Introduction
Oxidative stress is involved in the development and
progression of various neurodegenerative diseases, including
Parkinson's disease (PD), Alzheimer's disease and amyotrophic
lateral sclerosis (1). PD is a
progressive neurodegenerative disease with an unknown pathogenesis,
and the loss of substantia nigra dopaminergic neurons is
characteristic of its lesions (2–5). Oxidative
stress-induced mitochondrial dysfunction is hypothesized to be the
main reason for the pathogenesis of PD (6). After neurotoxin
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induction, PD
can be modeled in vitro. MPTP can cross the blood-brain
barrier, and is converted into its active metabolite
methyl-4-phenylpyridine (MPP+), which due to affinity with the
dopamine transporter, is thus selectively transported to the
mitochondria of the dopaminergic neurons. As a neurotoxic
metabolite, MPP+ can block cell respiration and promote active
oxygen [reactive oxygen species (ROS) formation], thus inducing the
death of dopaminergic neurons (7).
While traditional levodopa replacement therapy and
certain non-steroidal anti-inflammatory drugs can reduce the
clinical symptoms, they do not prevent progression of the disease
and long-term medication can cause serious adverse reactions
(8,9).
Matrine can be obtained from plants of the Sophora genus and
has always been used in traditional Chinese medicine to treat
inflammation (10). Matrine has been
shown to produce a wide range of pharmacological effects and has
been used to treat a variety of diseases, including viral
hepatitis, neuropathic pain and isoproterenol-induced heart disease
(11–13). In addition, significant antitumor
effects have been found in gastric cancer, rhabdomyosarcoma, acute
myeloid leukemia and breast cancer (14,15), and
studies have shown that matrine exhibits antioxidant effects in a
number of diseases. PD is mainly caused by damage to dopamine
neurons, and oxidative stress is one of its important pathogenetic
factors. There is little literature on the interaction between
matrine and the MPTP-induced damage to mouse dopaminergic neurons
in PD. Accordingly, the present study investigated whether matrine
has a protective effect on dopaminergic neurons, and the viral
mechanisms involved were studied.
Materials and methods
Materials
C57BL, 7 to 8-month-old, male mice (weighing 20–25
g) were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). The mice were housed in a
thermostatically controlled environment with set lighting
conditions (lighting time, 7:30 a.m. to 7:30 p.m.). A total of 25
mice were randomly divided into five groups, namely the control
group (group A), the MPTP group (group B) and three matrine (4, 8
and 16 mg/kg) plus MPTP treatment groups (groups C, D and E,
respectively). The control group received saline by intraperitoneal
injection (30 mg/kg/day for 4 days), and the MPTP group was
continuously administered an intraperitoneal injection of 30 mg/kg
MPTP for 4 days (once a day) to create the PD mouse model. The
matrine + MPTP groups were treated with different doses of matrine
(4, 8 and 16 mg/kg) in advance, 8 h prior to intraperitoneal
injection with MPTP.
The study was approved by the Ethics Committee of
the College of Basic Medical Sciences, Jilin University (Changchun,
Jilin, China).
Equipment, drugs and reagents
An ultra-pure water system (Milli-Q Synthesis) was
purchased from Millipore (Darmstadt, Germany) and an automatic
embedding machine (model no. EG-1140C) was purchased from Leica
Microsystems, Inc. (Buffalo Grove, IL, USA). A slicing machine
(model no. X-202A) was purchased from Guangdong Yi Mai Technology
Co., Ltd. (Guangdon, China), an inverted phase contrast microscope
was obtained from Olympus Corporation (model no. BX51), and
constant current regulator electrophoresis (model no. DYC-40C) and
semi-dry transfer membrane (model no. DYY-8B) instruments were
purchased from Beijing Liuyi Biotechnology Co., Ltd. (Beijing,
China). Matrine (catalog no. CDS016735), MPTP (catalog no. M0896)
and rabbit anti-mouse tyrosine hydroxylase (TH) antibody (catalog
no. T8700) were purchased from Sigma-Aldrich (Millipore). Rabbit
anti-mouse nuclear factor E2-related factor 2 (Nrf2; catalog no.
12721P) and rabbit anti-mouse β-actin (catalog no. 12620; dilution,
1:10,000) antibodies were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). The concentrated DAB kit was purchased
from Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing,
China; catalog no. ZLI-9017), the superoxide dismutase (SOD) test
kit (catalog no. A001-3) and the glutathione (GSH) test kit
(catalog no. A006) were purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, Jiangsu, China), and the
CytoBuster protein extraction reagent was purchased from Novagen
Inc. (Madison, WI, USA; catalog no. 71009-3).
Establishment and execution of a mouse
model of PD
Under a constant temperature and lighting conditions
(lighting time, 7:30 a.m. to 7:30 p.m.), the mice were continuously
treated for 4 days, and suspension and climbing experiments were
conducted every day. On the fourth day, all mice were decapitated,
then the mouse brain striatum and substantia nigra were isolated;
one portion was used for protein extraction, and the other portion
was stored at room temperature after being embedded in
paraffin.
Suspension experiment
The C57BL mice were placed on a horizontal wire of
~1.5 mm in diameter, suspended 30 cm from the ground, and the hang
time was recorded to detect mouse limb coordination. Scoring
criteria: 0–5 sec, 0 points; 6–10 sec 2 points; 11–15 sec, 3
points; 16–20 sec, 4 points; and >20 sec, 5 points.
Pole-climbing test
A tube of ~30 cm in length and ~1 cm in diameter was
gauze wrapped and a wooden ball was attached to the top. Mice were
placed on top of the wooden ball and the time required for the
mouse to traverse from the top to the bottom of the tube was
recorded. Time were compared prior to and after PD modeling.
SOD and GSH detection
The levels of SOD and GSH were detected according to
the manufacturer's instructions provided in the SOD (catalog no.
A001-3) and GSH (catalog no. A006) test kits (Nanjing Jiancheng
Bioengineering Institute).
TH antigen test
The paraffin sections were made into frozen sections
and dried at room temperature for 15 min, the stained tissue was
marked by Pap Pen, soaked for 10 min in PBS to remove the OCT and
then film-fixed at room temperature for 30 min with 4%
paraformaldehyde. The sections were treated for 10 min with 2.2%
Triton X-100, and then washed with PBS twice and blocked with 10%
normal goat serum in PBS at room temperature for 1 h. TH antibody
was added (1:3,000 dilution) and the section was incubated
overnight at 4°C, followed by being washed with PBS twice. The
Alexa Fluor 488-labeled goat anti-rabbit immunoglobulin G secondary
antibody was added and the section was incubated at 37°C for 1 h
prior to being washed again with PBS twice. Fluorescence microscopy
was performed on five randomly selected fields, and the number of
positive cells was counted and analyzed statistically.
Nigrostriatal protein extracts
The nigrostriatal tissue was homogenized, then
washed twice with ice-cold PBS and centrifuged at 400 × g
for 5 min. The supernatant was discarded, and the 100X protease
inhibitors and CytoBuster protein extraction reagent were added to
the precipitate, pipetted and mixed at room temperature for 15 min,
prior to centrifugation at 12,000 × g for 15 min at 4°C. The
resulting supernatant contained the desired protein.
Western blotting
The extracted protein was isolated using 10%
SDS-PAGE gel separation and a 5% stacking gel, and then
semi-transferred to nitrocellulose membranes using incubation in
TBST containing 5% BSA at room temperature for 2 h. Nrf2 rabbit
anti-mouse antibodies (1:1,000 dilution)were added and the
membranes were incubated at 4°C overnight. The next day, the
membrane was washed with 0.1% TBST 3 times, for 5 min each, and the
horseradish peroxidase (HRP)-labeled goat anti-rabbit secondary
antibody was added for incubation at room temperature for 1 h. 0.1%
TBST was used to wash the membrane, using Supersignal West
Femto/Pico HRP-sensitive chemiluminescent substrate for coloration.
Rabbit anti-mouse β-actin (1:10,000 dilution) was used as an
internal control. All experiments were repeated at least 3
times.
Statistical
Using SPSS 15.0 statistical software (SPSS, Inc.,
Chicago, IL, USA), multiple sets of data were compared using a
one-way analysis of variance, and differences between two groups of
data were compared using the Student-Newman-Keuls analysis method.
All data are presented as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
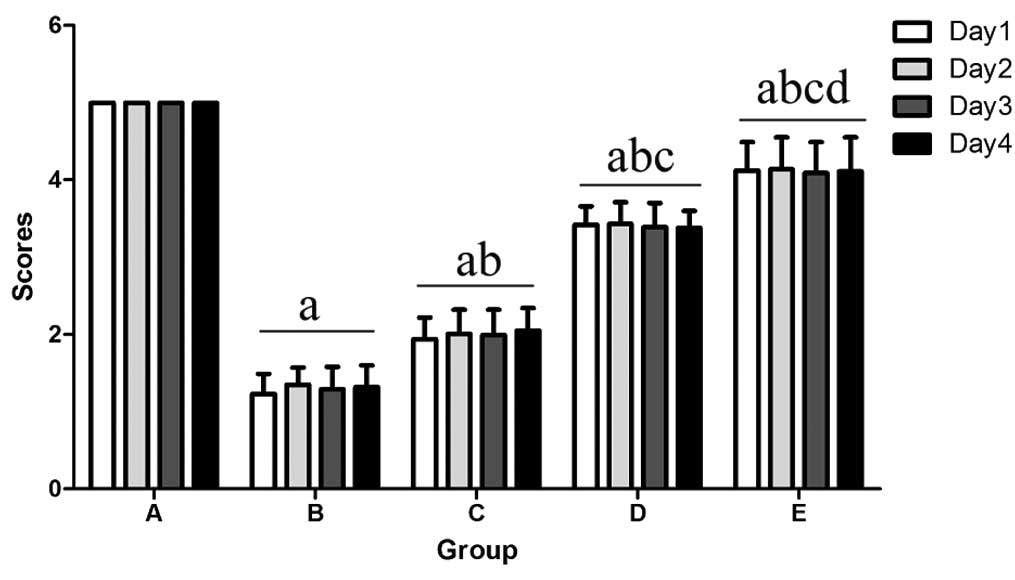
Effect of matrine on the suspension
ability of mice with PD
The control mice exhibited a mean suspension ability
score of 5. Compared with the control group, the mean score in the
MPTP mice was significantly lower (P<0.001), while matrine
administration significantly alleviated this phenomenon (P=0.004).
The suspension ability score increased with increasing matrine
concentration (P<0.001) (Fig.
1).
Effect of matrine on pole-climbing
ability
Compared with the control group, MPTP mice exhibited
a significantly reduced capacity for pole-climbing, with a
significantly longer climb time (P<0.001). The administration of
matrine was able to significantly alleviate this phenomenon
(P=0.008), and the pole-climbing ability recovered more
significantly with increasing matrine concentration (P<0.001)
(Fig. 2).
Mouse brain tissue SOD and GSH
assay
Compared with the control group, the MPTP mice
exhibited significantly lower SOD and GSH activity in the brain
tissues (P<0.001), while the administration of matrine
significantly alleviated this phenomenon (P=0.006). The mouse brain
activity recovered more significantly with increasing matrine
concentration (P<0.001) (Fig.
3).
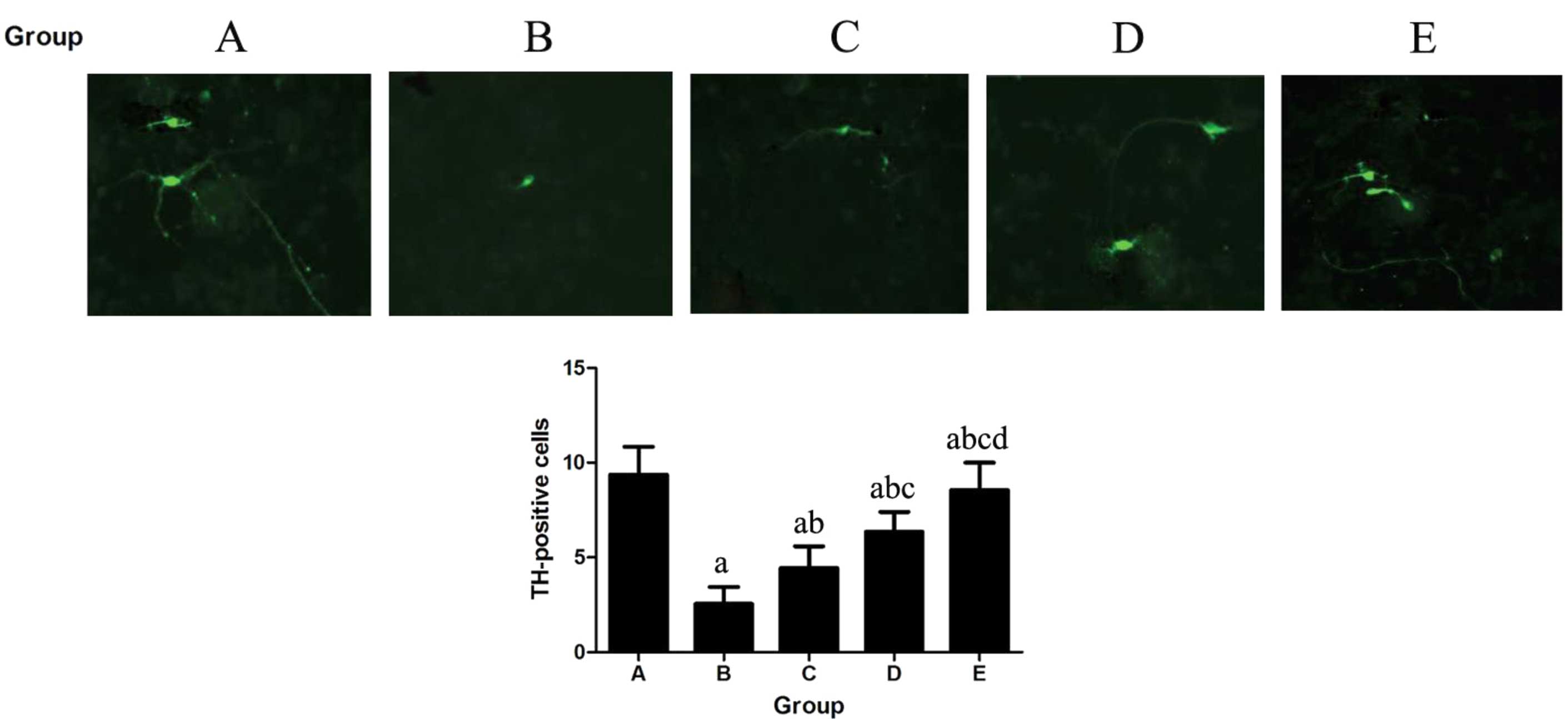
Immunohistochemistry
In the comparison of dopamine TH expression in the
substantia nigra and the striatum, relative to the control group,
the number of TH-positive cells of the MPTP group significantly
decreased (P<0.001). In the matrine-treated mice, a higher
expression level of TH and a greater number of TH-positive cells
was evident (Fig. 4).
Western blot analysis detecting the
expression of Nrf2
The relative intensity of antioxidant-related Nrf2
molecules decreased significantly in the MPTP group, while in the
matrine-treated mice, stronger Nrf2 expression was exhibited in the
substantia nigra and striatum, which increased in a
concentration-dependent manner (Fig.
5).
Discussion
PD is a progressive neurodegenerative disease whose
main symptoms include bradykinesia, resting tremors and rigidity
(16–18). In the present study, using an
MPTP-induced mouse PD model, reduced motion bradykinesia, which is
typical of PD, was apparent along with other symptoms of the
disease. Oxidative stress is the cause of a number of
neurodegenerative diseases, including PD (6). Due to a high metabolic rate, brain
tissue is prone to hypoxia, and this may lead to an increase in
reactive oxygen species (ROS) and oxidative stress. SOD is one of
the key enzymes in the oxidative stress defense and is key to good
health (19). GSH, formed from a
combination of glutamic acid, cysteine and glycine, is a tripeptide
containing a thiol group, and has antioxidant and detoxification
integration (20). The activity of
SOD and GSH may reflect the free radical scavenging ability of the
body.
When PD occurs, brain tissue SOD and GSH activity is
significantly reduced. In the present study, SOD and GSH activity
was significantly reduced in the cerebral tissue of the MPTP mice.
In addition, immunofluorescence showed a significant reduction in
the TH expression of MPTP mice at the substantia nigra and the
striatum. The described animal model meets the requirements
established for PD in this study.
Matrine obtained from plants of the Sophora
genus has always been used in traditional Chinese medicine to treat
inflammation (10). Studies have
shown that matrine can prevent the steatohepatitis caused by a high
carbohydrate diet (21) through its
antioxidant effects. PD is mainly caused by a loss of dopamine
neurons, and oxidative stress is important in the pathogenesis.
Little literature is available on whether matrine can protect
dopamine neurons in mice with MPTP-induced PD and the specific
mechanisms involved. The results of the present study showed that
following the administration of matrine treatment, a number of the
typical symptoms of PD significantly improved, SOD and GSH activity
appeared significantly increased in the brain tissue, and a higher
level of TH expression and more TH-positive cells were evident.
This suggests that matrine may have a significant therapeutic
effect on PD.
Nrf2 can regulate the expression of 200 genes,
including a number of antioxidant genes (22). Nrf2 protects against the islet β cell
damage caused by acute oxidative stress (23). The present study further examined the
matrine treatment of PD by regulating the expression of Nrf2 to
investigate how matrine protect dopamine neurons. The results
showed that Nrf2 expression in the MPTP group was significantly
decreased, while the administration of matrine resulted in stronger
Nrf2 expression in the substantia nigra and the striatum, in a
concentration-dependent manner. In summary, the results of the
present study confirmed that matrine exhibited a significant
therapeutic effect in mice with PD and demonstrated that the
mechanism of matrine treatment in PD may be the inhibition of
oxidative damage of dopamine neurons by the promotion of
antioxidant-related Nrf2 signaling pathways.
References
|
1
|
Barnham KJ, Masters CL and Bush AI:
Neurodegenerative diseases and oxidative stress. Nat Rev Drug
Discov. 3:205–214. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wood JM and Gupta S: Vitamin D and
neurocognitive disorder due to Alzheimer's disease: A review of the
literature. Ann Clin Psychiatry. 27:e1–e7. 2015.PubMed/NCBI
|
|
3
|
Sargolzaei S, Sargolzaei A, Cabrerizo M,
Chen G, Goryawala M, Noei S, Zhou Q, Duara R, Barker W and Adjouadi
M: A practical guideline for intracranial volume estimation in
patients with Alzheimer's disease. BMC Bioinformatics. 16:(Suppl
7). S82015. View Article : Google Scholar
|
|
4
|
Zhang C, Hu WH, Wu L, Zhang K and Zhang
JG: Behavioral effects of deep brain stimulation of the anterior
nucleus of thalamus, entorhinal cortex and fornix in a rat model of
Alzheimer's disease. Chin Med J (Engl). 128:1190–1195. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lara H Herman, Alanís-Garza EJ, Puente MF
Estrada, Mureyko LL, DA Alarcón Torres and Ixtepan Turrent L:
Nutritional approaches to modulate oxidative stress that induce
Alzheimer's disease. Nutritional approaches to prevent Alzheimer's
disease. Gac Med Mex. 151:245–251. 2015.(In Spanish). PubMed/NCBI
|
|
6
|
Langston JW and Irwin I: MPTP: Current
concepts and controversies. Clin Neuropharmacol. 9:485–507. 1986.
View Article : Google Scholar
|
|
7
|
Alcaraz-Zubeldia M, Rojas P, Boll C and
Rios C: Neuroprotective effect of acute and chronic administration
of copper (II) sulfate against MPP+ neurotoxicity in mice.
Neurochem Res. 26:59–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yacoubian TA and Standaert DG: Targets for
neuroprotection in Parkinson's disease. Biochim Biophys Acta.
1792:676–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Samii A, Etminan M, Wiens MO and Jafari S:
NSAID use and the risk of Parkinson's disease: Systematic review
and meta-analysis of observational studies. Drugs Aging.
26:769–779. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Wang B, Zhou C and Bi Y: Matrine
induces apoptosis in angiotensin II-stimulated hyperplasia of
cardiac fibroblasts: Effects on Bcl-2/Bax expression and caspase-3
activation. Basic Clin Pharmacol Toxicol. 101:1–8. 2007. View Article : Google Scholar
|
|
11
|
Long Y, Lin XT, Zeng KL and Zhang L:
Efficacy of intramuscular matrine in the treatment of chronic
hepatitis B. Hepatobiliary Pancreat Dis Int. 3:69–72.
2004.PubMed/NCBI
|
|
12
|
Haiyan W, Yuxiang L, Linglu D, Tingting X,
Yinju H, Hongyan L, Lin M, Yuanxu J, Yanrong W and Jianqiang Y:
Antinociceptive effects of matrine on neuropathic pain induced by
chronic constriction injury. Pharm Biol. 51:844–850. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Zhou R, Zheng P, Yan L, Wu Y, Xiao X
and Dai G: Cardioprotective effect of matrine on
isoproterenol-induced cardiotoxicity in rats. J Pharm Pharmacol.
62:514–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Zhang J, Ma H, Chen X, Liu T, Jiao
Z, He W, Wang F, Liu X and Zeng X: Protective role of autophagy in
matrine-induced gastric cancer cell death. Int J Oncol.
42:1417–1426. 2013.PubMed/NCBI
|
|
15
|
Guo L, Xue TY, Xu W and Gao JZ: Effects of
matrine on the proliferation and apoptosis of human
rhabdomyosarcoma RD cells. Zhongguo Dang Dai Er Ke Za Zhi.
14:780–784. 2012.(In Chinese). PubMed/NCBI
|
|
16
|
Haga R, Sugimoto K, Nishijima H, Miki Y,
Suzuki C, Wakabayashi K, Baba M, Yagihashi S and Tomiyama M:
Clinical utility of skin biopsy in differentiating between
Parkinson's disease and multiple system atrophy. Parkinsons Dis.
2015:1670382015.PubMed/NCBI
|
|
17
|
Akbar U, He Y, Dai Y, Hack N, Malaty I,
McFarland NR, Hess C, Schmidt P, Wu S and Okun MS: Weight loss and
impact on quality of life in Parkinson's disease. PLoS One.
10:e01245412015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu HG, Zhang K, Yang AC and Zhang JG:
Deep brain stimulation of the subthalamic and pedunculopontine
nucleus in a patient with Parkinson's disease. J Korean Neurosurg
Soc. 57:303–306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun WH, Liu F, Chen, Y and Zhu YC:
Hydrogen sulfide decreases the levels of ROS by inhibiting
mitochondrial complex IV and increasing SOD activities in
cardiomyocytes under ischemia/reperfusion. Biochem Biophys Res
Commun. 421:164–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bo Young Chung, So Ra Choi, Ik Jun Moon,
et al: The glutathione derivative, GSH monoethyl ester, may
effectively whiten skin but GSH does not. Int J Mol Sci.
17:E6292016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang HF, Shi LJ, Song GY, Cai ZG, Wang C
and An RJ: Protective effects of matrine against progression of
high-fructose diet-induced steatohepatitis by enhancing antioxidant
and anti-inflammatory defences involving Nrf2 translocation. Food
Chem Toxicol. 55:70–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abdul-Aziz A, MacEwan DJ, Bowles KM and
Rushworth SA: Oxidative stress responses and NRF2 in human
leukaemia. Oxid Med Cell Longev. 2015:4546592015.PubMed/NCBI
|
|
23
|
Fu J, Zheng H, Wang H, Yang B, Zhao R, Lu
C, Liu Z, Hou Y, Xu Y, Zhang Q, et al: Protective role of nuclear
factor E2-related factor 2 against acute oxidative stress-induced
pancreatic β-cell damage. Oxid Med Cell Longev.
2015:6391912015.PubMed/NCBI
|