Introduction
According to the 2014 World Cancer Report, kidney
cancer was the sixteenth most common cause of cancer-associated
mortality worldwide in 2012 (1).
Although targeted drug therapy for renal cell carcinoma (RCC) has
been improved, the response rates of targeted drugs in metastatic
RCC (mRCC) remain poor, and resistance to chemotherapy is a primary
obstacle in RCC treatment (1,2).
Mechanistic target of rapamycin (mTOR) signaling is
highly induced in almost all types of cancer cell and is important
in cell proliferation, metabolism and survival. Temsirolimus is an
mTOR inhibitor used in the first-line chemotherapeutic treatment of
mRCC; however, the majority of patients acquire temsirolimus
resistance during the course of treatment (3,4).
Therefore, novel therapeutic strategies to overcome drug
resistance, improve clinical effects and decrease toxicity are
required.
Curcumin, a polyphenolic phytochemical derived from
the rhizomes of the Curcuma longa plant, is one of the
best-studied plant derivatives in the world (5,6). Curcumin
has been used as a therapeutic agent in the treatment of various
types of disease due to its apoptotic inductive, chemopreventive,
anti-angiogenic and anti-invasive/metastatic properties (7). Curcumin may induce apoptosis through the
reshaping of multiple molecular targets, including the upregulation
of death receptor 4/5 expression, activation of caspase-3, release
of cytochrome c, inhibition of B-cell lymphoma (Bcl)-extra
large protein, Bcl-2 protein and c-Myc mRNA expression, and
activation of the mitochondrial signaling pathway (5,8–10). Furthermore, curcumin has been reported
to increase radio- and chemosensitivity (11,12). Seo
et al (12) reported that
combined curcumin and NVP-BEZ235 treatment had a synergistic effect
on apoptosis through the inhibition of Bcl-2 expression in a
p53-dependent manner, however the underlying mechanism remains
unclear.
Previously, it has been observed that curcumin is
able to regulate the expression of YAP in bladder cancer cells
(6). Therefore, in the present study,
the combined effect of curcumin and temsirolimus treatment on
apoptosis in RCC cells was investigated, and it was determined
whether the enhanced inhibitory effect of temsirolimus was caused
by curcumin-mediated Yes-associated protein (YAP)/p53
induction.
Materials and methods
Cell culture and temsirolimus/curcumin
treatment
Human RCC cell lines Caki-1 and OS-RC-2, purchased
from American Type Culture Collection (Manassas, VA, USA), were
maintained in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% (v/v) fetal bovine serum (FBS;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in a
humidified 5% CO2 incubator. Caki-1 cells were treated
with final concentrations of 10 µM temsirolimus alone, 15 µM
curcumin alone or 10 µM temsirolimus and 15 µM curcuma and
incubated at 37°C for 48 h; cells were treated with dimethyl
sulfoxide (DMSO) as a control. OS-RC-2 cells were treated with
final concentrations of 15 µM temsirolimus alone, 10 µM curcumin
alone or 15 µM temsirolimus and 10 µM curcumin, or DMSO as a
control, and subsequently incubated at 37°C for 48 h.
Cell flow cytometric analysis
Human RCC cell lines Caki-1 and OS-RC-2 were
cultured in RPMI-1640 medium supplemented with 10% FBS in 6
cm-dishes. Prior to treatment, cells were provided with fresh media
and subsequently incubated with the aforementioned concentrations
of temsirolimus, curcumin and temsirolimus combined with curcumin,
for 48 h. The cells were resuspended and washed with 500 ml
phosphate-buffered saline (PBS), and incubated with
Annexin-V-Fluorescein (Roche Applied Science, Penzberg, Germany) in
a 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer
containing propidium iodide at room temperature for 20 min. The
stained cells were analyzed by fluorescence activated cell sorting
using a FACSCalibur™ flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA).
TUNEL analysis
Cells cultured in a Millicell® EZ SLIDE
8-well glass (Merck Millipore, Darmstadt, Germany) were washed with
PBS three times, fixed with 4% paraformaldehyde for 30 min, washed
with PBS again and treated with permeabilization solution (1%
Triton X-100™ (Sigma-Aldrich; Merck Millipore) in PBS) for 5 min.
Subsequently, incubated with terminal deoxynucleotidyl
transferase-containing reaction mixture, which was part of the One
Step TUNEL Apoptosis Assay kit (Beyotime Institute of
Biotechnology, Shanghai, China), for 60 min at 37°C in the dark.
Cells were washed with PBS three times and stained with
streptavidin-tetramethylrhodamine for 30 min at 37°C in the dark.
Subsequently, cells were washed with PBS three times and stained
with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min in the dark.
Finally, the samples were visualized using a confocal laser
scanning microscope (Nikon A1R/A1; Nikon Corporation, Tokyo,
Japan).
Western blot analysis
Total cellular protein was extracted from cells
lysed in radioimmunoprecipitation assay buffer [50 mM Tris (pH
8.0), 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% NP-40 and
0.5% sodium deoxycholate] containing protease inhibitors: 1%
protease inhibitor cocktail and 1 mM phenylmethanesulfonyl fluoride
(Sigma-Aldrich; Merck Millipore). The concentration of protein was
detected by Bradford assay protein quantitation kit (Abcam,
Cambridge, UK). A total of 30 µg protein was separated by 12%
SDS-polyacrylamide gel electrophoresis and transferred onto
nitrocellulose membranes. The membranes were blocked with 5%
skimmed milk reconstituted in Tris-buffered saline with 0.1% Tween
20 (pH 7.6) at room temperature for 1 h, and washed with PBS three
times, followed by incubation at 4°C overnight with primary
antibody. Primary antibodies used were as follows: Poly ADP-ribose
polymerase (PARP) (dilution 1:1,000; #9532), caspase 3 (dilution,
1:1,000; #9662), YAP (dilution, 1:1,000; #14074; All Cell Signaling
Technology, Inc., Danvers, MA, USA), p53 (dilution, #sc-47698
1:400; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH; dilution 1:10,000;
#KC-5G4; KangChen Biotech, Shanghai, China). Following incubation
with the primary antibodies, membranes were incubated with
secondary antibody [peroxidase-conjugated affinipure goat
anti-Rabbit IgG (#ZB-2301; dilution, 1:2,000; Beijing Zhongshan
Golden Bridge Biotechnology, Co., Ltd., Beijing, China) and
peroxidase-conjugated affinipure goat anti-Mouse IgG (#ZB-2305;
dilution, 1:2,000; Beijing Zhongshan Golden Bridge Biotechnology,
Co., Ltd.)] for 1 h at room temperature. Immunoreactive signals
were detected using a WesternBright Quantum HRP substrate kit
(Advansta, Inc., Menlo Park, CA, USA), visualized by a Molecular
Imager ChemiDoc XRS system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Immunoblotting for GAPDH was performed as an internal
control.
Immunofluorescence assay
Cells were cultured in a Millicell EZ SLIDE 8-well
glass and washed with PBS three times, prior to being fixed in 4%
paraformaldehyde for 30 min. Cells were subsequently washed with
PBS again, treated with permeabilization solution (1% Triton X-100
in PBS), washed with PBS three times, and blocked with 1% bovine
serum albumin (BSA; Sigma-Aldrich; Merck Millipore) in PBS for 1 h.
Samples were subsequently incubated with anti-p53 primary antibody
in antibody dilution buffer (1% BSA in 1xPBS) overnight at 4°C.
Samples were washed with PBS three times and incubated with
secondary antibody tetramethylrhodamine-conjugated affinipure goat
anti-Mouse IgG (#ZF-0313; dilution, 1:200; Beijing Zhongshan Golden
Bridge Biotechnology, Co., Ltd.) and fluorescein
isothiocyanate-conjugated affinipure goat anti-Rabbit IgG
(#ZF-0315; dilution, 1:200; Beijing Zhongshan Golden Bridge
Biotechnology, Co., Ltd.) for 60 min at room temperature, followed
by DAPI staining (1:5,000) for 5 min in the dark. Samples were
examined using laser scanning confocal microscopy (Nikon
A1R/A1).
Small interfering RNA (siRNA)
transfection
The YAP-siRNA (siYAP) and non-specific control (NC)
siRNA (siNC) used in the present study were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The sequence of
siYAP was: 5′-GCGTAGCCAGTTACCAACA-3′ and the sequence of siNC was:
5′-UUCUCCGAACGUGUCACGUTT-3′. Cells were transfected with siRNA
using the X-tremeGENE™ siRNA Transfection Reagent (Roche Applied
Science) according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using the RNA fast 200 kit
(Feijie Biotech, Shanghai, China) and reverse-transcribed into cDNA
using the PrimeScript RT-PCR kit (Takara Biotechnology Co., Ltd.,
Dalian, China). Relative gene expression was studied using the
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.) and
SYBR Green PCR Master mix (Takara Biotechnology Co., Ltd.). The
thermocycling conditions were as follows: 95°C for 30 sec, followed
by 40 cycles at 95°C for 5 sec, and 60°C for 30 sec. Primer
sequences used are presented in Table
I. Transcriptional expression of specific genes was calculated
using the 2−ΔΔCq method (13). GAPDH was used for normalization.
 | Table I.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| GAPDH |
ATGGGGAAGGTGAAGGTCGG |
GACGGTGCCATGGAATTTGC |
| YAP |
AATTTGCCCAGTTATACCTCAGTG |
CACATCAAGGCTATGATTCAAACTC |
Statistical analysis
Results are presented as the mean ± standard error
of triplicate experiments. Differences between two groups were
compared by the Student's t-test. All data analyses were
performed using GraphPad Prism version 6 software (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Synergistic effect of temsirolimus and
curcumin treatment on apoptosis
To investigate whether combined treatment with
curcumin and temsirolimus could induce cell death in RCC cells, the
Caki-1 and OS-RC-2 renal cancer cells were treated with these two
agents. Apoptosis was detected using flow cytometry and the TUNEL
assay. As demonstrated in Fig. 1A,
Caki-1 and OS-RC-2 cells treated with temsirolimus or curcumin
alone did not exhibit increased apoptosis, however combined
curcumin and temsirolimus treatment did cause increased apoptosis
in RCC cells. The percentage of apoptotic Caki-1 cells following
combined treatment was 52.90%, which was significantly increased
compared with temsirolimus (6.78%) and curcumin (8.38%) treatment
alone (Fig. 1B; P=0.013 and P=0.009,
respectively). The percentage of apoptotic OS-RC-2 cells following
combined treatment was 41.82%, which was significantly increased
compared with temsirolimus (3.05%) and curcumin (2.52%) treatment
alone (Fig. 1C; P=0.005 and P=0.003,
respectively).
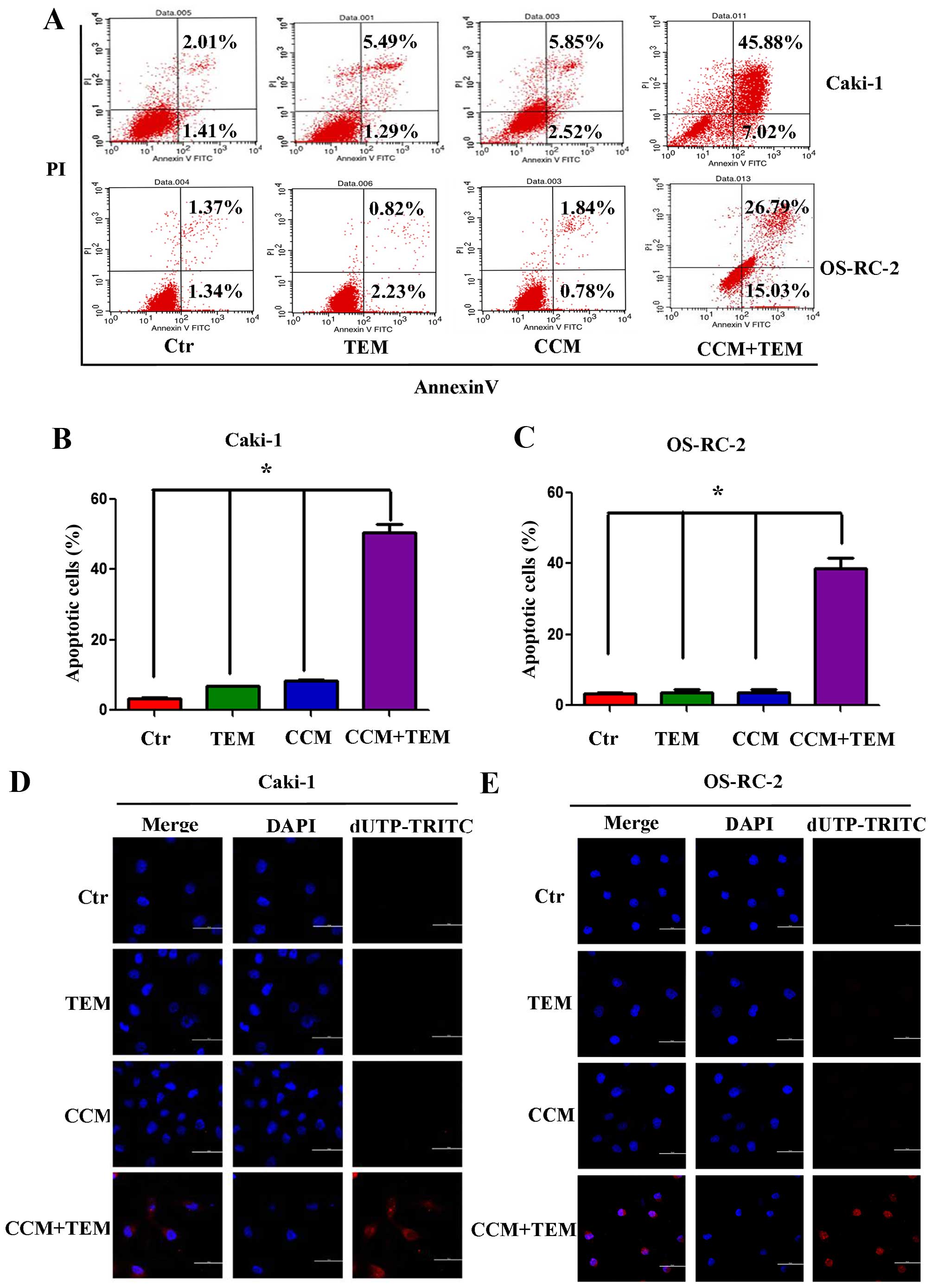 | Figure 1.Synergistic effect of treatment with
TEM and CCM on apoptosis in renal cell carcinoma cell lines. (A)
Caki-1 and OS-RC-2 cells were treated with TEM alone, CCM alone,
TEM and CCM, or the Ctr for 48 h. Cells were stained using
Annexin-V-Fluorescein and PI at room temperature for 20 min, and
apoptosis was measured using flow cytometry. The percentage of
apoptotic cells in the (B) Caki-1 and (C) OS-RC-2 cell lines was
calculated and analyzed using GraphPad Prism software. Values are
presented as the mean ± standard deviation from three independent
samples (*P<0.05 vs. Ctr). Following treatment with TEM alone,
CCM alone, TEM and CCM, or the Ctr for 48 h, DNA strand breakage
was detected using the terminal deoxynucleotidyl
transferase-mediated dUTP Nick-End Labeling assay and observed
using a confocal laser scanning microscope in (D) Caki-1 cells and
(E) OS-RC-2 cells. TEM, temsirolimus; CCM, curcumin; Ctr, control;
PI, propidium iodide; dUTP-TRITC, 2′-deoxyuridine,
5′-triphosphate-tetramethylrhodamine; DAPI,
4′,6-diamidino-2-phenylindole. |
Furthermore, the TUNEL assay was used to detect DNA
fragmentation following the treatment of RCC cells with
temsirolimus/curcumin alone or in combination. As shown in Fig. 1D and E, treatment with temsirolimus or
curcumin alone did not induce DNA fragmentation in the RCC cell
lines, however co-treatment increased DNA fragmentation in Caki-1
(Fig. 1D) and OS-RC-2 (Fig. 1E) cells. The results of the present
study indicate that temsirolimus and curcumin have a synergistic
effect in inducing apoptosis in RCC cells.
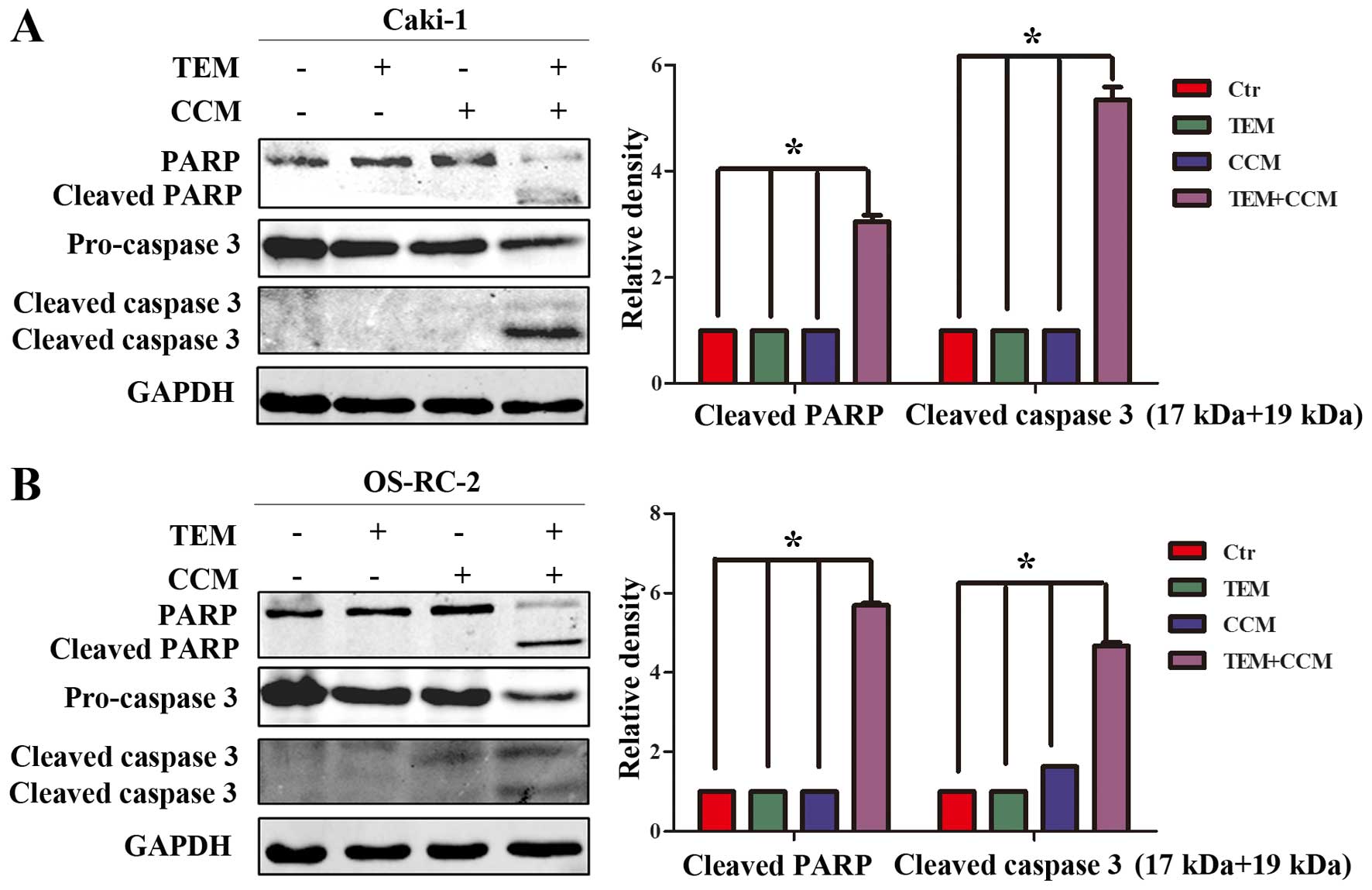
Effect of combined treatment with
temsirolimus and curcumin on apoptosis-associated proteins
In order to elucidate the mechanism of combined
temsirolimus/curcumin-mediated apoptosis in human RCC cells,
western blot analysis was performed to detect the expression of
caspase 3 and PARP, which serve important roles in apoptotic
progression. Pro-caspase 3 levels were significantly downregulated
in Caki-1 cells treated with combined temsirolimus and curcumin,
compared with Caki-1 cells treated with temsirolimus or curcumin
alone (Fig. 2A; P<0.05 vs.
control). Similarly, pro-caspase 3 levels were significantly
downregulated in OS-RC-2 cells treated with combined temsirolimus
and curcumin compared with OS-RC-2 cells treated with temsirolimus
or curcumin alone (Fig. 2B; P=0.025
and P=0.039, respectively). Furthermore, cleaved caspase 3 (17 and
19 kDa) was observed only in the combined treatment groups. In
addition, cleaved PARP, an index of caspase 3, was only observed in
the combined treatment groups. The results of the present study
indicate that combined temsirolimus and curcumin treatment induces
apoptosis in a caspase-dependent manner.
Combined treatment with temsirolimus
and curcumin induces apoptosis through altered p53 expression and
distribution
Cellular p53 is activated as a result of stress,
including DNA damage (14), and is an
essential regulator of apoptosis (15). Therefore, the effect of curcumin and
temsirolimus treatment on p53 expression was analyzed. p53
expression was markedly upregulated in RCC cells treated with
curcumin alone between 4 and 24 h, while in the combined treatment
groups, p53 protein expression was only upregulated between 4 and
12 h, and decreased at 24 h (Fig.
3A). No changes in p53 expression were observed in the DMSO or
temsirolimus alone treatment groups (Fig.
3A).
 | Figure 3.Combined treatment with TEM and CCM
induces apoptosis through alterations in the expression and
distribution profile of p53. (A) Caki-1 and OS-RC-2 cells were
treated with TEM alone, CCM alone, TEM and CEM, or the Ctr, and p53
expression levels were determined using western blot analysis at
various time points. (B) Caki-1 and OS-RC-2 cells were treated with
TEM alone, CEM alone, TEM and CEM, or the Ctr for 6 h. p53
expression and distribution was detected using immunofluorescence.
TEM, temsirolimus; CCM, curcumin; Ctr, control; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; DAPI,
4′,6-diamidino-2-phenylindole. |
The effect of curcumin on p53 expression and
distribution was further examined using an immunofluorescence
assay. As shown in Fig. 3B, p53 was
translocated into the nucleus following 6 h treatment with curcumin
alone or in combination with temsirolimus, however this phenomenon
was not observed in cells treated with temsirolimus alone or in the
control group. The results of the immunofluorescence assay suggest
that p53 is activated by curcumin and explain how combined
treatment induces apoptosis. The results of the present study
indicate that combined treatment with temsirolimus and curcumin
increases apoptosis, which is at least partly due to
curcumin-mediated p53 induction. However, the underlying mechanism
of curcumin-mediated induced p53 expression remains unclear.
Curcumin-induced expression of
YAP/p53
YAP is the major downstream effector of the Hippo
signaling pathway and is able to directly regulate the
transcription of p53 (16).
Previously, it was demonstrated that curcumin is able to regulate
the expression of YAP in bladder cancer cells (6), therefore curcumin may regulate the
expression of YAP in RCC cells. To investigate changes in YAP
expression following treatment with temsirolimus plus curcumin, YAP
expression was detected by western blot analysis at the following
time points: 0, 1, 2, 4, 8, 12 and 24 h. As shown in Fig. 4A, YAP protein expression increased
between 2 and 12 h in a time-dependent manner and subsequently
decreased at 24 h, which is concurrent with the expression pattern
of p53 following treatment with curcumin alone. However, the
induction of p53 was ~2 h behind the induction of YAP. The results
of the present study suggest that expression of YAP and p53 is
increased in RCC cells following treatment with curcumin.
 | Figure 4.YAP is necessary in the activation of
p53. (A) Caki-1 and OS-RC-2 cells were treated with TEM and CCM.
YAP and p53 expression levels were determined using western blot
analysis at various time points. Caki-1 cells were transfected with
siYAP or siNC. A total of 48 h following transfection, cells were
lysed and the expression of YAP was analyzed by (B) reverse
transcription-quantitative polymerase chain reaction and (C)
western blot analysis. (D) A total of 48 h following transfection,
cells were treated with 10 µm/ml CCM, and YAP and p53 expression
levels were subsequently analyzed at various time points by western
blot analysis. (E) Caki-1/siNC and Caki-1/siYAP cells were treated
with 10 µm/ml CCM and 15 µm/ml TEM, and YAP and p53 expression
levels were subsequently analyzed at various time points using
western blot analysis (*P<0.05 vs. Ctr). YAP, Yes-associated
protein; TEM, temsirolimus; CCM, curcumin; si, small interfering;
NC, non-specific control; Ctr, control; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
As YAP and p53 are activated by combined treatment
with temsirolimus and curcumin, and YAP has an important function
as an activator of p53 (16), it was
further investigated whether curcumin activated p53 through YAP.
Following transfection with YAP siRNA for 48 h, Caki-1 cells
exhibited a decrease in YAP mRNA and protein expression, when
compared with the negative control cells (Fig. 4B and C). Caki-1/siNC and Caki-1/siYAP
cells were subsequently treated with 10 µM/ml curcumin, and YAP and
p53 protein levels were measured at various time points using
western blot analysis. As shown in Fig.
4D, p53 expression in Caki-1/si YAP was not increased following
treatment with temsirolimus plus curcumin for 8 h, compared with
the control cells. As shown in Fig.
4E, increased p53 protein expression was not induced in
Caki-1/siYAP cells 8 h following treatment with curcumin, however
significantly increased p53 expression was induced in Caki-1/siNC
cells 4 h following treatment with curcumin (P<0.05 vs.
control). Furthermore, Caki-1 cells were treated with temsirolimus
plus curcumin following transfection with siRNA for 48 h. The
results of the present study reveal that activation of p53 by
curcumin in RCC cells is dependent on YAP.
Discussion
Deregulated apoptosis is a hallmark of cancer
(15). p53 is reported to be a tumor
suppressor protein involved in DNA repair, cell cycle arrest,
senescence and apoptosis (15,17). In
the present study, Caki-1 and OS-RC-2 RCC cell lines, which contain
wild type p53, were used as a cell model (18). The present results demonstrated that
the expression of p53 is increased following combined treatment
with temsirolimus and curcumin in Caki-1 and OS-RC-2 cells for 48
h. However, this effect is not observed following treatment with
temsirolimus or curcumin alone. In addition, cellular p21
expression is increased following treatment with curcumin (data not
shown). Previous research has demonstrated that when p53 is induced
as a result of limited cell damage it activates growth-inhibitory
genes to cause transient cell cycle arrest. However, p53 triggers
the activation of apoptosis-associated genes when cells are exposed
to extensive and irreparable damage (15,19,20). Cells
sustained mild damage following treatment with low concentration
curcumin, leading to p53-mediated activation of p21 expression and
subsequent cell cycle arrest. However, combined treatment with
temsirolimus and curcumin caused severe damage to cells and this
led to apoptosis.
YAP, the effector of the Hippo signaling pathway, is
reported to be an oncoprotein involved in tumor progression,
metastasis, proliferation, transformation, migration and invasion
in RCC (21–23). However, the results of the present
study suggest that YAP enhances the chemosensitivity of RCC cells
by directly activating p53 expression. It was demonstrated that YAP
protein was immediately increased following treatment with
curcumin. Furthermore, following transfection with YAP siRNA, it
was observed that YAP is essential for p53 activation. The results
of the present study indicate that YAP functions as an apoptotic
enhancer of chemotherapeutics in RCC and this is consistent with
observations in other carcinoma cells (16). Chemoresistance in cells appears to be
regulated by proliferative and anti-apoptotic signals, as well as
metabolic pathways and changes in drug treatment. YAP may not only
be regulated by the Hippo signaling pathway.
Curcumin is used widely in combinatory cancer
therapy due to its anti-inflammatory and anticarcinogenic
properties, as well as its low toxicity (8). The results of the present study
demonstrated that curcumin combined with temsirolimus induces
apoptosis. This is consistent with a previous study, in which
combined treatment with curcumin and an mTOR inhibitor was
demonstrated to induce apoptosis through p53-mediated
downregulation of Bcl-2 pathway (12). However, the mechanism of altered p53
expression remained unclear. In the present study, it was observed
that treatment with low concentration curcumin activates the
expression of YAP and p53 in a time-dependent manner but does not
induce apoptosis. However combined treatment with low concentration
curcumin and temsirolimus significantly induces apoptosis. Notably,
treatment with high concentration curcumin alone induced apoptosis
in Caki-1 and OS-RC-2 cell lines (data not shown). The molecular
mechanism may be that low concentration curcumin activates YAP
expression, which induces peak p53 expression and translocation to
the nucleus. A second drug or stress may subsequently utilize p53
to induce the expression of apoptosis-associated genes, leading to
apoptosis. The results demonstrate that YAP is a novel candidate
target for curcumin-activated apoptosis.
In conclusion, the results of the present study
demonstrate that co-treatment with temsirolimus and curcumin
induces apoptosis in RCC cells through an increase in YAP/p53
expression. However, treatment with temsirolimus or curcumin alone
does not induce apoptosis-associated gene expression. Furthermore,
the molecular mechanism of temsirolimus and curcumin-induced
apoptosis is the upregulation of YAP protein expression, and
subsequently the upregulation of p53 expression. The present
results suggest that combined treatment with curcumin and
anti-cancer drugs has a synergistic effect in RCC cells.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos., 81602244,
81372279, and 81171953).
References
|
1
|
Frew IJ and Moch H: A clearer view of the
molecular complexity of clear cell renal cell carcinoma. Annu Rev
Pathol. 10:263–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Axelson H and Johansson ME: Renal stem
cells and their implications for kidney cancer. Semin Cancer Biol.
23:56–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kapoor A and Figlin RA: Targeted
inhibition of mammalian target of rapamycin for the treatment of
advanced renal cell carcinoma. Cancer. 115:3618–3630. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerullis H, Ecke TH, Eimer C, Heuck CJ and
Otto T: mTOR-inhibition in metastatic renal cell carcinoma. Focus
on temsirolimus: A review. Minerva Urol Nefrol. 62:411–423.
2010.PubMed/NCBI
|
|
5
|
Tuorkey MJ: Curcumin a potent cancer
preventive agent: Mechanisms of cancer cell killing. Interv Med
Appl Sci. 6:139–146. 2014.PubMed/NCBI
|
|
6
|
Gao Y, Shi Q, Xu S, Du C, Liang L, Wu K,
Wang K, Wang X, Chang LS, He D and Guo P: Curcumin promotes KLF5
proteasome degradation through downregulating YAP/TAZ in bladder
cancer cells. Int J Mol Sci. 15:15173–15187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rahmani AH, Al Zohairy MA, Aly SM and MA:
Curcumin Khan: A potential candidate in prevention of cancer via
modulation of molecular pathways. Biomed Res Int. 2014:7616082014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Troselj KG and Kujundzic RN: Curcumin in
combined cancer therapy. Curr Pharm Des. 20:6682–6696. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rana C, Piplani H, Vaish V, Nehru B and
Sanyal SN: Downregulation of PI3-K/Akt/PTEN pathway and activation
of mitochondrial intrinsic apoptosis by Diclofenac and Curcumin in
colon cancer. Mol Cell Biochem. 402:225–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rashid K and Sil PC: Curcumin enhances
recovery of pancreatic islets from cellular stress induced
inflammation and apoptosis in diabetic rats. Toxicol Appl
Pharmacol. 282:297–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Toden S, Okugawa Y, Jascur T, Wodarz D,
Komarova NL, Buhrmann C, Shakibaei M, Boland CR and Goel A:
Curcumin mediates chemosensitization to 5-fluorouracil through
miRNA-induced suppression of epithelial-to-mesenchymal transition
in chemoresistant colorectal cancer. Carcinogenesis. 36:355–367.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seo BR, Min KJ, Cho IJ, Kim SC and Kwon
TK: Curcumin significantly enhances dual PI3K/Akt and mTOR
inhibitor NVP-BEZ235-induced apoptosis in human renal carcinoma
Caki cells through down-regulation of p53-dependent Bcl-2
expression and inhibition of Mcl-1 protein stability. PLoS One.
9:e955882014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El-Deiry WS: The role of p53 in
chemosensitivity and radiosensitivity. Oncogene. 22:7486–7495.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aylon Y and Oren M: Living with p53, dying
of p53. Cell. 130:597–600. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bai N, Zhang C, Liang N, Zhang Z, Chang A,
Yin J, Li Z, Luo N, Tan X, Luo N, et al: Yes-associated protein
(YAP) increases chemosensitivity of hepatocellular carcinoma cells
by modulation of p53. Cancer Biol Ther. 14:511–520. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vousden KH and Prives C: Blinded by the
light: The growing complexity of p53. Cell. 137:413–431. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou X, Tolstov Y, Arslan A, Roth W,
Grüllich C, Pahernik S, Hohenfellner M and Duensing S: Harnessing
the p53-PUMA Axis to overcome DNA damage resistance in renal cell
carcinoma. Neoplasia. 16:1028–1035. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harris SL and Levine AJ: The p53 pathway:
Positive and negative feedback loops. Oncogene. 24:2899–2908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laptenko O and Prives C: Transcriptional
regulation by p53: One protein, many possibilities. Cell Death
Differ. 13:951–961. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma Y, Yang Y, Wang F, Wei Q and Qin H:
Hippo-YAP signaling pathway: A new paradigm for cancer therapy. Int
J Cancer. 137:2275–2286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Piccolo S, Dupont S and Cordenonsi M: The
biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev.
94:1287–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barron DA and Kagey JD: The role of the
Hippo pathway in human disease and tumorigenesis. Clin Transl Med.
3:252014. View Article : Google Scholar : PubMed/NCBI
|