Introduction
Curcumin, which is commonly called
diferuloylmethane, is derived from Curcuma longa, a plant of
the ginger family (1). Extensive
research over the last half century has revealed the therapeutic
potential of curcumin in tumor progression, including inducing
apoptosis, inhibiting angiogenesis and enhancing susceptibility to
chemotherapy and radiotherapy (1,2).
Furthermore, the anticancer effect of curcumin has been confirmed
in a number of clinical trials, in which is has been used as a
natural chemoprevention agent in colorectal and pancreatic cancer
(3–5).
Accumulating evidence suggests that curcumin has a diverse range of
molecular targets, including c-Myc, cyclooxygenase-2, Notch1,
nuclear factor-κB and p53 (2,6–8).
The tumor suppressor p53 plays a pivotal role in the
etiology of human cancers; it not only controls the cellular
proliferation of tumor cells, but is also capable of inducing cell
apoptosis (9). Previous studies
reported that curcumin induced p53 expression in prostate cancer,
B-cell lymphoma (Bcl) cells and breast cancer, and thereby
activated the pro-apoptotic downstream genes p21 and
Bcl-2-associated X protein (Bax) and inhibited Bcl-2
(anti-apoptosis) expression to induce apoptotic progress (2,10,11). Furthermore, curcumin induced
cell-cycle arrest by downregulating cyclin D1 expression (2,10,11).
In gastric cancer, curcumin attenuated in
vivo tumor growth induced by N-methyl-N-nitrosourea by
downregulating the expression of cyclin D1 in tumor cells (12). In in vitro studies, curcumin
induced cell apoptosis by reducing Bcl-2 expression or enhancing
reactive oxygen species production, and induced a G1 cell cycle
arrest by downregulating cyclin D1 expression (12–15).
Activation of the phosphoinositide 3-kinase (PI3K)/AKT pathway was
also inhibited by curcumin, and played a role in promoting cell
apoptosis (16). Although Bcl-2 and
cyclin D1 are downstream molecules of p53 (17), and LY294002 (a PI3K inhibitor) was
shown to induce p53 expression and p53-dependent apoptosis in
gastric cancer cells by inhibiting the activation of PI3K/AKT
signaling (18), there is not yet
sufficient evidence to confirm that curcumin regulates p53
expression in gastric cancer cells.
The long non-coding RNA (lncRNA) H19 is produced
from the paternally imprinted H19 gene and is considered an
oncogenic lncRNA in various cancers (19–22).
Furthermore, previous studies have reported that H19 is abnormally
upregulated in gastric cancer (23–25) and
contributes to cellular proliferation by directly inactivating p53
(26). Notably, curcumin
downregulated H19 gene transcription and c-Myc expression in human
tumor cells (2,27,28). In
addition, the c-Myc oncogene was shown to directly induce H19
expression by binding to the H19 promoter, and thereby promoted the
proliferation of gastric cancer cells (29,30).
The present study aimed to determine whether
curcumin suppresses the proliferation of gastric cancer cells by
regulating c-Myc/H19/p53 signaling. It was confirmed that curcumin
inhibited the proliferation of gastric cancer cells, suppressed H19
and c-Myc expression, and enhanced p53 expression in a time- and
concentration-dependent manner. Overexpression of H19 in gastric
cancer cells reversed curcumin-induced cell apoptosis and the
inhibitory effect on cell proliferation, as well as decreasing p53
expression in the presence of curcumin. Furthermore, exogenous
c-Myc enhanced H19 expression in gastric cancer cells in the
presence of curcumin. Together, these results suggested that
curcumin exploited a novel mechanism to inhibit gastric cancer cell
growth.
Materials and methods
Reagent and cell culture
Curcumin (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) was dissolved in dimethyl sulfoxide (Sigma-Aldrich; Merck
Millipore) and stored at −20°C until use. Active human c-Myc
full-length protein was purchased from Abcam (Cambridge, MA, USA)
and added to media for a final concentration of 5 µg/ml (31). The human gastric cancer cell line
SGC7901 and the immortalized human gastric epithelial mucosa cell
line GES-1 were obtained from the American Type Culture Collection
(Manassas, VA, USA). All cell lines were maintained in RPMI-1640
medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and cultured in a humidified
incubator containing 5% CO2 at 37°C. For the c-Myc
protocol, recombinant human c-Myc protein (5 µg/ml) was added to
the media of SGC7901 cells in the presence of 50 µM curcumin.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA was extracted from the cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. RNase-free DNase I
(Thermo Fisher Scientific, Inc.) treatment was performed to remove
any contaminating DNA. RT-qPCR was performed using the ReverTra
Ace-α first-strand cDNA synthesis kit and the SYBR Green Real-time
PCR Master mix kit (both Toyobo Co., Ltd., Osaka, Japan). For mRNA
detection, the primers used in this study were as follows: H19
forward, 5′-TACAACCACTGCACTACCTG-3′ and reverse,
5′-TGGAATGCTTGAAGGCTGCT-3′ (32); and
GAPDH (as an internal control) forward, 5′-ACCTGACCTGCCGTCTAGAA-3′
and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′ (33). The ABI StepOne Plus (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used to perform
qPCR. PCR reactions were performed at 95°C for 5 min, followed by
40 cycles of 95°C for 15 sec and 60°C for 1 min. Each experiment
was performed in triplicate. The relative mRNA expression levels
were determined using the 2−ΔΔCq method (34).
Transfection
H19 cDNA (GenBank accession no. NR_002196.1) was
inserted into the multiple cloning sites of the pcDNA3.1 vector
(Invitrogen; Thermo Fisher Scientific, Inc.), as described
previously (33). A total of
1×105 cells were plated onto 24-well plates for 24 h and
then transfected with 0.5 µg plasmid using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h. The cells
were then subjected to RNA/protein extraction or further functional
assays.
Cell proliferation assay
Cell proliferation assays were performed using a
Cell Counting kit-8 (CCK-8; Beyotime Institute of Biotechnology,
Shanghai, China), as described previously (35). Briefly, SGC7901 cells
(1×104 cells/well) were plated onto 96-well plates, and
then treated with curcumin or pre-transfected with pcDNA3.1-H19 or
empty vector for 48 h. The number of cells per well was detected by
measuring the absorbance (450 nm) of reduced WST-8 at various time
points using the SpectraMax® i3x microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA).
Cell apoptosis
Evaluation of cell apoptosis was performed using the
FITC Annexin V Apoptosis Detection kit with PI (BioLegend, Inc.,
San Diego, CA, USA). Briefly, the cells were washed twice with cold
BioLegend's Cell Staining Buffer, and then resuspended in Annexin V
Binding Buffer at a concentration of 0.25–1.0×107
cells/ml. This suspension (100 µl) was stained with 5 µl
FITC/Annexin V and 10 µl PI, after which the cells were gently
vortexed and incubated for 15 min at room temperature (25°C) in the
dark. Subsequently. 400 µl Annexin V Binding Buffer was added to
each tube, which were analyzed by flow cytometry.
Ki67 staining
The cells were washed twice with PBS by
centrifugation at 350 × g for 5 min at 4°C, and then
resuspended in 3 ml cold 70% ethanol and incubated at −20°C for 1
h. Subsequently, the cells were resuspended in 100 µl PBS in the
presence of phycoerythrin-conjugated anti-human Ki67 antibody
(1:20; cat. no., 350504; BioLegend, Inc.), and then incubated at
room temperature in the dark for 30 min. Next, 500 µl PBS was added
to resuspend the cells for flow cytometric analysis.
Western blotting
Proteins were extracted from the cells using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) and were quantified using a BCA Protein Assay kit
(Beyotime Institute of Biotechnology). Proteins (30 µg) were
separated by 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membrane was blocked with 5% nonfat milk and incubated with diluted
antibodies at 4°C overnight. Primary antibodies against p53
(1:1,000; cat. no. 1C12), Bax (1:1,000; cat. no. D2E11), Bcl-2
(1:1,000; cat. no. 50E3), c-Myc (1:1,000; cat. no. D84C12) and
β-actin (1:1,000; cat. no. 13E5) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Subsequently, the membranes
were incubated with a horseradish peroxidase-conjugated secondary
antibody (1:2,000; cat. no., sc-2055; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at 37°C for 1 h. The immunoreactive bands
were visualized using the Immobilon™ Western Chemiluminescent HRP
Substrate (EMD Millipore) and the UVP Bioimaging system (UVP, Inc.,
Upland, CA, USA).
Statistical analysis
All experiments were performed three times. Data are
presented as the mean ± standard deviation and analyzed using
GraphPad Prism 5.00 software (GraphPad Software, Inc., La Jolla,
CA, USA). Differences among the groups were assessed by one-way
analysis of variance followed by Neuman-Keuls post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Curcumin inhibits gastric cancer cell
proliferation and H19 expression
Initially, the effect of curcumin on the
proliferation of the gastric cancer cell line SGC7901 was analyzed
by CCK-8 assays in the presence of various concentrations of
curcumin for 12, 24, 48 and 72 h. As shown in Fig. 1A, curcumin inhibited the growth of
SGC7901 cells in a concentration- and a time-dependent manner. In
comparison with the untreated cells, cell proliferation was
significantly inhibited after 48 h of treatment with 50 µM curcumin
(P<0.01). The relative mRNA expression level of H19 was
decreased in a dose-dependent manner following treatment with
various concentrations of curcumin (Fig.
1B), and, as compared with SGC7901 cells in the absence of
curcumin, showed the lowest level at 50 µM (P<0.0001). As for
p53 expression in SGC7901 cells, curcumin markedly increased the
expression level of p53 after 12 h (Fig.
1C and attained a peak at 48 h following treatment with 25 µM
curcumin (Fig. 1D).
Ectopic expression of H19 reverses
curcumin-mediated inhibition of proliferation
To further elucidate the role of H19 in
curcumin-induced proliferative inhibition of gastric cancer cells,
H19 was overexpressed in SGC7901 cells, which were subsequently
treated with 50 µM curcumin, as this concentration of curcumin
induced the highest level of proliferative inhibition (Fig. 1A). As compared with the empty vector
control, ectopic expression of H19 significantly enhanced cell
proliferation in the presence of curcumin, as determined using the
CCK-8 assay (P<0.05; Fig. 2A) or
Ki67 staining (Fig. 2B), which is a
nuclear antigen only present in proliferating cells (36). In pcDNA3.1-H19-transfected cells,
curcumin downregulated H19 expression (Fig. 2C), but did not enhance p53 expression
(Fig. 2D). As H19 directly binds to
p53 and deactivates p53 expression (26), curcumin may depend on the inhibition
of H19 expression to enhance the tumor-suppressive activity of p53.
These results suggest that curcumin enhances p53 expression by
downregulating H19 expression.
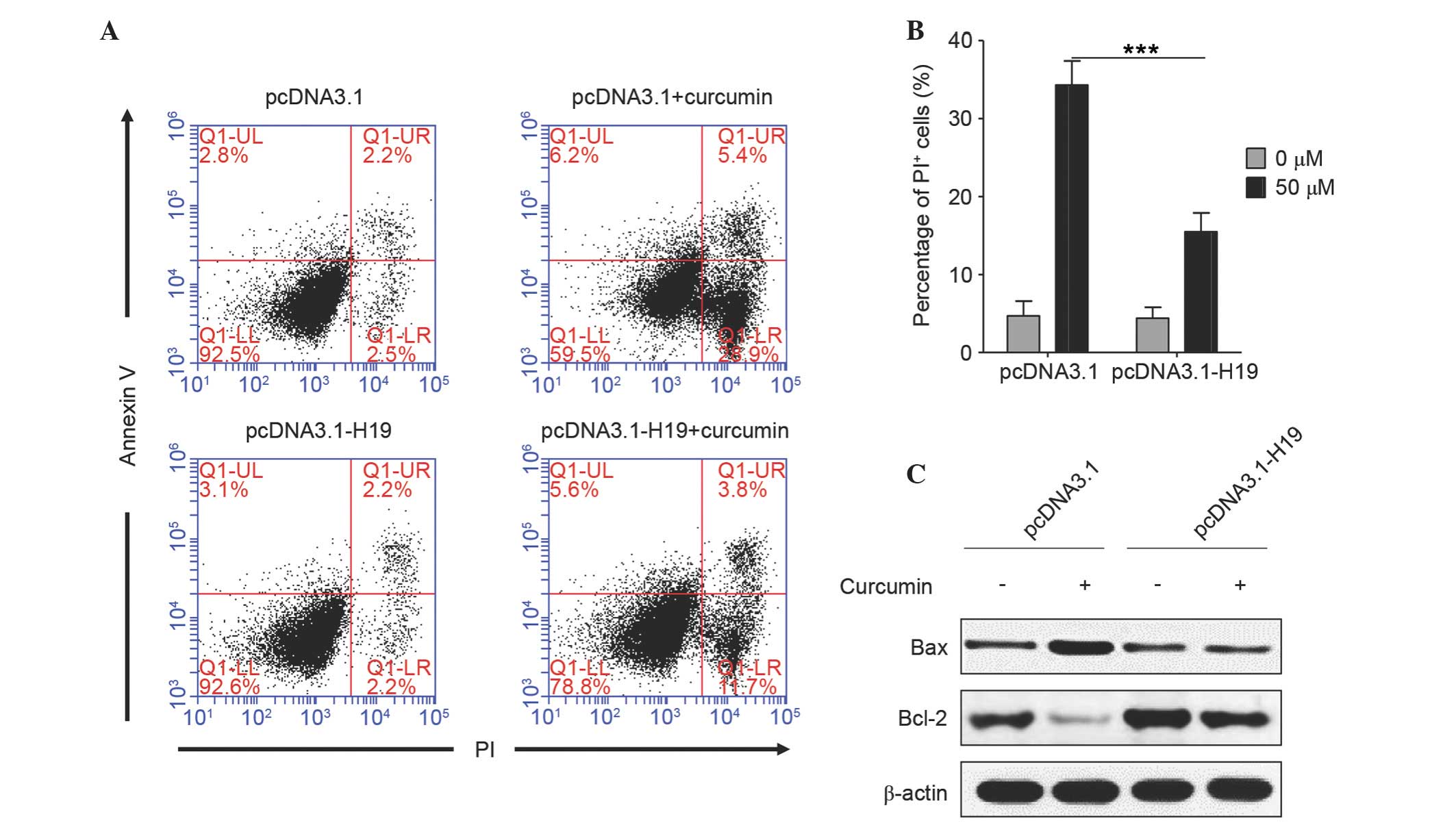
Ectopic expression of H19 reverses
curcumin-induced cell apoptosis
Subsequently, the role of H19 in curcumin-induced
apoptosis of SGC7901 cells was analyzed. As shown in Fig. 3A and B, there was no significant
difference in cell apoptosis between the cells transfected with
empty vector and pcDNA3.1-H19 (PI-positive, 4.7 vs. 4.4%), which
suggested that plasmid transfection did not induce a difference in
cell apoptosis. Curcumin significantly induced the apoptosis of
cells transfected with empty vector (~34.3% were PI-positive),
whereas, in H19-transfected cells, the percentage of apoptotic
cells was ~15.5%, which was significantly lower compared with cells
transfected with empty vector (P<0.0001). An increase in the
ratio of Bax/Bcl-2 is known to initiate apoptosis (17); it was noted that curcumin markedly
increased Bax expression and decreased Bcl-2 expression in empty
vector-transfected cells, while this effect was almost diminished
in H19-overexpressed cells (Fig. 3C).
These results suggest that curcumin induces cell apoptosis by
downregulating H19 expression.
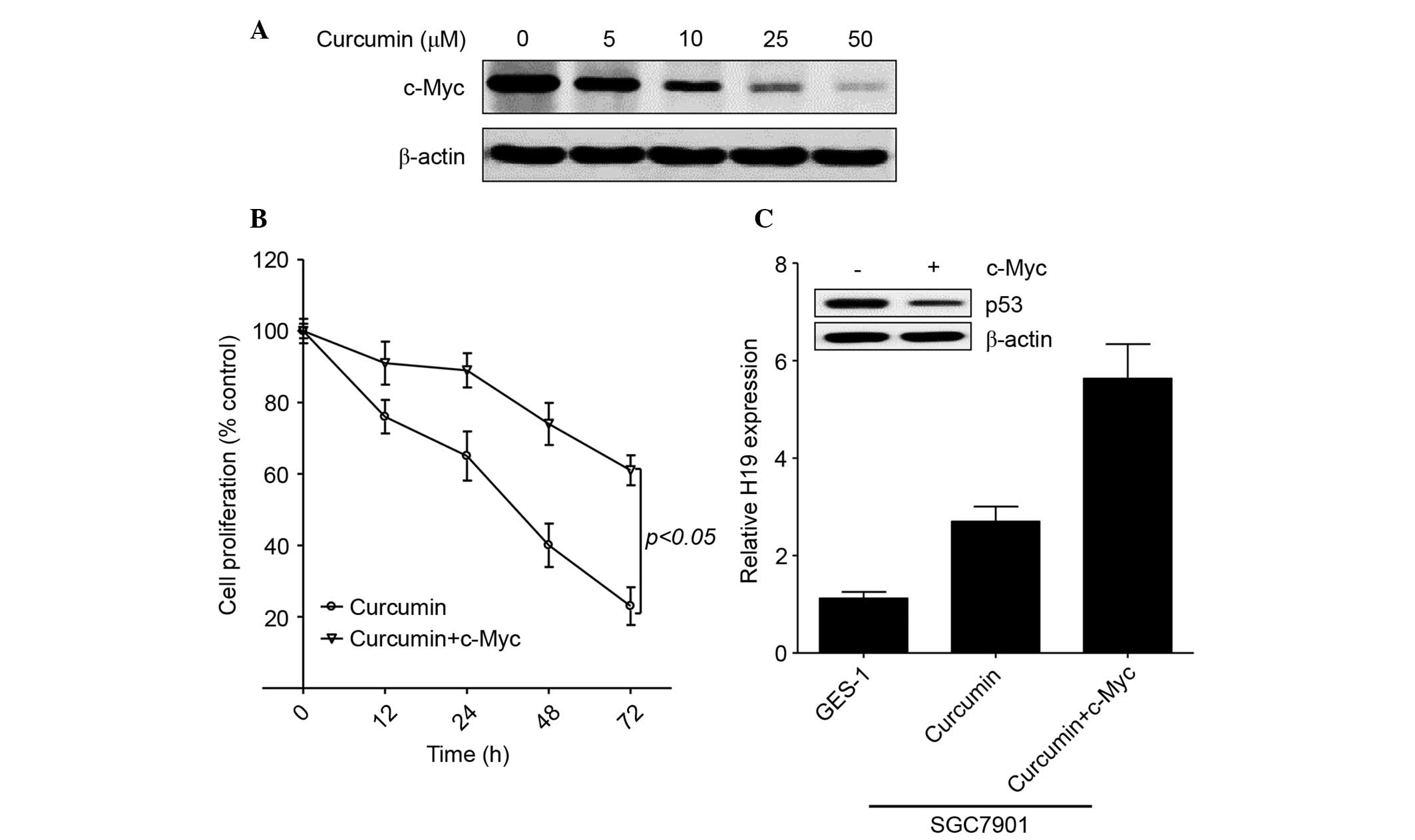
c-Myc enhances H19 expression in
curcumin-treated gastric cancer cells
As an oncogene, the expression of c-Myc has been
shown to be upregulated in patients with gastric cancer and to
induce H19 expression in gastric cancer cells (29,30).
Furthermore, curcumin inhibited c-Myc expression in Bcl and skin
cancer (2,28). Therefore, the present study further
evaluated the role of c-Myc in regulating H19 expression in the
presence of curcumin. As shown in Fig.
4A, curcumin markedly decreased c-Myc expression in gastric
cancer cells in a concentration-dependent manner. Similar to H19,
exogenous c-Myc induced cell proliferation in the presence of 50 µM
curcumin (Fig. 4B). In addition,
exogenous c-Myc enhanced H19 expression and decreased p53
expression in curcumin-treated SGC7901 cells (Fig. 4C). These results confirm that curcumin
inhibits H19 expression by regulating c-Myc expression in gastric
cancer.
Discussion
Gastric cancer is the fifth most common malignancy
and the third leading cause of cancer-associated mortality
worldwide, with an estimated 952,000 new cases diagnosed and
723,000 deaths registered in 2012 (37). Previous studies have demonstrated that
H19 plays an oncogenic role in gastric cancer and predicts a poor
prognosis in patients with gastric cancer (25,26,29,33,38).
However, an agent that is able to downregulate H19 expression in
tumor cells has rarely been reported (39). The present study demonstrated that
curcumin, a naturally occurring phytochemical, was able to inhibit
H19 expression in gastric cancer cells and thereby induce apoptosis
and inhibit cellular proliferation.
Curcumin is able to suppress the proliferation and
survival of cancer cells by directly or indirectly binding to
various targets, including transcription factors, growth factors
and several proteins that are involved in cell signal transduction
pathways (40). c-Myc is an important
oncogene that has been shown to be downregulated by curcumin
(2). Similarly, the present study
observed that curcumin decreased c-Myc expression in a
concentration-dependent manner in gastric cancer. c-Myc regulates
numerous gene targets that subsequently execute its many biological
activities, including cell proliferation, transformation,
angiogenesis and apoptosis (41).
Furthermore, elevated expression of c-Myc correlates with a poor
prognosis in various cancers, including head and neck squamous cell
carcinoma, breast cancer and hepatocellular carcinomas (42–45). The
present study also demonstrated that exogenous c-Myc was able to
reverse curcumin-induced proliferative inhibition in gastric cancer
cells.
Previous studies have indicated that c-Myc promotes
cancer progression by upregulating tumor-promotive lncRNAs,
including prostate cancer gene expression marker 1 and HOX
transcript antisense RNA (46,47). In
addition, c-Myc has been reported to directly bind to the promoter
of H19 in order to induce its expression and potentiate tumor
progression in primary breast and lung carcinomas (30). In gastric cancer, c-Myc has been shown
to induce H19 expression, and its expression was positively
correlated with H19 expression in gastric cancer patients (29). The present study demonstrated that
exogenous c-Myc enhanced H19 expression in the presence of
curcumin, which provided evidence to explain how curcumin inhibited
H19 expression, and provides a direct molecular link between
curcumin and H19. However, whether c-Myc is indispensable for
curcumin to regulate H19-mediated p53 deactivation still needs to
be clarified in future.
The role of H19 in the progression of gastric cancer
may be due to its association with p53 (26). p53, which is an important tumor
suppressor, plays a pivotal role in inhibiting the proliferation
and inducing the apoptosis of cancer cells (11). In the present study, curcumin
significantly enhanced p53 expression, and simultaneously induced
cell apoptosis and inhibited proliferation of gastric cancer cells.
Conversely, ectopic expression of H19 abrogated curcumin-induced
p53 expression, and the following effects on proliferation and
apoptosis of cancer cells.
In conclusion, the major findings of this study can
be summarized as follows: i) Curcumin inhibits H19 expression in
gastric cancer cells; ii) H19 plays a pivotal role in
curcumin-induced proliferative inhibition and apoptosis of gastric
cancer cells; and iii) c-Myc can be downregulated by curcumin and
is an important mediator between curcumin and H19. To the best of
our knowledge, the present study demonstrated, for the first time,
a novel mechanism by which curcumin exploits a lncRNA to inhibit
gastric cancer growth. Therefore, curcumin may be considered a
value therapeutic strategy for the treatment of gastric cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81370562).
References
|
1
|
Bar-Sela G, Epelbaum R and Schaffer M:
Curcumin as an anti-cancer agent: Review of the gap between basic
and clinical applications. Curr Med Chem. 17:190–197. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han SS, Chung ST, Robertson DA, Ranjan D
and Bondada S: Curcumin causes the growth arrest and apoptosis of B
cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-kappa
B, and p53. Clin Immunol. 93:152–161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dhillon N, Aggarwal BB, Newman RA, Wolff
RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V and Kurzrock
R: Phase II trial of curcumin in patients with advanced pancreatic
cancer. Clin Cancer Res. 14:4491–4499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma RA, Euden SA, Platton SL, Cooke DN,
Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer
SM, et al: Phase I clinical trial of oral curcumin: Biomarkers of
systemic activity and compliance. Clin Cancer Res. 10:6847–6854.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carroll RE, Benya RV, Turgeon DK, Vareed
S, Neuman M, Rodriguez L, Kakarala M, Carpenter PM, McLaren C,
Meyskens FL Jr and Brenner DE: Phase IIa clinical trial of curcumin
for the prevention of colorectal neoplasia. Cancer Prev Res
(Phila). 4:354–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goel A, Boland CR and Chauhan DP: Specific
inhibition of cyclooxygenase-2 (COX-2) expression by dietary
curcumin in HT-29 human colon cancer cells. Cancer Lett.
172:111–118. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z, Zhang Y, Banerjee S, Li Y and
Sarkar FH: Notch-1 down-regulation by curcumin is associated with
the inhibition of cell growth and the induction of apoptosis in
pancreatic cancer cells. Cancer. 106:2503–2513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marin YE, Wall BA, Wang S, Namkoong J,
Martino JJ, Suh J, Lee HJ, Rabson AB, Yang CS, Chen S and Ryu JH:
Curcumin downregulates the constitutive activity of NF-kappaB and
induces apoptosis in novel mouse melanoma cells. Melanoma Res.
17:274–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagamine M, Okumura T, Tanno S, Sawamukai
M, Motomura W, Takahashi N and Kohgo Y: PPAR gamma ligand-induced
apoptosis through a p53-dependent mechanism in human gastric cancer
cells. Cancer Sci. 94:338–343. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choudhuri T, Pal S, Agwarwal ML, Das T and
Sa G: Curcumin induces apoptosis in human breast cancer cells
through p53-dependent Bax induction. FEBS Lett. 512:334–340. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choudhuri T, Pal S, Das T and Sa G:
Curcumin selectively induces apoptosis in deregulated cyclin
D1-expressed cells at G2 phase of cell cycle in a p53-dependent
manner. J Biol Chem. 280:20059–20068. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sintara K, Thong-Ngam D, Patumraj S and
Klaikeaw N: Curcumin attenuates gastric cancer induced by
N-methyl-N-nitrosourea and saturated sodium chloride in rats. J
Biomed Biotechnol. 2012:9153802012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai XZ, Wang J, Li XD, Wang GL, Liu FN,
Cheng MS and Li F: Curcumin suppresses proliferation and invasion
in human gastric cancer cells by downregulation of PAK1 activity
and cyclin D1 expression. Cancer Biol Ther. 8:1360–1368. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai XZ, Huang WY, Qiao Y, Du SY, Chen Y,
Chen D, Yu S, Che RC, Liu N and Jiang Y: Inhibitory effects of
curcumin on gastric cancer cells: A proteomic study of molecular
targets. Phytomedicine. 20:495–505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang T, Zhang X, Xue W, Zhao S, Zhang X
and Pei J: Curcumin induced human gastric cancer BGC-823 cells
apoptosis by ROS-mediated ASK1-MKK4-JNK stress signaling pathway.
Int J Mol Sci. 15:15754–15765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song G, Ming Y, Mao Y, Bao S and Ouyang G:
Osteopontin prevents curcumin-induced apoptosis and promotes
survival through Akt activation via alpha v beta 3 integrins in
human gastric cancer cells. Exp Biol Med (Maywood). 233:1537–1545.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanigawa S, Fujii M and Hou DX:
Stabilization of p53 is involved in quercetin-induced cell cycle
arrest and apoptosis in HepG2 cells. Biosci Biotechnol Biochem.
72:797–804. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xing CG, Zhu BS, Liu HH, et al: LY294002
induces p53-dependent apoptosis of SGC7901 gastric cancer cells.
Acta pharmacologica Sinica. 29:489–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adriaenssens E, Dumont L, Lottin S, Bolle
D, Leprêtre A, Delobelle A, Bouali F, Dugimont T, Coll J and Curgy
JJ: H19 overexpression in breast adenocarcinoma stromal cells is
associated with tumor values and steroid receptor status but
independent of p53 and Ki-67 expression. Am J Pathol.
153:1597–1607. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ariel I, Miao HQ, Ji XR, Schneider T, Roll
D, de Groot N, Hochberg A and Ayesh S: Imprinted H19 oncofetal RNA
is a candidate tumour marker for hepatocellular carcinoma. Mol
Pathol. 51:21–25. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu
J: Long non-coding RNA H19 increases bladder cancer metastasis by
associating with EZH2 and inhibiting E-cadherin expression. Cancer
Lett. 333:213–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi Y, Wang Y, Luan W, Wang P, Tao T,
Zhang J, Qian J, Liu N and You Y: Long non-coding RNA H19 promotes
glioma cell invasion by deriving miR-675. PLoS One. 9:e862952014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Song YX and Wang ZN: Non-coding
RNAs in gastric cancer. Gene. 560:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li PF, Chen SC, Xia T, Jiang XM, Shao YF,
Xiao BX and Guo JM: Non-coding RNAs and gastric cancer. World J
Gastroenterol. 20:5411–5419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song H, Sun W, Ye G, Ding X, Liu Z, Zhang
S, Xia T, Xiao B, Xi Y and Guo J: Long non-coding RNA expression
profile in human gastric cancer and its clinical significances. J
Transl Med. 11:2252013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J
and Fang G: Up-regulated long non-coding RNA H19 contributes to
proliferation of gastric cancer cells. FEBS J. 279:3159–3165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kujundzić R Novak, Grbesa I, Ivkić M,
Katdare M and Gall-Troselj K: Curcumin downregulates H19 gene
transcription in tumor cells. J Cell Biochem. 104:1781–1792. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kakar SS and Roy D: Curcumin inhibits TPA
induced expression of c-fos, c-jun and c-myc proto-oncogenes
messenger RNAs in mouse skin. Cancer Lett. 87:85–89. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang EB, Han L, Yin DD, Kong R, De W and
Chen J: c-Myc-induced, long, noncoding H19 affects cell
proliferation and predicts a poor prognosis in patients with
gastric cancer. Med Oncol. 31:9142014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barsyte-Lovejoy D, Lau SK, Boutros PC,
Khosravi F, Jurisica I, Andrulis IL, Tsao MS and Penn LZ: The c-Myc
oncogene directly induces the H19 noncoding RNA by allele-specific
binding to potentiate tumorigenesis. Cancer Res. 66:5330–5337.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geiler C, Andrade I and Greenwald D:
Exogenous c-Myc Blocks Differentiation and Improves Expansion of
Human Erythroblasts In vitro. International journal of stem cells.
7:153–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsang WP, Ng EK, Ng SS, Jin H, Yu J, Sung
JJ and Kwok TT: Oncofetal H19-derived miR-675 regulates tumor
suppressor RB in human colorectal cancer. Carcinogenesis.
31:350–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhuang M, Gao W, Xu J, Wang P and Shu Y:
The long non-coding RNA H19-derived miR-675 modulates human gastric
cancer cell proliferation by targeting tumor suppressor RUNX1.
Biochem Biophys Res Commun. 448:315–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie B, Zhou J, Shu G, Liu DC, Zhou J, Chen
J and Yuan L: Restoration of klotho gene expression induces
apoptosis and autophagy in gastric cancer cells: Tumor suppressive
role of klotho in gastric cancer. Cancer Cell Int. 13:182013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li N, Deng W, Ma J, et al: Prognostic
evaluation of Nanog, Oct4, Sox2, PCNA, Ki67 and E-cadherin
expression in gastric cancer. Med Oncol. 32:4332015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li H, Yu B, Li J, Su L, Yan M, Zhu Z and
Liu B: Overexpression of lncRNA H19 enhances carcinogenesis and
metastasis of gastric cancer. Oncotarget. 5:2318–2329. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sorin V, Ohana P, Mizrahi A, et al:
Regional therapy with DTA-H19 vector suppresses growth of colon
adenocarcinoma metastases in the rat liver. International journal
of oncology. 39:1407–1412. 2011.PubMed/NCBI
|
|
40
|
Zang S, Liu T, Shi J and Qiao L: Curcumin:
A promising agent targeting cancer stem cells. Anticancer Agents
Med Chem. 14:787–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dang CV: c-Myc target genes involved in
cell growth, apoptosis, and metabolism. Mol Cell Biol. 19:1–11.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Field JK, Spandidos DA, Stell PM, Vaughan
ED, Evan GI and Moore JP: Elevated expression of the c-myc
oncoprotein correlates with poor prognosis in head and neck
squamous cell carcinoma. Oncogene. 4:1463–1468. 1989.PubMed/NCBI
|
|
43
|
Deming SL, Nass SJ, Dickson RB and Trock
BJ: C-myc amplification in breast cancer: A meta-analysis of its
occurrence and prognostic relevance. Br J Cancer. 83:1688–1695.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nair R, Roden DL, Teo WS, McFarland A,
Junankar S, Ye S, Nguyen A, Yang J, Nikolic I, Hui M, et al: c-Myc
and Her2 cooperate to drive a stem-like phenotype with poor
prognosis in breast cancer. Oncogene. 33:3992–4002. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jang KY, Noh SJ, Lehwald N, Tao GZ,
Bellovin DI, Park HS, Moon WS, Felsher DW and Sylvester KG: SIRT1
and c-Myc promote liver tumor cell survival and predict poor
survival of human hepatocellular carcinomas. PLoS One.
7:e451192012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ma MZ, Li CX, Zhang Y, Weng MZ, Zhang MD,
Qin YY, Gong W and Quan ZW: Long non-coding RNA HOTAIR, a c-Myc
activated driver of malignancy, negatively regulates miRNA-130a in
gallbladder cancer. Mol Cancer. 13:1562014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hung CL, Wang LY, Yu YL, Chen HW,
Srivastava S, Petrovics G and Kung HJ: A long noncoding RNA
connects c-Myc to tumor metabolism. Proc Natl Acad Sci USA.
111:18697–18702. 2014. View Article : Google Scholar : PubMed/NCBI
|