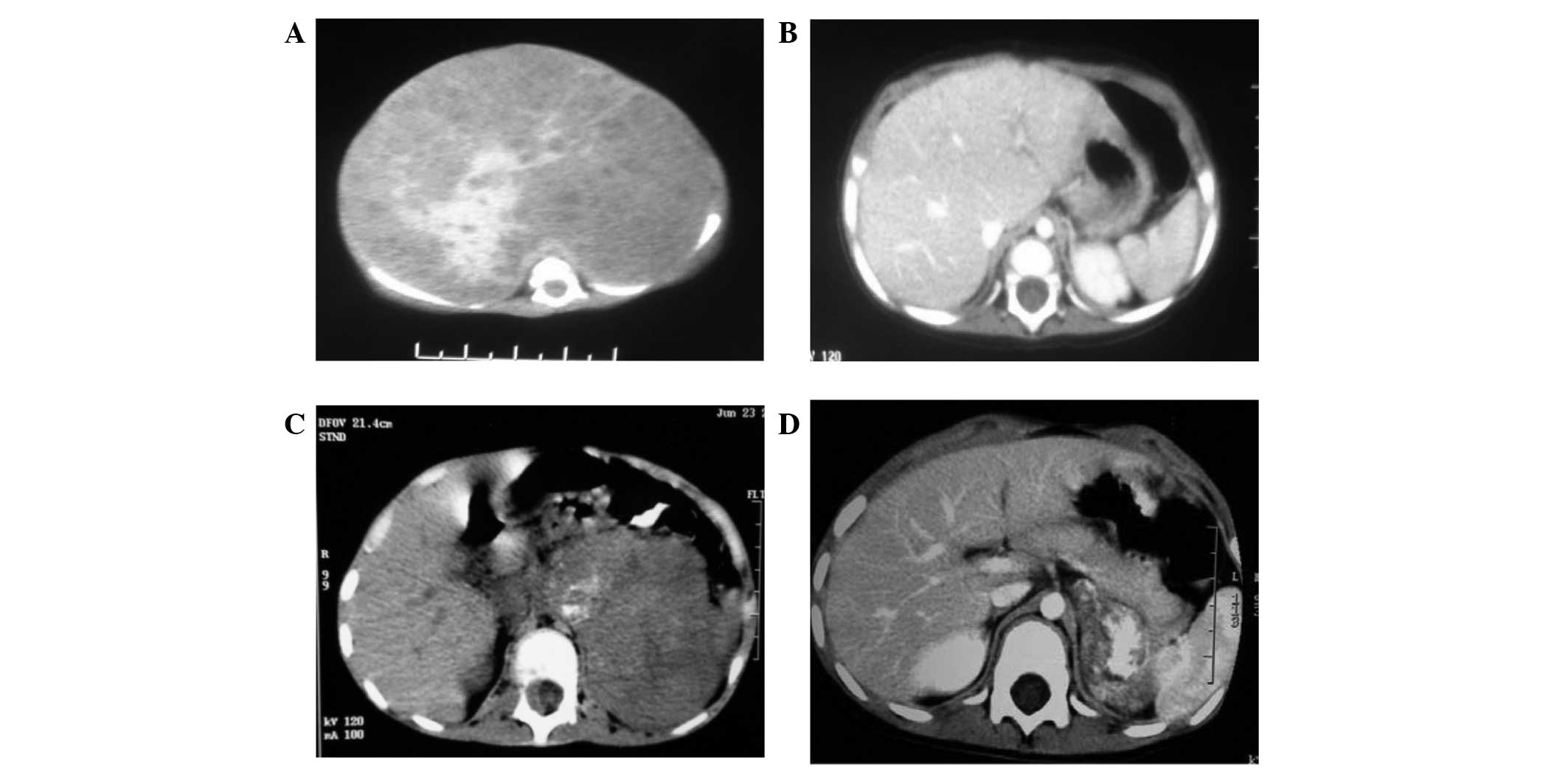
and 1 case with a PR (case number 12). The CT images from a CR case
(case number 7) prior to and after the surgery and PECA treatment
are shown in Fig. 1A and B. Case
number 12 underwent surgery consisting of liver metastasis
resection and eventually achieved a CR. The efficiency of the PECA
strategy was 100%. Furthermore, the toxicity evaluation for those
patients was stage I (slight loss of appetite, vomiting and bone
marrow suppression), but all patients recovered after appropriate
treatment.
High-dose chemotherapy combined with
APBSCT
In this study, 2 patients at stage 4 were treated
with high-dose chemotherapy combined with APBSCT after regular
surgery and chemotherapy (case numbers 8 and 15). Case number 8 was
an 11-month-old who had presented with a tumor in the left
retroperitoneal space, with metastasis to the lymph nodes at the
left iliac fossa, liver and skull. This patient received 2 cycles
of chemotherapy prior to surgery and 8 cycles of chemotherapy after
surgery, which did not lead to a CR. Hence, this case was further
treated with CEM combined with APBSCT. Pirarubicin resulted in
cardiomegaly and stage III chronic cardiac insufficiency after
regular chemotherapy. APBSCT treatment and oral administration of
small-dose digoxin (0.125 mg/day) for 6 months aided the recovery
of echocardiography results and cardiac function. By the time of
the last follow-up, the patient had survived for 75 months
(Fig. 1C and D).
Case number 15 initially exhibited a fever and
diarrhea. Biopsy and FISH detection showed that this patient had
strong N-myc amplification (>10 copies). The patient was treated
with 3 cycles of regular chemotherapy, then surgery followed by 6
cycles of chemotherapy, and a second surgery. Treatment was
continued with 131I-MIBG combined with APBSCT in another
hospital. At 2 months post-transplantation, extensive metastasis to
the skull occurred, which was followed by 11 cycles of chemotherapy
with a total dose of 20 Gy. Until the date of follow-up, this
patient had survived with PD, and the time course of the disease
was 47 months (Fig. 2).
Toxicity evaluation
The toxicity evaluation for these 16 patients was as
follows: 14 cases were at stage I, 1 case was at stage II (case
number 7) and 1 case was at stage III (case number 8). Cases with
toxicity at stage I showed bone marrow inhibition and the leukocyte
count in the peripheral blood reached the lowest value of
0.85±0.23×109/l (normal range,
6.0–17.5×109/l). The average recovery time was 6.2±1.3
days. Case number 7 with toxicity at stage II showed bone marrow
inhibition and an increase in platelet count in the peripheral
blood (1,100×109/l; normal range,
220–460×109/l). Case number 8 with toxicity at stage III
showed bone marrow inhibition and dilated cardiomyopathy. Following
regular treatment with small-dose digoxin for 6 months, the dilated
cardiomyopathy was relieved.
Discussion
NB in children usually initiates in the
retroperitoneal space, adrenal area, mediastinum, spine or pelvic
cavity (22). NB in infants,
particularly those <6 months old, is usually found at stage 4s
(22). Characteristics of this
disease include a young onset of disease, incomplete development of
visceral organ function, a low body weight and huge tumors
(22). Certain tumors are found
during the prenatal diagnosis prior to delivery (22). In the present study, patients < 6
months old were all at stage 4s, which was consistent with the
results of previous studies (23,24).
According to the COG classification, the majority of cases were in
the low- and intermediate-risk groups (9 cases), and the rest were
in the high-risk group (7 cases). According to the results,
although NB malignancy is high and the progression is fast, the
risk of NB cases occurring within patients <1 year old is
relatively mild.
NSE is a glycolytic enzyme involved in the
glycolysis pathway. NSE is usually expressed in neurons, peripheral
nerve tissues and neuroendocrine tissues (such as brain spinal cord
and branching peripheral nerves), and in the cell system (such as
endocrine cells, which can take in amine and amine precursor and
produce peptide and/or amine hormones) (25–28). NSE
is released prior to cell damage and serve as specific markers for
mature neuroendocrine neoplasms (29). Recently, NB immunohistochemistry
revealed that NB patients are all NSE-positive (with better
staining intensity in intermediate-risk NB) (30). In the present study, the serum NSE
levels in the 16 cases were all above the normal range at the time
of diagnosis. Following treatment, the NSE levels were decreased to
normal in 75% of cases, indicating that although the malignance of
infant NB was slightly less than that in elder children, and
certain patients at stage 4s experience tumor regression without
any treatment, the serum NSE level remains one of the important
indicators for diagnosis and treatment response.
It has been reported that the different primary
sites of NB exhibit important differences in terms of clinical and
biological features (31). In the
present study, a direct correlation was not observed between
primary tumor sites and prognosis, most likely due to the limited
number of cases. However, the primary tumor site affects numerous
aspects of NB treatment and progression, including the decision for
primary surgery, residual diseases, and tumor metastasis and
invasion. Therefore, the primary tumor site still impacts the
clinical outcome. For instance, an initial resection of the tumor
in case number 14 was not performed, as this case had a primary
tumor site at the front pelvic sacrum, and during chemotherapy
prior to surgery, spinal canal invasion was observed in this
case.
According to the COG and Pediatrics Oncology Group
guidelines for NB treatment, and the characteristics of NB during
diagnosis (the size and location of the primary tumor, metastasis
and the tolerance degree to surgery), the NB treatments include
capacity reduction chemotherapy prior to surgery, chemotherapy
after surgery, and high-dose chemotherapy combined with ABPSCT
(31,32). However, 30% of NB infants at stage 4s,
particularly those <6 months old, experience tumor regression.
Hence, the choice of whether to treat with chemotherapy, the
decision on the number of cycles of chemotherapy and the choice of
whether to treat with APBSCT have not been standardized in China,
as well as in other countries. Akramipour et al (33) reported that 1 patient at stage 1
survived event-free after surgery only and 36 months of follow-up.
In 2012, Nuchtern et al (34)
reported that 87 NB cases <6 months old had a >90% 3-year
survival rate after surgery only and observation. However, it is
believed that mild- and high-risk cases should be treated with
chemotherapy. Capasso et al (35) showed that during 2007–2012, the
condition of 5 cases at stage 4s treated with a cyclophosphamide,
vincristine and Adriamycin, and carboplatin and etoposide
strategies was completely relieved, while 1 case had not accepted
chemotherapy for 15 months, and was then treated with 2 cycles of
chemotherapy during disease progression until the condition was
eventually relieved. In the present study, all 16 cases were
treated with chemotherapy. Case number 8 was partially relieved
after surgery combined with 10 cycles of chemotherapy, and case
number 15 at stage 4 with amplification of N-myc was treated with
high-dose chemotherapy combined with APBSCT. The former survived
without disease and the latter experienced progression of the
recurrence after APBSCT. Hence, based on the international and
domestic studies, and the efficacy and the safety evaluation of
these 16 cases, with regard to chemotherapy for NB infants, the
cases in the mild- and high-risk groups were treated with
chemotherapy. Depending on the size of primary tumors and whether
surgery can achieve complete excision, cases in the low-risk group
can be treated with chemotherapy. NB infants, who are less than 6
months old and have no N-myc gene amplification, may undergo
surgery followed by low-dose chemotherapy to reduce the time of
tumor relief and improve survival quality. Chemotherapy could be
carefully chosen according to the original diagnosis to achieve
tolerance and efficiently inhibit tumor growth. However, for APBSCT
application, although 2 cases in the present study were treated
with APBSCT, 1 patient experienced recurrence after 2 months of
treatment. Therefore, we believe that after considering the
advantages and disadvantages, NB infants, particularly those at
stage 4s, are not suitable for APBSCT treatment. Although the
responses for cases at stage 4 were not totally satisfactory, only
1 case exhibited recurrence, 2 cases were partially relieved and
survived with tumors, and the remaining 4 cases had a CR and
event-free survival, indicating that NB infants have a high
response rate and a high survival rate. Hence, the treatment
confidence of family members of patients should be buoyed to
further improve the cure rate of NB patients.
In conclusion, the main treatments for NB infants
were surgery and chemotherapy. Although NB infants have a low onset
age, a serious initial condition, a higher risk of chemotherapy and
more difficulties during surgery than older children, appropriate,
reasonable and comprehensive treatment can achieve high relief and
survival rates.
Acknowledgements
This study was supported by the #215 High-Level
Talent Program of Beijing Municipal Health Bureau (grant no.
2015-3-018, to Y.Z.).
References
|
1
|
Evans A: Neuroblastoma: A historical
perspective 1864–1998Neuroblastoma. Brodeur GM, Sawada T, Tsuchida
Y and Voûte PA: Elsevier Science; Amsterdam: pp. 1–7. 2000
|
|
2
|
Yáñez Y, Grau E, Rodríguez-Cortez VC,
Hervás D, Vidal E, Noguera R, Hernández M, Segura V, Cañete A,
Conesa A, et al: Two independent epigenetic biomarkers predict
survival in neuroblastoma. Clin Epigenetics. 7:162015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
London WB, Castleberry RP, Matthay KK,
Look AT, Seeger RC, Shimada H, Thorner P, Brodeur G, Maris JM,
Reynolds CP and Cohn SL: Evidence for an age cutoff greater than
365 days for neuroblastoma risk group stratification in the
children's oncology group. J Clin Oncol. 23:6459–6465. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Look AT, Hayes FA, Shuster JJ, Douglass
EC, Castleberry RP, Bowman LC, Smith EI and Brodeur GM: Clinical
relevance of tumor cell ploidy and N-myc gene amplification in
childhood neuroblastoma: A pediatric oncology group study. J Clin
Oncol. 9:581–591. 1991.PubMed/NCBI
|
|
5
|
Caron H: Allelic loss of chromosome 1 and
additional chromosome 17 material are both unfavourable prognostic
markers in neuroblastoma. Med Pediatr Oncol. 24:215–221. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lampert F, Rudolph B, Christiansen H and
Franke F: Identical chromosome 1p breakpoint abnormality in both
the tumor and the constitutional karyotype of a patient with
neuroblastoma. Cancer Genet Cytogenet. 34:235–239. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cohn SL, Pearson AD, London WB, Monclair
T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, et
al: The international neuroblastoma risk group (INRG)
classification system: An INRG task force report. J Clin Oncol.
27:289–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spitz R, Hero B, Simon T and Berthold F:
Loss in chromosome 11q identifies tumors with increased risk for
metastatic relapses in localized and 4S neuroblastoma. Clin Cancer
Res. 12:3368–3373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Castleberry RP: Consistently investigating
the inconsistent nature of neuroblastoma. J Pediatr Hematol Oncol.
21:178–180. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Irwin MS and Park JR: Neuroblastoma:
Paradigm for precision medicine. Pediatr Clin North Am. 62:225–256.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Monclair T, Brodeur GM, Ambros PF, Brisse
HJ, Cecchetto G, Holmes K, Kaneko M, London WB, Matthay KK,
Nuchtern JG, et al: The international neuroblastoma risk group
(INRG) staging system: An INRG task force report. J Clin Oncol.
27:298–303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brodeur GM, Seeger RC, Schwab M, Varmus HE
and Bishop JM: Amplification of N-myc in untreated human
neuroblastomas correlates with advanced disease stage. Science.
224:1121–1124. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seeger RC, Brodeur GM, Sather H, Dalton A,
Siegel SE, Wong KY and Hammond D: Association of multiple copies of
the N-myc oncogene with rapid progression of neuroblastomas. N Engl
J Med. 313:1111–1116. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimada H, Ambros IM, Dehner LP, Hata J,
Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK, et
al: The international neuroblastoma pathology classification (the
Shimada system). Cancer. 86:364–372. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng JM, Hiemstra JL, Schneider SS,
Naumova A, Cheung NK, Cohn SL, Diller L, Sapienza C and Brodeur GM:
Preferential amplification of the paternal allele of the N-myc gene
in human neuroblastomas. Nat Genet. 4:191–194. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Landier W, Bhatia S, Eshelman DA, Forte
KJ, Sweeney T, Hester AL, Darling J, Armstrong FD, Blatt J,
Constine LS, et al: Development of risk-based guidelines for
pediatric cancer survivors: the Children's Oncology Group Long-Term
Follow-Up Guidelines from the Children's Oncology Group Late
Effects Committee and Nursing Discipline. J Clin Oncol.
22:4979–4990. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malogolowkin MH, Katzenstein HM, Krailo M,
Chen Z, Quinn JJ, Reynolds M and Ortega JA: Redefining the role of
doxorubicin for the treatment of children with hepatoblastoma. J
Clin Oncol. 26:2379–2383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Franklin HR, Simonetti GP, Dubbelman AC,
ten Bokkel Huinink WW, Taal BG, Wigbout G, Mandjes IA, Dalesio OB
and Aaronson NK: Toxicity grading systems. A comparison between the
WHO scoring system and the Common Toxicity Criteria when used for
nausea and vomiting. Ann Oncol. 5:113–117. 1994.PubMed/NCBI
|
|
20
|
Brodeur GM, Seeger RC, Barrett A, Berthold
F, Castleberry RP, D'Angio G, De Bernardi B, Evans AE, Favrot M,
Freeman AI, et al: International criteria for diagnosis, staging,
and response to treatment in patients with neuroblastoma. J Clin
Oncol. 6:1874–1881. PubMed/NCBI
|
|
21
|
Cohn SL, Pearson AD, London WB, Monclair
T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, et
al: The International Neuroblastoma Risk Group (INRG)
classification system: an INRG Task Force report. J Clin Oncol.
27:289–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dumba M, Jawad N and McHugh K:
Neuroblastoma and nephroblastoma: A radiological review. Cancer
Imaging. 15:52015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schleiermacher G, Rubie H, Hartmann O,
Bergeron C, Chastagner P, Mechinaud F and Michon J: Neuroblastoma
Study Group of the French Society of Paediatric Oncology: Treatment
of stage 4s neuroblastoma-report of 10 years' experience of the
French Society of Paediatric Oncology (SFOP). Br J Cancer.
89:470–476. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hiyama E, Iehara T, Sugimoto T, Fukuzawa
M, Hayashi Y, Sasaki F, Sugiyama M, Kondo S, Yoneda A, Yamaoka H,
et al: Effectiveness of screening for neuroblastoma at 6 months of
age: a retrospective population-based cohort study. Lancet.
371:1173–1180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kushner BH, Modak S, Kramer K, LaQuaglia
MP, Yataghene K, Basu EM, Roberts SS and Cheung NK: Striking
dichotomy in outcome of MYCN-amplified neuroblastoma in the
contemporary era. Cancer. 120:2050–2059. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ando S, Suzuki M, Yamamoto N, Iida T and
Kimura H: The prognostic value of both neuron-specific enolase
(NSE) and Cyfra21-1 in small cell lung cancer. Anticancer Res.
24:1941–1946. 2004.PubMed/NCBI
|
|
27
|
Karnak D, Beder S, Kayacan O, Ibis E and
Oflaz G: Neuron-specific enolase and lung cancer. Am J Clin Oncol.
28:586–590. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tapia FJ, Polak JM, Barbosa AJ, Bloom SR,
Marangos PJ, Dermody C and Pearse AG: Neuron-specific enolase is
produced by neuroendocrine tumours. Lancet. 1:808–811. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gallagher KK, Spector ME, Pepper JP,
McKean EL, Marentette LJ and McHugh JB: Esthesioneuroblastoma:
Updating histologic grading as it relates to prognosis. Ann Otol
Rhinol Laryngol. 123:353–358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vo KT, Matthay KK, Neuhaus J, London WB,
Hero B, Ambros PF, Nakagawara A, Miniati D, Wheeler K, Pearson AD,
et al: Clinical, biologic, and prognostic differences on the basis
of primary tumor site in neuroblastoma: A report from the
international neuroblastoma risk group project. J Clin Oncol.
32:3169–3176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stram DO, Matthay KK, O'Leary M, Reynolds
CP, Haase GM, Atkinson JB, Brodeur GM and Seeger RC: Consolidation
chemoradiotherapy and autologous bone marrow transplantation versus
continued chemotherapy for metastatic neuroblastoma: A report of
two concurrent children's cancer group studies. J Clin Oncol.
14:2417–2426. 1996.PubMed/NCBI
|
|
32
|
Vogelzang NJ, Benowitz SI, Adams S,
Aghajanian C, Chang SM, Dreyer ZE, Janne PA, Ko AH, Masters GA,
Odenike O, et al: Clinical cancer advances 2011: Annual report on
progress against cancer from the American society of clinical
oncology. J Clin Oncol. 30:88–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akramipour R, Zargooshi J and Rahimi Z:
Infant with concomitant presence of hernia/hydrocele and primary
paratesticular neuroblastoma: A diagnostic and therapeutic
challenge. J Pediatr Hematol Oncol. 31:3492009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nuchtern JG, London WB, Barnewolt CE,
Naranjo A, McGrady PW, Geiger JD, Diller L, Schmidt ML, Maris JM,
Cohn SL and Shamberger RC: A prospective study of expectant
observation as primary therapy for neuroblastoma in young infants:
A children's oncology group study. Ann Surg. 256:573–580. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Capasso M, Cinalli G, Nastro A, Giuliano
M, Errico ME, Caccioppoli U, Turco R, Ruotolo S, Vetrella S, De
Bernardi B, et al: Symptomatic epidural compression in infants with
neuroblastoma: A single-center experience with 5 cases. J Pediatr
Hematol Oncol. 35:260–266. 2013. View Article : Google Scholar : PubMed/NCBI
|