Introduction
Colorectal cancer, also termed colon cancer or
rectal cancer, results from abnormal multiplication of cells in the
colon or rectum that are able to spread to other parts of the body
(1). Statistics indicated that
136,830 new patients with colorectal cancer and 50,310 mortalities
from colorectal cancer occurred in the USA in 2014 (2). In China, colorectal cancer is also one
of the most widespread malignant tumors, and its incidence is
increasing (3). Chemotherapy is
widely used in colorectal cancer treatment. However, cancer cells
usually show resistance to the drugs, which is the main cause of
treatment failure (4–7). Overcoming drug resistance will be
significant to improve prognosis and survival. 5-Fluorouracil
(5-FU), an anti-cancer drug, is used as one of the standard
chemotherapy regimens for colorectal cancer treatment (8). 5-FU acts as an antimetabolite that
irreversibly inhibits thymidylate synthase enzyme, resulting in
defective synthesis of DNA and RNA, and thus induces apoptosis and
inhibits cell growth (9). However, it
has been reported that the therapeutic effectiveness of 5-FU is
often limited due to the development of drug resistance and
toxicity at high doses (10). Thus,
an effective treatment strategy is required to repress resistance
to 5-FU and resensitize cancer cells to the drug.
Caveolins are a family of membrane-associated
proteins that have three members in vertebrates: Caveolin-1
(Cav-1), caveolin-2 (Cav-2) and caveolin-3 (Cav-3), which are the
main components of cholesterol-enriched invaginations of the plasma
membrane termed caveola membranes (11). Caveola membranes are pivotally
involved in receptor-independent endocytosis (11–13),
caveolae biogenesis, signal transduction and cholesterol
homeostasis (14–16). The cell plasma membrane is the main
entry point for chemotherapeutic agents, and membrane-associated
proteins are speculated to be involved in the development of
resistance, though this phenomenon may be attributed to multiple
mechanisms (17). Cav-1, as the
principal component of caveolae, plays an important role in
material transportation, endothelial infiltration and tumorigenesis
(18). Cav-1 acts as a scaffolding
protein by interacting with signaling molecules through a caveolin
scaffolding domain to modulate gene expression, signal transduction
and protein translocation in the cell membrane (18). It is highlighted that Cav-1 plays a
crucial role in tumor progression, cell growth, invasion and
metastasis (19–22). Additionally, it has been shown that
Cav-1 is closely associated with the development of drug resistance
(23–25).
In the present study, drug-resistant colorectal
cancer HCT116 cells were cultivated, and the expression of Cav-1 in
these drug-resistant cells (DRC) was explored. Using the Cav-1
specific inhibitor methyl β-cyclodextrin (MCD) and its small
interfering RNA (siRNA), the present study determined that Cav-1
was involved in the development of resistance of colorectal cancer
HCT116 cells to 5-FU. The current study suggested that targeting
the chemoresistance-associated protein Cav-1 may improve the
efficiency of chemotherapy with 5-FU.
Materials and methods
Cell culture
The human colorectal cancer HCT116 cell line
(American Type Culture Collection, Manassas, VA, USA) was cultured
at the Department of Oncology, Affiliated Hospital of Hangzhou
Normal University (Hangzhou, China). Cells were maintained in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% heat-inactivated fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml of penicillin and
100 µg/ml of streptomycin in a 37°C incubator with a humidified
atmosphere containing 5% (v/v) CO2.
Development of 5-FU-resistant HCT116
cells
To obtain DRC, human colorectal cancer HCT116 cells
were exposed to increasing concentrations of 5-FU ranging from 5 to
40 mg/l in complete medium. Briefly, HCT116 cells were cultured in
60-mm culture plates for 24 h, and 5 mg/l of 5-FU was added in the
medium for another 48 h. The medium was then replaced with
drug-free fresh medium to incubate the cells until 90% confluence
was reached. Subsequently, the cells were trypsizined, re-plated
and re-exposed to a double dose of drug. This process was repeated
until cells exhibited resistance to 40 mg/l of 5-FU. Subsequent to
exposure to increasing concentrations of 5-FU for ≥3 months, living
cells were collected and termed DRC, which were used for additional
experiments.
Cell survival assay
Cell survival was evaluated by MTT assay. Briefly,
1×104 cells were seeded in a 96-well plate and incubated
at 37°C until 80% confluence was reached. The cells were then
treated with 40 mg/l of 5-FU for 0, 24, 48 and 72 h, followed by
incubation with 20 µl of 5 mg/ml MTT (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) for additional 4 h. Finally, 200 µl
of dimethyl sulfoxide was added to lyse the cells, and the
absorbance was determined using an ELISA reader (Tecan Austria
GmbH, Grödig, Austria) at 570 nm.
Morphological observation of
5-FU-resistant cells
HCT116 cells and DRC were cultured in 60-mm culture
dishes for 24 h, and then treated with or without 40 mg/l of 5-FU
for 72 h. Next, the medium was removed and the cells were washed
once with RPMI-1640 medium. Cell morphology was observed and images
were captured using a vertical microscope (Olympus Corporation,
Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using the Total
RNA Isolation kit (A&A Biotechnology, Gdynia, Poland) following
the manufacturer's protocol. Complementary DNA was obtained by RT
of RNA using the PrimeScript II First Strand cDNA Synthesis kit
(Takara Biotechnology, Co., Ltd., Dalian, China) and amplified
using TaqMan® Gene Expression Assay (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with fluorogenic fluorescein
amidite-labeled probes. The primers for Cav-1 and the internal
control GAPDH were obtained from Takara Biotechnology, Co., Ltd.
The sequences of the primers were as follows:
5-CTCGAGATGTCTGGGGGCAAATACG-3′ (forward) and
5-GAATTCTATCTCTTTCTGCGTGCTG-3′ (reverse) for Cav-1; and
5-GGCCGTGAAGTCGTCAGAAC-3′ (forward) and 5-GCCACGATGCCCAGGAA-3′
(reverse) for GAPDH. Cav-1 expression was normalized to GAPDH
levels and calculated using the 2−ΔΔCq method (26). The relative expression of Cav-1
messenger RNA (mRNA) in DRC was indicated as the percentage of mRNA
in HCT116 cells.
Western blot analysis
Cells were lysed in cell lysis buffer for western
blotting and immunoprecipitation (catalogue no. P0013; Beyotime
Institute of Biotechnology, Haimen, China) containing a protease
inhibitor cocktail (Roche Applied Science, Madison, WI, USA).
Protein samples (50 µg) were separated by 12% SDS-PAGE and
transferred to Immobilon-P membranes (EMD Millipore, Billerica, MA,
USA). The membrane was blocked with 5% non-fat dried milk in TBS
containing Tween 20 for 1 h at room temperature, and then incubated
overnight at 4°C with an anti-Cav-1 mouse monoclonal antibody (mAb)
(1:1,000; sc-135860), which was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). GAPDH was probed using an
anti-GAPDH rabbit mAb (1:1,000; 5174P; Cell Signaling Technology,
Inc., Danvers, MA, USA) overnight at 4°C as a loading control. Goat
anti-mouse (1:2,000; sc-2005; Santa Cruz Biotechnology, Inc.) and
goat anti-rabbit immunoglobulin G secondary antibodies (1:2,000;
sc-2004; Santa Cruz Biotechnology, Inc.) were then incubated for 2
h at room temperature. An enhanced chemiluminescent-detecting
reagent (GE Healthcare Life Sciences, Chalfont, UK) was used for
development. The protein blots were quantified by densitometry
using QuantityOne software version 4.6.7 (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), and the levels were expressed relative to
the internal reference GAPDH.
siRNA transfection
Cell transfections were conducted using
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Cells were additionally
grown for 24 h, followed by the addition of 5 mg/l of 5-FU for
another 72 h. Whole cell lysates were either prepared for
immunoblotting, or MTT assay was performed.
Statistical analysis
All the experiments were repeated ≥3 times.
Statistical significance was analyzed by Students t-test using SPSS
11.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference. Data
was presented as the mean ± standard error of the mean.
Results
Development of drug-resistant
colorectal cancer cells to 5-FU
To study the underlying molecular mechanism of drug
resistance development in colorectal cancer cells to 5-FU, the
5-FU-resistant colorectal cancer HCT116 cell model was firstly
established. Cell survival was evaluated by MTT assay, and growth
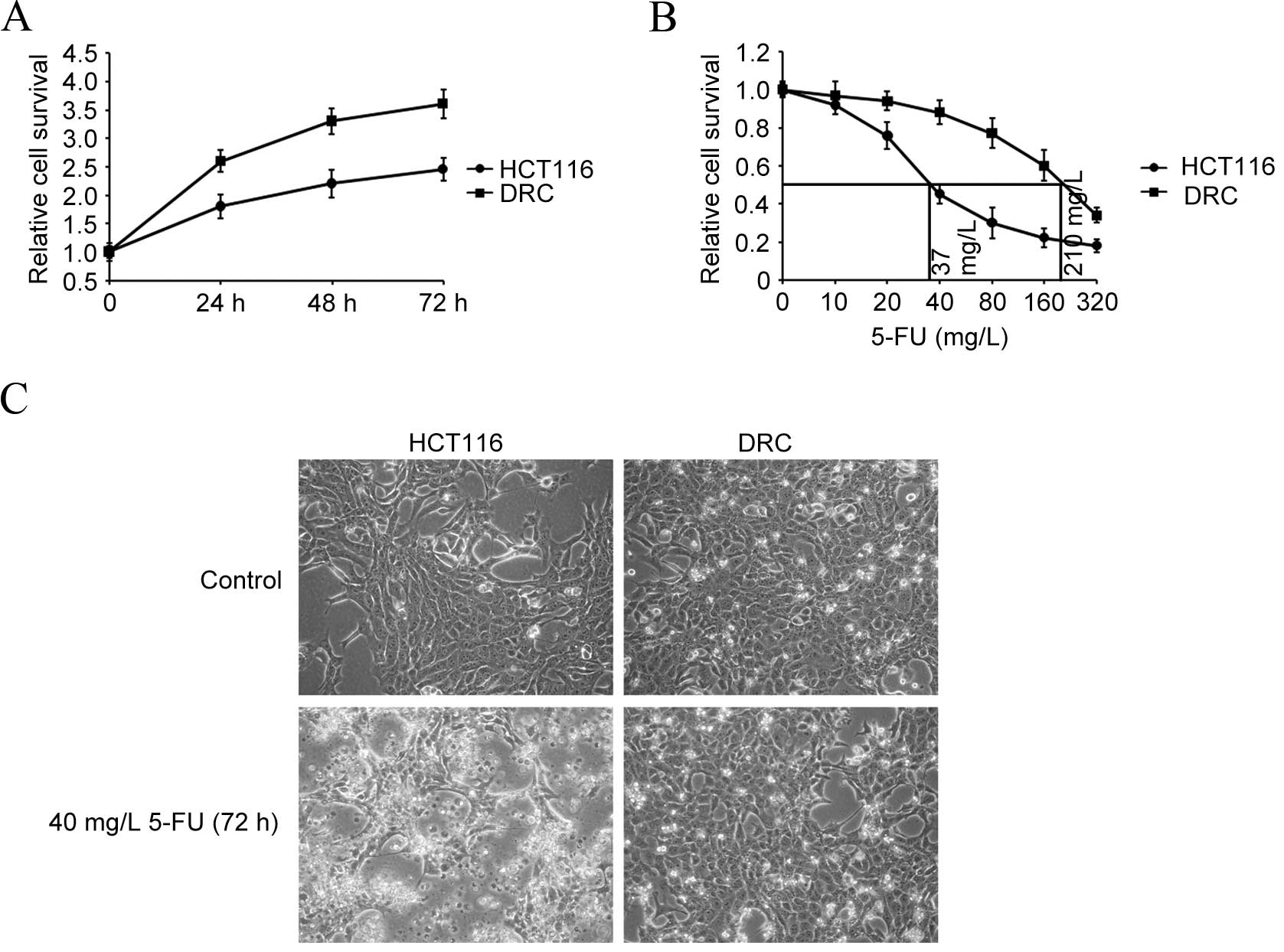
curves of DRC and HCT116 cells were drawn. DRC grew faster compared
withHCT116 cells. As shown in Fig.
1A, the doubling time for the two cell lines was respectively
calculated to be 24 and 36 h. Additionally, the concentration
required for 50% inhibition (IC50) of 5-FU was
determined by exposing DRC and HCT116 cells to different
concentrations of 5-FU for 72 h. The IC50 value of DRC
was calculated to be 210 mg/l, while that of HCT116 cells was 37
mg/l, at 72 h (Fig. 1B).
Additionally, cell survival capabilities were compared by treating
the two cell lines with 40 mg/l of 5-FU for 72 h and visualizing
their morphology. As expected, HCT116 cells were rounded off and
displayed membrane blebbing, which is an apoptotic feature
(27). However, no obvious changes in
DRC morphology were observed (Fig.
1C).
Expression of Cav-1 in DRC and HCT116
cells
To reveal whether the drug resistance-associated
protein Cav-1 is involved in the development of resistance to 5-FU
in DRC, the expression of Cav-1 was detected. RT-qPCR demonstrated
that the mRNA expression level of Cav-1 in DRC was significantly
higher than that in HCT116 cells (P=0.006) (Fig. 2A). In addition, western blot analysis
indicated that Cav-1 protein expression was also increased in DRC
compared with that in HCT116 cells (Fig.
2B), suggesting that Cav-1 may serves an important role in the
5-FU resistance development in colorectal cancer HCT116 cells.
Inhibition of Cav-1 resensitizes
resistant cells to 5-FU
To investigate the role of Cav-1 in the development
of 5-FU resistance, a molecular inhibitor and siRNA for Cav-1 were
used to inhibit the function of Cav-1. MCD, a potent inhibitor of
Cav-1, suppressed the growth of DRC and HCT116 cells in a
dose-dependent manner (Fig. 3A).
Additionally, there was no significant inhibitory effect on the
survival of 1 mM MCD-treated cells at 72 h. Combination treatment
of MCD and 5-FU markedly decreased cell growth of DRC compared that
caused by 5-FU treatment alone, while it did not significantly
affect that of HCT116 cells compared with 5-FU treatment alone
(Fig. 3B). To verify the function of
Cav-1, Cav-1 in cells was silenced by siRNA. Western blotting
demonstrated that Cav-1 expression in DRC was inhibited by 5-fold
(Fig. 3C). Control siRNA- and Cav-1
siRNA-treated cells were exposed to 5-FU for 72 h, and cell
survival was assessed. MTT assay indicated that cell survival in
Cav-1 siRNA-transfected DRC was repressed by 5-FU treatment
relative to that in control siRNA-transfected DRC treated with 5-FU
alone (P=0.008) (Fig. 3D). However,
under identical experimental conditions, the viability of HCT116
cells was unaffected. These data suggested that downregulation of
Cav-1 in DRC enhanced their sensitivity to 5-FU.
 | Figure 3.Inhibition of Cav-1 resensitizes DRC
to 5-FU. (A) Cells were exposed to medium containing MCD for 4 h.
The medium was then removed, and cells were incubated with fresh
medium for additional 72 h. Cell survival was examined by MTT
assay. (B) Cells were treated with 1 mM MCD for 4 h, and then
incubated in fresh medium with or without 40 mg/l of 5-FU for 72 h.
Subsequently, MTT assay was performed. (C) DRC were treated with
control or Cav-1 siRNA. Following 36 h, Cav-1 expression was
assessed by western blot analysis. (D) DRC and HCT116 cells were
transfected with control or Cav-1 siRNA. After 36 h, fresh medium
with or without 5-FU was added for additional 72-h incubation. Cell
survival was analyzed using MTT assay. **P<0.01 vs. 5-FU
treatment alone. Cav-1, caveolin-1; DRC, drug-resistant cells;
5-FU, 5-fluorouracil; MCD, methyl β-cyclodextrin; si/siRNA small
interfering RNA; N.S., no significance; Ctrl, control. |
Discussion
Colorectal cancer is one of the most common causes
of cancer-associated mortality (28).
While non-invasive colorectal cancer may be curable with surgery,
for invasive and metastatic cancer, surgery is insufficient for
final treatment (29). Chemotherapy
is the alternative therapy strategy (30). However, the development of resistance
during treatment limits the effectiveness of chemotherapy, as tumor
cells may not only obtain resistance to the drug originally used,
but may also exhibit cross-resistance to other drugs, which may be
triggered possibly by multiple factors with different mechanisms
(31–33). Thus, exploring the mechanism of
chemoresistance is important to improve cancer treatment.
In the present study, a DRC model was established by
varying the concentration of 5-FU treatment that mimicked the
phenotype of resistance development in vivo. The survival
and growth of DRC and parental HCT116 cells were compared to
determine the resistance phenotype. The growth of DRC was increased
and was inhibited by 5-FU in a slower manner compared with that of
HCT116 cells. It has been reported that elevated expression of
Cav-1 is associated with the development of resistance in
hepatocellular cancer cells to paclitaxel (27). Additionally, Cav-1 participates in
cell survival, tumor progression, metastasis and poor prognosis
(19–22). Cav-1 has been found to be correlated
with colon cancer growth, metastasis and tumorigenicity (34,35). The
present study demonstrated that the expression of Cav-1 was
increased in DRC relative to that in HCT116 cells, suggesting that
it was involved in drug resistance. In addition, whether
cross-resistance of DRC against other therapeutic drugs is
generated remains to be explored, which may be valuable for the
treatment of cancer.
To verify the hypothesis, a specific inhibitor and
siRNA for Cav-1 were used to inhibit the function of Cav-1, which
accelerated cell death and resensitized DRC to 5-FU. Taken
together, Cav-1 is an important regulator in the development of
drug resistance to 5-FU in colorectal cancer HCT116 cells. The
present data indicate that chemotherapeutic agents combined with
pharmacological inhibitors or siRNAs targeting
resistance-associated proteins such as Cav-1 may exhibit increased
therapeutic effects for colorectal cancer.
Acknowledgements
The present study was supported by the Science and
Technology Bureau of Hangzhou City (Hangzhou, China; grant nos.
20130733Q38 and 20130733Q45).
References
|
1
|
Defining Cancer, . National Cancer
Institute. https://www.cancer.gov/about-cancer/understanding/what-is-cancerAccessed
June 10, 2014.
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu J, Qiu T, Pan P, Yu D, Ju Z, Qu X, Gao
X, Mao C and Wang L: Detection of serum anti-p53 antibodies from
patients with colorectal cancer in china using a combination of
p53- and phage-ELISA. Asian Pac J Cancer Prev. 12:2921–2924.
2011.PubMed/NCBI
|
|
4
|
Peetla C, Bhave R, Vijayaraghavalu S,
Stine A, Kooijman E and Labhasetwar V: Drug resistance in breast
cancer cells: Biophysical characterization of and doxorubicin
interactions with membrane lipids. Mol Pharm. 7:2334–2348. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seddon AM, Casey D, Law RV, Gee A, Templer
RH and Ces O: Drug interactions with lipid membranes. Chem Soc ReV.
38:2509–2519. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Righetti SC, Perego P, Carenini N, Corna
E, Dal Bo L, Cedrola S, La Porta CA and Zunino F: Molecular
alterations of cells resistant to platinum drugs: Role of PKCalpha.
Biochim Biophys Acta. 1763:93–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai Z, Huang Y and Sadée W: Growth factor
signaling and resistance to cancer chemotherapy. Curr Top Med Chem.
4:1347–1356. 2004.PubMed/NCBI
|
|
8
|
Rossi S: Australian Medicines Handbook
(2013 edition). The Australian Medicines Handbook Unit Trust;
Adelaide: 2013
|
|
9
|
Tolba MF and Abdel-Rahman SZ:
Pterostilbine, an active component of blueberries, sensitizes colon
cancer cells to 5-fluorouracil cytotoxicity. Sci Rep. 5:152392015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohtsu A: Chemotherapy for metastatic
gastric cancer: Past, present, and future. J Gastroenterol.
43:256–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Williams TM and Lisanti MP: The caveolin
proteins. Genome Biol. 5:2142004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang Z, Scherer PE, Okamoto T, Song K, Chu
C, Kohtz DS, Nishimoto I, Lodish HF and Lisanti MP: Molecular
cloning of caveolin-3, a novel member of the caveolin gene family
expressed predominantly in muscle. J Biol Chem. 271:2255–2261.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scherer PE, Okamoto T, Chun M, Nishimoto
I, Lodish HF and Lisanti MP: Identification, sequence, and
expression of caveolin-2 defines a caveolin gene family. Proc Natl
Acad Sci USA. 93:131–135. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hill MM, Bastiani M, Luetterforst R,
Kirkham M, KirKham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM,
Martin S, et al: PTRF-Cavin, a conserved cytoplasmic protein
required for caveola formation and function. Cell. 132:113–124.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hansen CG and Nichols BJ: Exploring the
caves: Cavins, caveolins and caveolae. Trends Cell Biol.
20:177–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Briand N, Dugail I and Le Lay S: Cavin
proteins: New players in the caveolae field. Biochimie. 93:71–77.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gillet JP and Gottesman MM: Mechanisms of
multidrug resistance in cancer. Methods Mol Biol. 596:47–76. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Couet J and Lisanti MP: Src tyrosine
kinases, Galpha subunits, and H-Ras share a common
membrane-anchored scaffolding protein, caveolin. Caveolin binding
negatively regulates the auto-activation of Src tyrosine kinases. J
BiolChem. 271:29182–29013. 1996.
|
|
19
|
Selleri S, Arnaboldi F, Palazzo M, Hussein
U, Balsari A and Rumio C: Caveolin-1 is expressed on multipotent
cells of hair follicles and might be involved in their resistance
to chemotherapy. Br J Dermatol. 153:506–513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shajahan AN, Wang A, Decker M, Minshall
RD, Liu MC and Clarke R: Caveolin-1 tyrosine phosphorylation
enhances paclitaxel-mediated cytotoxicity. J BiolChem.
282:5934–5943. 2007.
|
|
21
|
Tse EY, Ko FC, Tung EK, Chan LK, Lee TK,
Ngan ES, Man K, Wong AS, Ng IO and Yam JW: Caveolin-1
overexpression is associated with hepatocellular carcinoma
tumourigenesis and metastasis. J Pathol. 226:645–653. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang Y, Zeng X, He F, Liao Y, Qian N and
Toi M: Caveolin-1 is related to invasion, survival, and poor
prognosis in hepatocellular cancer. Med Oncol. 29:977–984. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Selleri S, Arnaboldi F, Palazzo M, Hussein
U, Balsari A and Rumio C: Caveolin-1 is expressed on multipotent
cells of hair follicles and might be involved in their resistance
to chemotherapy. Br J Dermatol. 153:506–513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shajahan AN, Wang A, Decker M, Minshall
RD, Liu MC and Clarke R: Caveolin-1 tyrosine phosphorylation
enhances paclitaxel-mediated cytotoxicity. J BiolChem.
282:5934–5943. 2007.
|
|
25
|
Thomas S, Overdevest JB, Nitz MD, Williams
PD, Owens CR, Sanchez-Carbayo M, Frierson HF, Schwartz MA and
Theodorescu D: Src and Caveolin-1 reciprocally regulate metastasis
via a common downstream signaling pathways in bladder cancer.
Cancer Res. 71:832–841. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meena AS, Sharma A, Kumari R, Mohammad N,
Singh SV and Bhat MK: Inherent and acquired resistance to
paclitaxel in hepatocellular carcinoma: Molecular events involved.
PLoS One. 8:e615242013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
American Cancer Society, . Cancer Facts
& Figures 2014. American Cancer Society; Atlanta, GA: 2014
|
|
29
|
Colon Cancer Treatment
(PDQ®)–Patient Version. National Cancer Institute;
https://www.cancer.gov/types/colorectal/patient/colon-treatment-pdq#section/allAccessed
June 29, 2014.
|
|
30
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Germann UA and Chambers TC: Molecular
analysis of the multidrug transporter, P-glycoprotein.
Cytotechnology. 27:31–60. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu Y, Zhi F, Xu G, Tang X, Lu S, Wu J and
Hu Y: Overcoming multidrug-resistance in vitro and in vivo using
the novel P-glycoprotein inhibitor 1416. Biosci Rep. 32:559–566.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nimri L, Barak H, Graeve L and Schwartz B:
Restoration of caveolin-1 expression suppresses growth,
membrane-type-4 metalloproteinase expression and
metastasis-associated activities in colon cancer cells. Mol
Carcinog. 52:859–870. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bender FC, Reymond MA, Bron C and Quest
AF: Caveolin-1 levels are down-regulated in human colon tumors, and
ectopic expression of caveolin-1 in colon carcinoma cell lines
reduces cell tumorigenicity. Cancer Res. 60:5870–5888.
2000.PubMed/NCBI
|