Introduction
Glioblastoma multiforme (GBM) is the most malignant
type of brain cancer affecting the central nervous system. Patients
with GBM have a mean survival of only 14.6 months (1). Standard therapies for glioma, including
surgery, radiation and chemotherapy, are only effective in treating
high-grade tumors. The cancer's pathogenesis is not very well
defined, but invasive growth is a known hallmark of GBM; this is
also a major reason why GBM is associated with such poor prognosis
(2). Therefore, an increased
understanding of the biological and molecular nature of GBM is
required.
Targeting protein for Xenopus kinesin-like protein 2
(TPX2) is a cell cycle-regulated nuclear protein with roles in
proliferation and mitotic spindle assembly (3,4). TPX2
overexpression induces centrosome amplification and leads to DNA
polyploidy (5). Along these lines,
TPX2 has been implicated as an oncogene in several human cancers,
including lung cancer, cervical cancer, bladder cancer and human
malignant astrocytoma (6–9). A number of studies have established that
activated AKT signaling upregulates the expression of matrix
metallopeptidase (MMP)-2 and MMP-9 (10,11). TPX2
knockdown has been shown to suppress the proliferation and invasion
of hepatocellular carcinoma cells via inactivation of AKT signaling
and inhibition of the expression of MMP-2 and MMP-9 (12). Li et al also reported that TPX2
knockdown inhibited cell proliferation and increased early-stage
apoptosis in glioma U87 cells (9).
TPX2 knockdown was also shown to decrease the levels of Aurora A,
Ras-related nuclear protein (Ran) and cyclin B1, and to increase
the expression of c-Myc and p53 (9).
In the present study, increased expression of TPX2
was detected in multiple glioma cell lines. Additionally, TPX2
knockdown was able to inhibit glioma cell proliferation and
invasion via inactivation of the AKT pathway. It was also observed
that a small molecule inhibitor of AKT phenocopied the effects of
TPX2 knockdown. Our results demonstrate that TPX2 knockdown
suppresses cell proliferation and invasion via inactivation of AKT
signaling in glioma cells.
Materials and methods
Cell culture and transfection
Normal human astrocytes (NHAs) were purchased from
ScienCell Research Laboratories (Carlsbad, CA, USA). The glioma
cell lines U251, U87 and LN229 were obtained from the Institute of
Biochemistry and Cell Biology (Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences, Shanghai, China). Cells were
grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) and 1% penicillin/streptomycin at 37°C in an
atmosphere of 5% CO2. Specific small interfering RNA
(siRNA) targeting TPX2 (sc-37653) and control siRNA (sc-37007) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Glioma cells were divided into three groups, including the mock,
control siRNA and TPX2 siRNA groups. Glioma cells were seeded at a
concentration of 2×105 cells/well in a 6-well plate
(Corning Inc., Corning, NY, USA) and cultured overnight. Cells were
then transfected with 100 nM siRNA using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The mock cells were treated with
Lipofectamine 2000 only. The transfection medium was replaced with
complete medium at 24 h post-transfection, and cells were incubated
at 37°C in a 5% CO2 atmosphere. AKT inhibitor IV was
purchased from Calbiochem (EMD Millipore, Billerica, MA, USA).
Construction of the eukaryotic
expression vector pcDNA-TPX2
TPX2 full-length cDNA was amplified by polymerase
chain reaction (PCR) using the following TPX2-specific primers:
5′-CGGGATCCATGTCACAAGTTAAAAGCTC-3′ (forward) and
5′-GCTCTAGATTAGCAGTGGAATCGAGTGGAG-3′ (reverse). The cycling
conditions were as follows: 94°C for 4 min; 30 cycles at 94°C for
90 sec, 65°C for 30 sec and 72°C for 1 min; and 72°C for 5 min. The
TPX2 PCR product and empty vector pcDNA3.1 (Thermo Fisher
Scientific, Inc.) were digested with EcoRI and BamHI.
pcDNA-TPX2 was generated by subcloning TPX2 into pcDNA3.1 using T4
DNA ligase (Thermo Fisher Scientific, Inc.). Glioma cells were
divided into three groups, including the mock, pcDNA3.1 and
pcDNA-TPX2 groups. Glioma cells were seeded at a concentration of
2×105 cells/well in a 6-well plate and cultured
overnight. Cells were then transfected with pcDNA-TPX2 or
TPX2-siRNA (10 µl) in 250 µl Opti-MEM I reduced serum medium
(Thermo Fisher Scientific, Inc.) using Lipofectamine 2000. The mock
cells were treated with Lipofectamine 2000 only.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cultured cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. RNA samples were assessed
by their optical density at 260 nm, and then reverse transcribed
into cDNA using the RevertAid Premium First Strand cDNA Synthesis
kit (Fermentas; Thermo Fisher Scientific, Inc, Pittsburgh, PA,
USA). RT-qPCR was performed using the SYBR Green PCR Master Mix kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) in conjunction
with an ABI Prism 7300 Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The TPX2 primers were as follows:
Forward, 5′-ACCTTGCCCTACTAAGATT-3′ and reverse,
5′-AATGTGGCACAGGTTGAGC-3′. The β-actin primers were as follows:
Forward, 5′-GGGAAATCGTGCGTGACAT-3′ and reverse,
5′-CTGGAAGGTGGACAGCGAG-3′. The cycling conditions were as follows:
95°C for 10 min, followed by 40 cycles at 95°C for 15 sec, 60°C for
15 sec and 95°C for 15 sec. The relative fold-change in expression
of mRNAs was calculated using the 2-ΔΔCq method (13). Each sample was analyzed in
triplicate.
Western blotting
Total protein cell lysates were prepared by lysing
the cells in radioimmunoprecipitation assay buffer. Protein
concentrations were determined using the Pierce BCA Protein Assay
kit (Thermo Fisher Scientific, Inc.). In total, 40 µg protein from
each sample were resolved by 10% SDS-PAGE and transferred to
polyvinylidene difluoride membranes (Merck Millipore, Darmstadt,
Germany). Membranes were blocked for 1 h at room temperature with
5% non-fat dry milk, followed by incubation overnight at 4°C with
the following primary antibodies: Rabbit anti-human TPX2 polyclonal
antibody (1:200; sc-32863), rabbit anti-human AKT polyclonal
antibody (1:200; sc-8312), rabbit anti-human phosphorylated AKT
polyclonal antibody (1:200; sc-293095), rabbit anti-MMP-9
polyclonal antibody (1:200; sc-10737), polyclonal mouse anti-human
cyclin D1 (1:200; sc-29287) and polyclonal mouse anti-human p21
(1:200; sc-44271). Horseradish peroxidase-conjugated monoclonal
goat anti-rabbit or anti-mouse immunoglobulin G secondary
antibodies (1:1,000; W10804 and W10815, respectively; Zymed; Thermo
Fisher Scientific, Inc.) and enhanced chemiluminescence were used
for detection. Next, membranes were stripped and re-probed with a
primary antibody against GAPDH (1:1,000; AP0063; Bioworld
Technology, Inc., St. Louis Park, MN, USA), which was used for
normalization. Signal intensities were determined using ImageJ 1.37
gel analysis software (National Institutes of Health- Bethesda, MD,
USA).
Proliferation assays
Cells (5×103 cells/well) were plated in
96-well plates and allowed to grow for 24, 48 and 72 h after
transfection with the corresponding siRNAs or expression plasmids.
Cell proliferation was documented every 24 h for 4 days using an
MTT assay (Sigma-Aldrich; Merck Millipore). Absorbance was measured
at 570 nm using a microplate reader.
Cell cycle assay
At 48 h post-transfection, cells were harvested by
trypsinization, washed three times with ice-cold PBS and fixed with
70% ethanol overnight at 4°C. The fixed cells were rehydrated in
PBS and subjected to propidium iodide/RNase staining, followed by
fluorescence-activated cell sorting analysis (BD Biosciences, San
Jose, CA, USA). The percentage of cells in each phase of the cell
cycle was estimated using PVElite 2015 software (Intergraph
Corporation, Madison, AL, USA).
Transwell migration assay
Cell invasion was determined using Transwell assays.
Briefly, TPX2-overexpressing plasmid and siRNA to knockdown TPX2
expression were transfected into cells, and 48 h later,
3×104 cells were transferred to the top of
Matrigel-coated invasion chambers (BD Biosciences) in serum-free
DMEM. Next, DMEM containing 10% FBS was added to the lower chamber,
and 24 h later, the non-invading cells were removed, while the
invading cells were fixed with 95% ethanol and stained with 0.1%
crystal violet. Images were captured in a light microscope under
×100 magnification. Experiments were performed three independent
times.
Statistical analysis
All data are shown as the mean + standard deviation.
All experiments were repeated three times. All statistical analyses
were performed with a two-tailed Student's t-test using SPSS
version 12.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
TPX2 mRNA and protein expression in
glioma cell lines
RT-qPCR was performed to determine the mRNA
expression levels of TPX2 in the glioma cell lines U251, U87 and
LN229 and in primary NHAs. TPX2 transcript levels were
significantly lower in NHA cells compared with those in all the
glioma cell lines (all P<0.05; Fig.
1A). The TPX2 protein levels in all the cell lines mentioned
above were also examined, and our findings were consistent with
those obtained from RT-qPCR. That is, TPX2 protein levels were
lower in NHA cells compared with those in glioma cells (U251,
5.67-fold; U87, 5.27-fold; and LN229, 3.76-fold; all P<0.05;
Fig. 1B). These results suggest that
TPX2 may function as an oncogene in GBM.
TPX2 overexpression promotes cell
proliferation and invasion in U251 and U87 cells
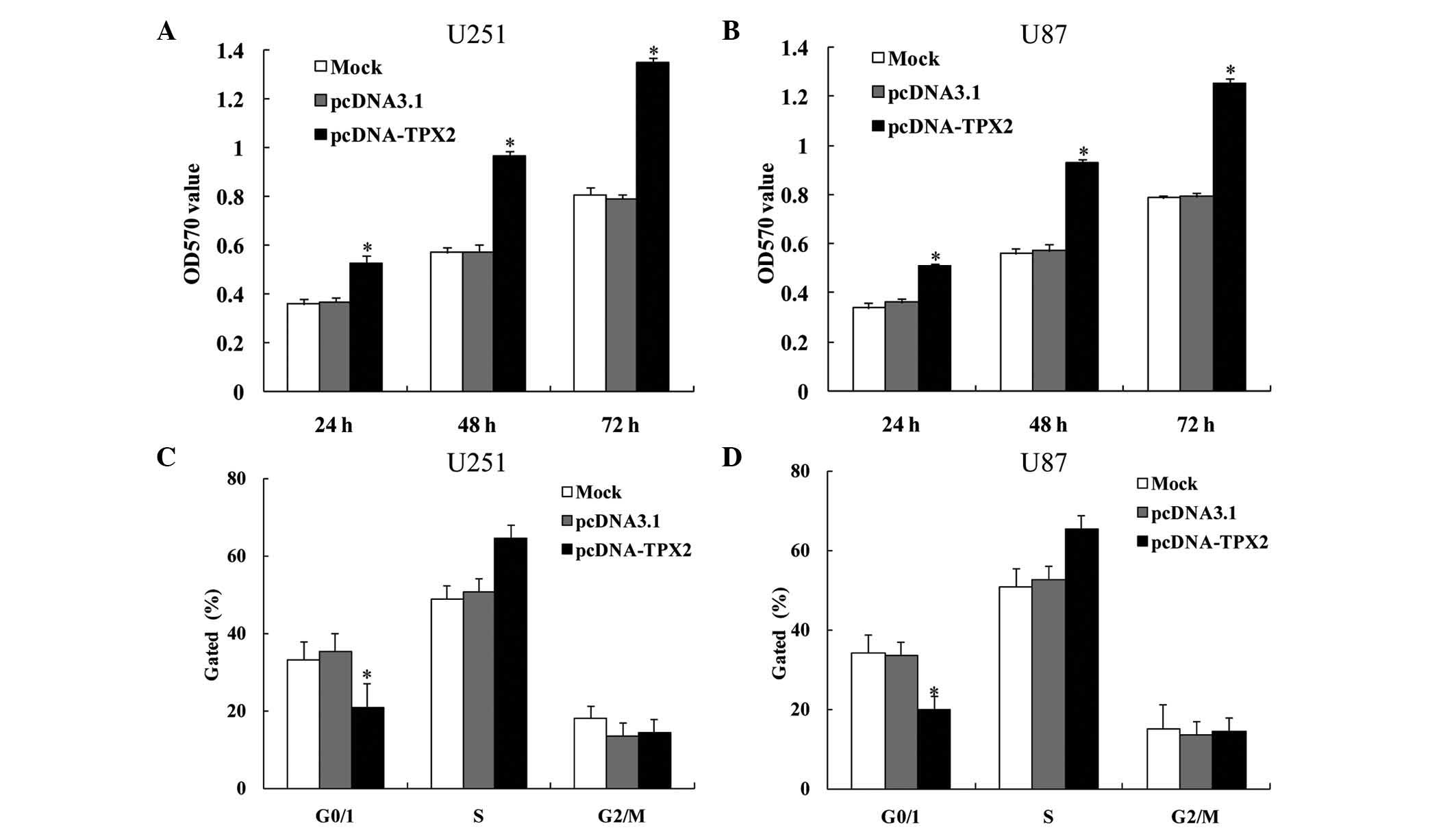
The effect of TPX2 overexpression in the glioma cell
lines U251 and U87 was examined. Cells were transfected with
pcDNA-TPX2 or pcDNA3.1 as control. MTT assay was performed to
measure proliferation, while Transwell assay was performed to
assess migration. Compared with the controls, TPX2 overexpression
promoted the proliferation of both U251 (24 h, 0.362±0.002,
0.366±0.008 and 0.522±0.005 for the mock, pcDNA3.1 and pcDNA-TPX2
groups, respectively; 48 h, 0.554±0.019, 0.565±0.027 and
0.959±0.023 for the mock, pcDNA3.1 and pcDNA-TPX2 groups,
respectively; and 72 h, 0.894±0.053, 0.843±0.062 and 1.253±0.172
for the mock, pcDNA3.1 and pcDNA-TPX2 groups, respectively;
P<0.05; Fig. 2A) and U87 cells (24
h, 0.358±0.019, 0.362±0.014 and 0.569±0.045 for the mock, pcDNA3.1
and pcDNA-TPX2 groups, respectively; 48 h, 0.555±0.017, 0.567±0.008
and 0.933±0.010 for the mock, pcDNA3.1 and pcDNA-TPX2 groups,
respectively; and 72 h, 0.790±0.018, 0.798±0.025 and 1.241±0.035
for the mock, pcDNA3.1 and pcDNA-TPX2 groups, respectively;
P<0.05; Fig. 2B). TPX2
overexpression also decreased the percentage of cells in the G0/G1
phase (U251 cells, 34.730±0.470, 35.481±0.671 and 21.092±0.708 for
the mock, pcDNA3.1 and pcDNA-TPX2 groups, respectively; U87 cells,
34.513±0.438, 34.114±0.909 and 19.623±0.578 for the mock, pcDNA3.1
and pcDNA-TPX2 groups, respectively; P<0.05; Fig. 2C and D, respectively). Overexpression
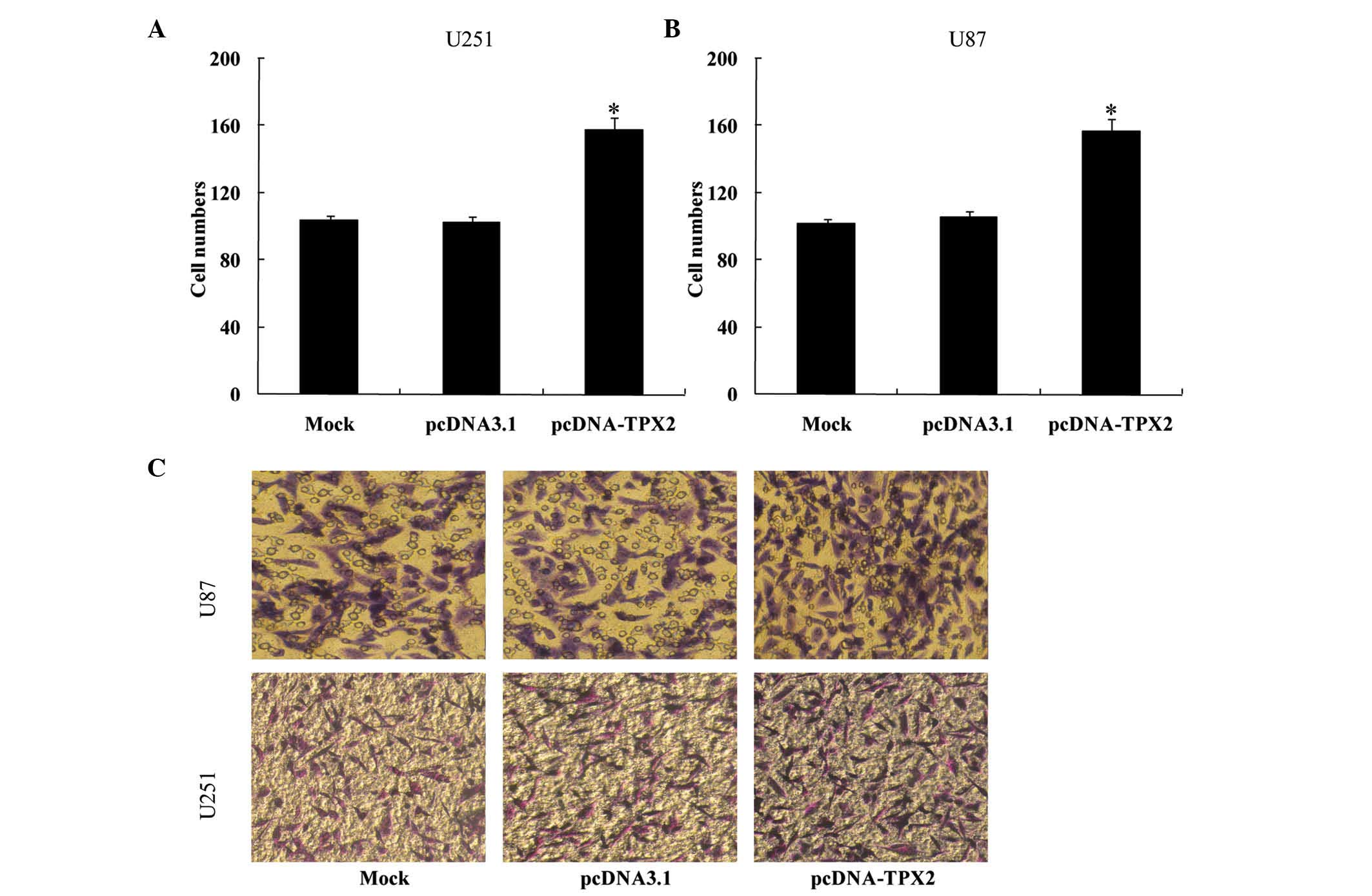
of TPX2 also increased cellular migration (U251 cells,
105.200±3.114, 100.600±3.361 and 153.000±5.612 for the mock,
pcDNA3.1 and pcDNA-TPX2 groups, respectively; U87 cells,
102.000±1.870, 104.800±1.303 and 152.200±3.114 for the mock,
pcDNA3.1 and pcDNA-TPX2 groups, respectively; P<0.05; Fig. 3). These results suggest that TPX2 may
promote cellular proliferation and invasion in glioma cells.
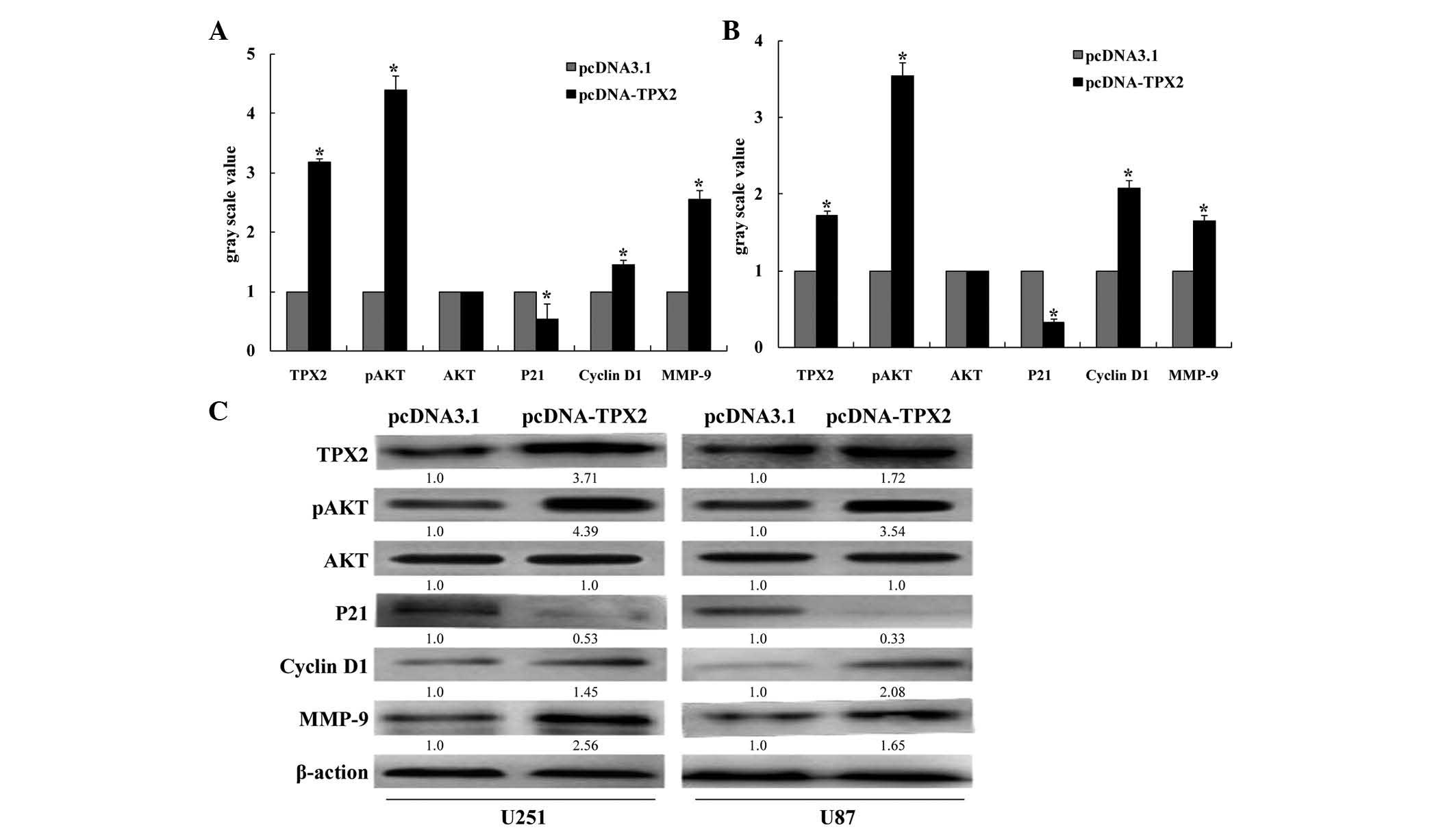
TPX2 overexpression activates AKT
signaling in U251 and U87 cells
AKT protein kinases are critical regulators of
multiple cellular functions, including cell growth, survival and
metabolism (14). Cyclin D1 and p21
are key pro- and anti-cell cycle regulators, respectively. In the
present study, TPX2 overexpression significantly increased AKT
phosphorylation in U251 and U87 cells (P<0.05; Fig 4). Additionally, overexpression of TPX2
decreased the levels of p21 and increased the levels of both cyclin
D1 and MMP-9 in U251 and U87 glioma cells.
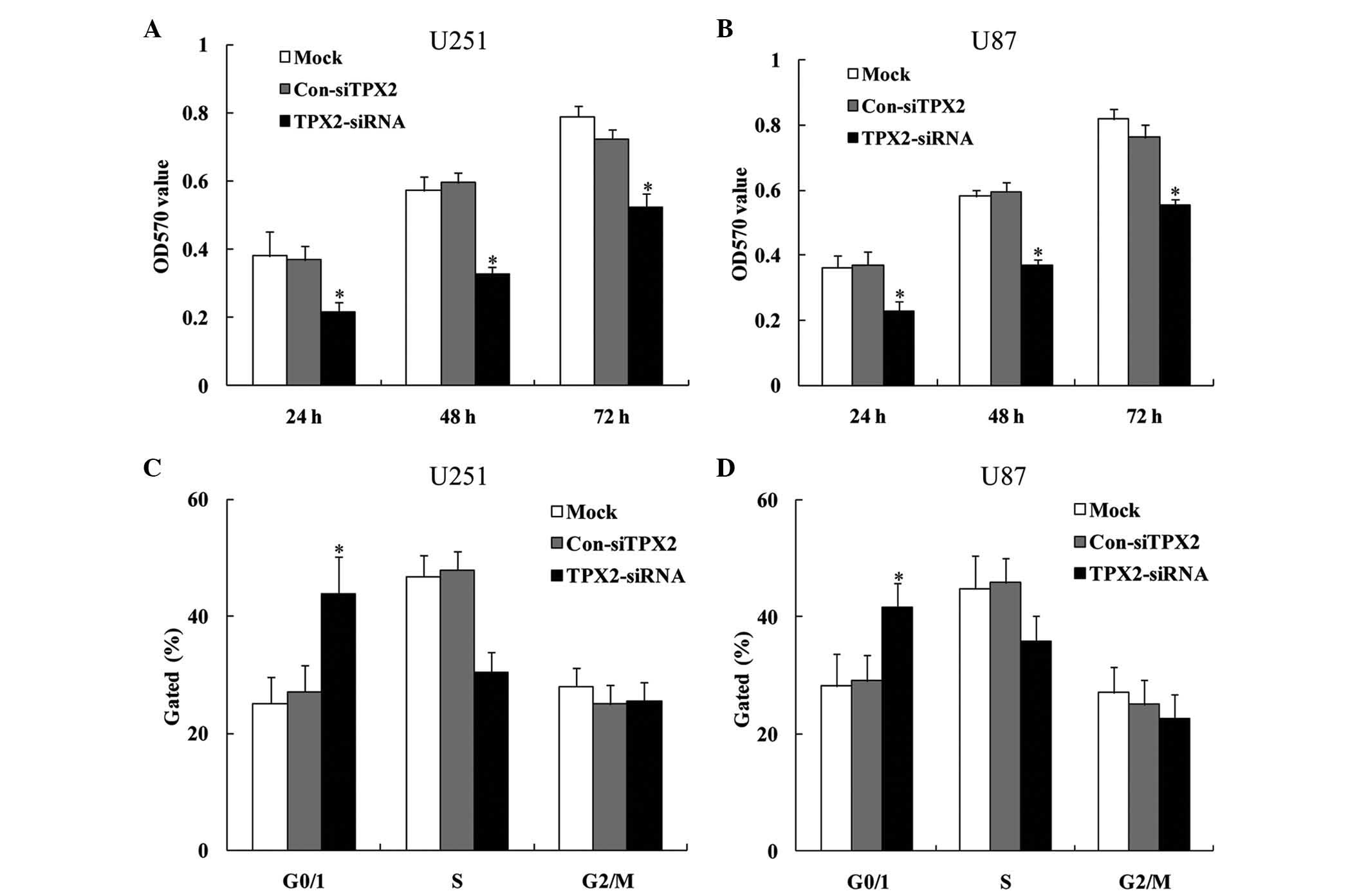
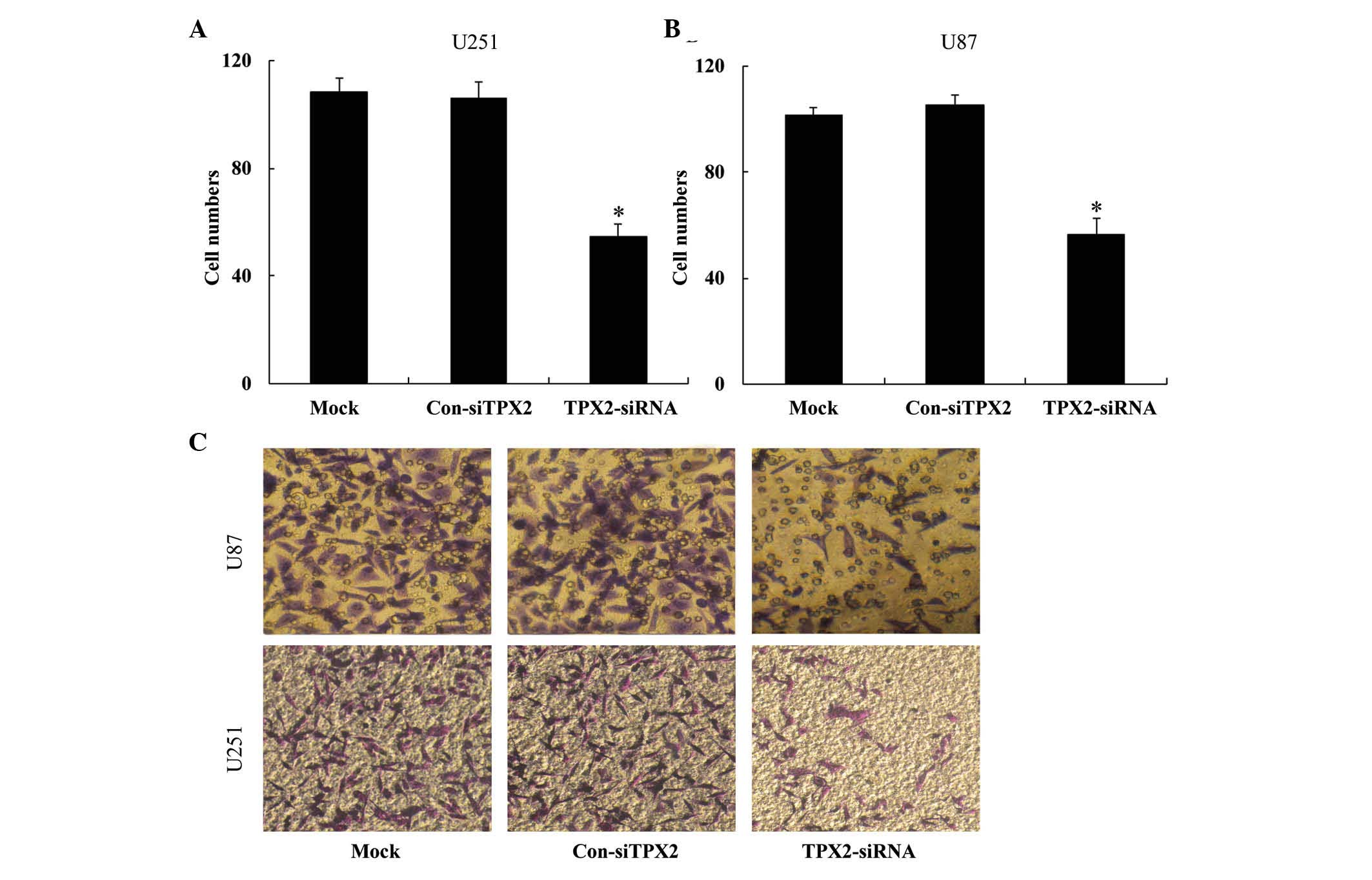
TPX2 knockdown suppresses
proliferation and invasion in U251 and U87 cells
The effect of TPX2 knockdown on the proliferation
and invasion abilities of the U251 and U87 glioma cell lines was
next examined. The results from MTT assay revealed that TPX2
knockdown decreased the cellular proliferation of both U251 (24 h,
0.386±0.006, 0.379±0.002 and 0.228±0.008 for the mock, pcDNA3.1 and
pcDNA-TPX2 groups, respectively; 48 h, 0.579±0.013, 0.587±0.007 and
0.327±0.009 for the mock, pcDNA3.1 and pcDNA-TPX2 groups,
respectively; and 72 h, 0.756±0.015, 0.747±0.039 and 0.517±0.008
for the mock, pcDNA3.1 and pcDNA-TPX2 groups, respectively;
P<0.05; Fig. 5A) and U87 cells (24
h, 0.368±0.009, 0.376±0.005 and 0.223±0.006 for the mock, pcDNA3.1
and pcDNA-TPX2 groups, respectively; 48 h, 0.577±0.009, 0.585±0.008
and 0.367±0.007 for the mock, pcDNA3.1 and pcDNA-TPX2 groups,
respectively; and 72 h, 0.814±0.012, 0.777±0.009 and 0.585±0.047
for the mock, pcDNA3.1 and pcDNA-TPX2 groups, respectively;
P<0.05; Fig. 5B) compared with the
mock control group and the control siRNA group. Consistent with
this, cell cycle analysis revealed that TPX2 knockdown increased
the percentage of cells in G0/G1 phase and decreased the percentage
of cells in S phase (U251 cells, 25.720±0.991, 27.246±0.503 and
45.034±4.496 for the mock, pcDNA3.1 and pcDNA-TPX2 groups,
respectively; Fig. 5C; U87 cells,
28.558±0.528, 29.174±0.578 and 41.006±1.158 for the mock, pcDNA3.1
and pcDNA-TPX2 groups, respectively; P<0.05; Fig. 5D). In addition to decreasing
proliferation, TPX2 knockdown also reduced the ability of U251
(108.2±2.863, 105.2±1.303 and 55.6±2.073 for the mock, pcDNA3.1 and
pcDNA-TPX2 groups, respectively; P<0.05; Fig. 6) and U87 cells (103.0±2.121,
105.6±1.517 and 55.6±2.073 for the mock, pcDNA3.1 and pcDNA-TPX2
groups, respectively; P<0.05; Fig.
6) to invade the Transwell chamber. Taken together, these
findings suggest that TPX2 knockdown suppresses the proliferation
and invasion capacities of glioma cells.
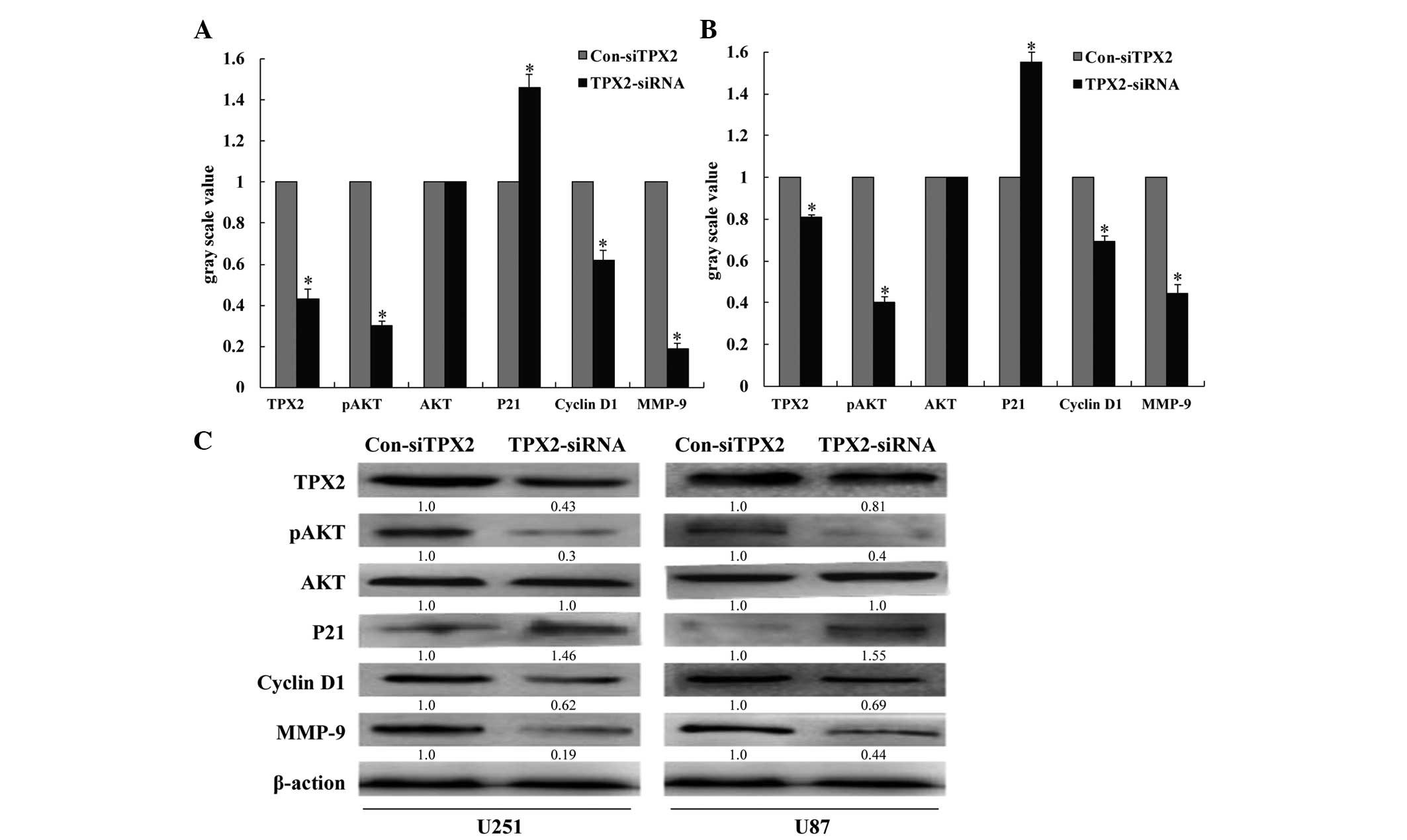
TPX2 knockdown inhibits AKT signaling
in U251 and U87 cells
The effect of TPX2 knockdown on AKT signaling in
various glioma cell lines was next examined. Compared with control
siRNA-transfected cells, TPX2 knockdown significantly decreased the
phosphorylation of AKT. Additionally, knockdown of TPX2 increased
the expression of the cell cycle inhibitor p21. By contrast, cyclin
D1 and MMP-9 were downregulated in U251 and U87 glioma cells
(P<0.05; Fig. 7) following
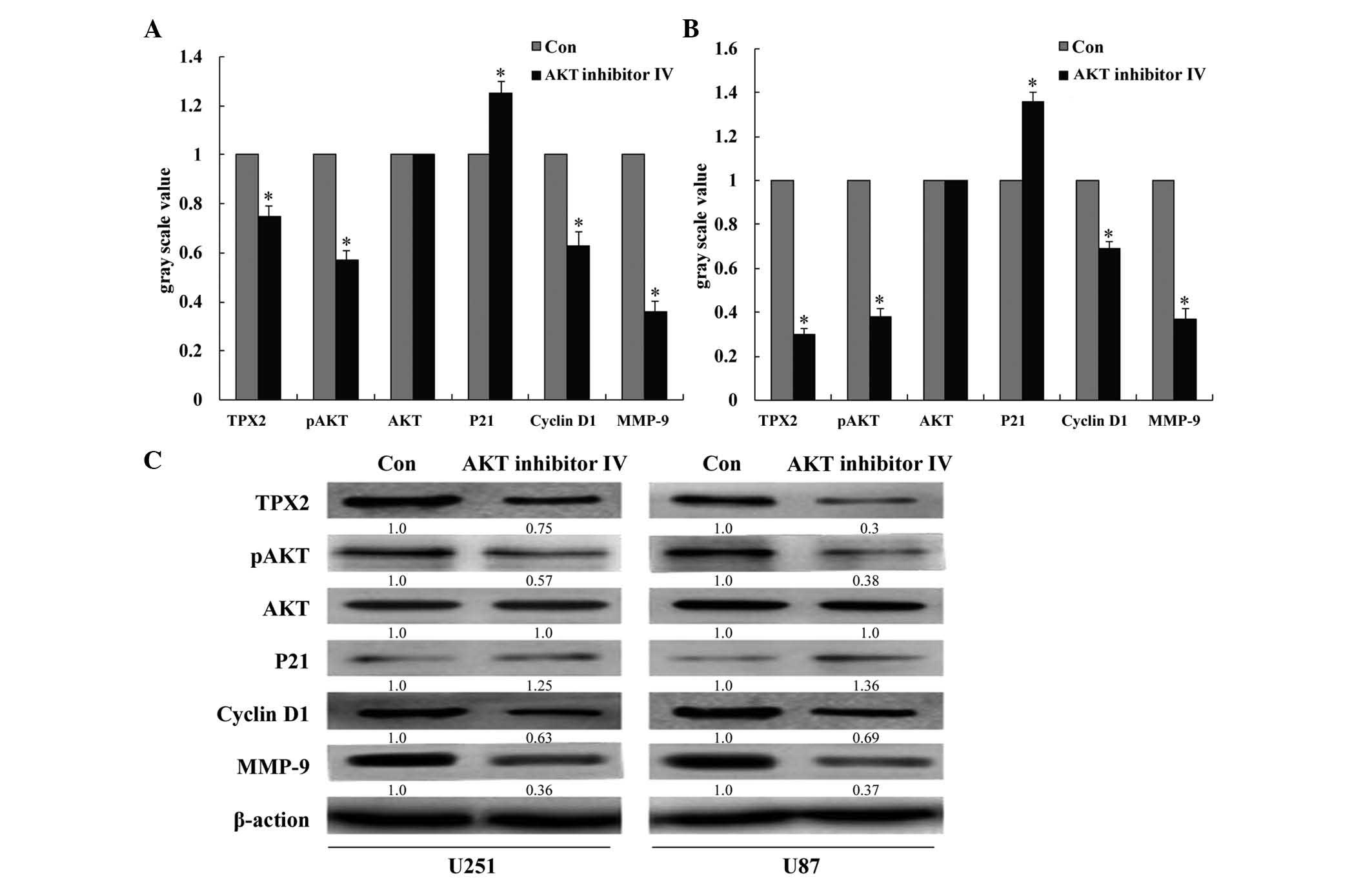
transfection with TPX2 siRNA. It was also observed that treatment
of cells with AKT inhibitor IV in large part phenocopied the effect
of TPX2 knockdown. That is, AKT inhibitor IV decreased the
phosphorylation of AKT, decreased the expression of MMP-9 and
cyclin D1, and increased the expression of p21 in U251 and U87
cells (P<0.05; Fig. 8). These
results suggest that TPX2-mediated control of glioma cell
proliferation and invasion may occur via the AKT signaling
pathway.
Discussion
GBM is the most common type of human brain tumor and
is essentially incurable. Despite recent advances in GBM treatment,
including the use of aggressive surgery combined with radiation,
chemotherapy and biological therapy, the median survival values
have not changed much over the past several years (15,16). Thus,
improved detection, prevention and treatment of GBM are important
unmet needs. Invasive growth is the most characteristic phenotype
of glioblastoma, which is a major reason why patients with this
type of tumor have such poor prognosis (2). Therefore, inhibition of invasion, for
example by interfering with the signaling pathways that regulate
this process, may be therapeutically useful for the treatment of
GBM.
TPX2 regulates multiple aspects of mitotic spindle
assembly and chromosome isolation (5,17). A
previous study revealed that TPX2 overexpression promotes invasion
and progression in vitro (18), and suggested that TPX2 is a potent
oncogene in human cancers (6–9). Li et al (9) reported that TPX2 expression was elevated
in glioma tissue compared with normal tissue. TPX2 knockdown has
been shown to inhibit cell proliferation and to increase
early-stage apoptosis in glioma U87 cells (9). Knockdown of TPX2 has also been shown to
decrease the levels of Aurora A, Ran and cyclin B1, and to increase
the expression of c-Myc and p53 (9).
Despite these findings, the precise role of TPX2 in glioma is
poorly understood.
The present study demonstrates that TPX2 expression
is significantly higher in several glioma cell lines compared with
that in NHAs. It also provides data showing that TPX2 regulates the
proliferation and invasion of abilities U251 and U87 cells.
Specifically, overexpression of TPX2 in either cell line promoted
cell proliferation and enhanced their invasive potential.
Importantly, knockdown of TPX2 had the exact opposite effect. Taken
together, these data suggest that TPX2 regulates cell proliferation
and invasion in glioma cells.
Previous in vivo and in vitro glioma
studies noticed that AKT activation and phosphorylation are common
occurrences in GBM (19,20). AKT protein kinases are critical
regulators of multiple cellular functions, including cell growth,
survival and metabolism (14). In
normal cells, the growth factor-dependent activation of
phosphoinositide 3-kinase (PI3K) is tightly controlled by the
potent tumor suppressor phosphatase and tensin homolog (PTEN).
Aberrant activation of AKT can result from mutations in upstream
regulators (such as receptor tyrosine kinases), overexpression of
AKT or deletions of negative regulators (such as PTEN) (21). AKT signaling regulates cell cycle
entry by phosphorylating cyclin D1-cyclin-dependent kinase 4
complexes (22,23). AKT promotes the G1/S transition by
antagonizing the accumulation of two G1/S transition inhibitors,
p21 and p27 (24). p21 can be
phosphorylated either directly by AKT or by kinases activated by
AKT (25). MMP-2 and MMP-9 are key
enzymes involved in the degradation of type IV collagen, which is a
component of the extracellular matrix. Increased expression of both
MMP-2 and MMP-9 has been associated with tumor cell growth and
invasion (26). Fu et al
demonstrated that activation of PI3K/AKT signaling suppressed the
expression of p53 and tissue inhibitor of MMP-2, resulting in
overexpression of proliferating cell nuclear antigen, cyclin D1,
MMP-2 and MMP-9, which promoted cell proliferation and invasion in
U251 glioma cells (27).
The data presented in the current study demonstrate
that TPX2 significantly increases AKT phosphorylation in U251 and
U87 glioma cells. This is associated with altered levels of cell
cycle regulators such as cyclin D1 and p21. It was also observed
that TPX2 regulates the levels of MMP-9, which may be associated
with the invasive potential of glioma cells. Taken together, our
results demonstrate that TPX2 promotes proliferation and invasion
via activating AKT signaling in glioma cells. Additionally, these
findings suggest that TPX2 may be a potential therapeutic target in
glioblastoma.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu DG, Wang YY, Fan LG, Luo H, Han B, Sun
LH, Wang XF, Zhang JX, Cao L, Wang XR, et al: MicroRNA-7 regulates
glioblastoma cell invasion via targeting focal adhesion kinase
expression. Chin Med J (Engl). 124:2616–2621. 2011.PubMed/NCBI
|
|
3
|
Heidebrecht HJ, Buck F, Steinmann J,
Sprenger R, Wacker HH and Parwaresch R: p100: A novel
proliferation-associated nuclear protein specifically restricted to
cell cycle phases S, G2, and M. Blood. 90:226–233. 1997.PubMed/NCBI
|
|
4
|
Kufer TA, Silljé HH, Körner R, Gruss OJ,
Meraldi P and Nigg EA: Human TPX2 is required for targeting
Aurora-A kinase to the spindle. J Cell Biol. 158:617–623. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gruss OJ, Wittmann M, Yokoyama H,
Pepperkok R, Kufer T, Silljé H, Karsenti E, Mattaj IW and Vernos I:
Chromosome-induced microtubule assembly mediated by TPX2 is
required for spindle formation in HeLa cells. Nat Cell Biol.
4:871–879. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Tang H, Sun Z, Bungum AO, Edell ES,
Lingle WL, Stoddard SM, Zhang M, Jen J, Yang P and Wang L:
Network-based approach identified cell cycle genes as predictor of
overall survival in lung adenocarcinoma patients. Lung Cancer.
80:91–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scotto L, Narayan G, Nandula SV,
Arias-Pulido H, Subramaniyam S, Schneider A, Kaufmann AM, Wright
JD, Pothuri B, Mansukhani M and Murty VV: Identification of copy
number gain and overexpressed genes on chromosome arm 20q by an
integrative genomic approach in cervical cancer: Potential role in
progression. Genes Chromosomes Cancer. 47:755–765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan L, Li S, Xu C, Zhao X, Hao B, Li H and
Qiao B: Target protein for Xklp2 (TPX2), a microtubule-related
protein, contributes to malignant phenotype in bladder carcinoma.
Tumour Biol. 34:4089–4100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li B, Qi XQ, Chen X, Huang X, Liu GY, Chen
HR, Huang CG, Luo C and Lu YC: Expression of targeting protein for
Xenopus kinesin-like protein 2 is associated with progression of
human malignant astrocytoma. Brain Res. 1352:200–207. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao J, Ding F, Liu Q and Yao Y: Knockdown
of MACC1 expression suppressed hepatocellular carcinoma cell
migration and invasion and inhibited expression of MMP-2 and MMP-9.
Mol Cell Biochem. 376:21–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang YH, Dong YY, Wang WM, Xie XY, Wang
ZM, Chen RX, Chen J, Gao DM, Cui JF and Ren ZG: Vascular
endothelial cells facilitated HCC invasion and metastasis through
the Akt and NF-κB pathways induced by paracrine cytokines. J Exp
Clin Cancer Res. 32:512013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Q, Yang P, Tu K, Zhang H, Zheng X, Yao
Y and Liu Q: TPX2 knockdown suppressed hepatocellular carcinoma
cell invasion via inactivating AKT signaling and inhibiting MMP-2
and MMP-9 expression. Chin J Cancer Res. 26:410–417.
2014.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clarke J, Butowski N and Chang S: Recent
advances in therapy for glioblastoma. Arch Neurol. 67:279–283.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu R, Page M, Solheim K, Fox S and Chang
SM: Quality of life in adults with brain tumors: Current knowledge
and future directions. Neuro Oncol. 11:330–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Neumayer G, Belzil C, Gruss OJ and Nguyen
MD: TPX2: Of spindle assembly, DNA damage response, and cancer.
Cell Mol Life Sci. 71:3027–3047. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang H, Wang J, Tian Y, Xu J, Gou X and
Cheng J: The TPX2 gene is a promising diagnostic and therapeutic
target for cervical cancer. Oncol Rep. 27:1353–1359.
2012.PubMed/NCBI
|
|
19
|
Choe G, Horvath S, Cloughesy TF, Crosby K,
Seligson D, Palotie A, Inge L, Smith BL, Sawyers CL and Mischel PS:
Analysis of the phosphatidylinositol3′-kinase signaling pathway in
glioma patients in vivo. Cancer Res. 63:2742–2746. 2003.PubMed/NCBI
|
|
20
|
Haas-Kogan D, Shalev N, Wong M, Mills G,
Yount G and Stokoe D: Protein kinase B (PKB/Akt) activity is
elevated in glioma cells due to mutation of the tumor suppressor
PTEN/MMAC. Curr Biol. 8:1195–1198. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robinson GL, Robinson JP, Lastwika KJ,
Holmen SL and Vanbrocklin MW: Akt signaling accelerates tumor
recurrence following ras inhibitionin the context of ink4a/arf
loss. Genes Cancer. 4:476–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diehl JA, Cheng M, Roussel MF and Sherr
CJ: Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis
and subcellular localization. Genes Dev. 12:3499–3511. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cross DA, Alessi DR, Cohen P, Andjelkovich
M and Hemmings BA: Inhibition of glycogen synthase kinase-3 by
insulin mediated by protein kinase B. Nature. 378:785–789. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee J and Kim SS: The function of p27 KIP1
during tumor development. Exp Mol Med. 41:765–771. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Child ES and Mann DJ: The intricacies of
p21 phosphorylation: Protein/protein interactions, subcellular
localization and stability. Cell Cycle. 5:1313–1319. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cawston TE and Wilson AJ: Understanding
the role of tissue degrading enzymes and their inhibitors in
development and disease. Best Pract Res Clin Rheumatol.
20:983–1002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu Y, Zhang Q, Kang C, Zhang J, Zhang K,
Pu P, Wang G and Wang T: Inhibitory effects of adenovirus mediated
Akt1 and PIK3R1 shRNA on the growth of malignant tumor cells in
vitro and in vivo. Cancer Biol Ther. 8:1002–1009. 2009. View Article : Google Scholar : PubMed/NCBI
|