Introduction
Chest pain is a common clinical symptom resulting
from various conditions, including trauma, acute coronary syndrome,
malignant tumors and aortic dissection (1–4).
Therefore, misdiagnosis may easily occur and a pulmonary embolism
developing as a result of an asymptomatic malignant tumor may go
undetected. The current study describes the case of a 43-year-old
male, who was referred to the Department of Cardiology, China-Japan
Union Hospital of Jilin University (Changchun, China) presenting
with sudden ‘squeezing’ pain in the chest and dyspnea. The symptoms
were suspected to be associated with a possible pulmonary embolism.
The patient was later diagnosed with multiple pulmonary emboli
occurring as a result of renal cell carcinoma (RCC).
Almost 58,000 new cases of RCC were expected in the
USA in 2009, and the morbidity rate is increasing each year. As the
increase in the number of diagnoses is not solely due to early
detection, the mortality rate of RCC is high and exhibits no
decreasing trend. Currently, RCC is the 7th most common cancer in
men and the 8th most common cancer in women in the USA (5). The chest pain due to pulmonary emboli
may be an initial symptom of RCC.
Case report
In December 2012, a 43-year-old male presented to
the Department of Cardiology, China-Japan Union Hospital of Jilin
University, complaining of sudden ‘squeezing’ pain in the chest and
dyspnea. The medical history of the patient was unremarkable with
no history of trauma, no family history of renal tumors and no
symptoms of hematuria or abdominal pain. The patient had a smoking
history of 25 years with an average of 140 cigarettes per week.
Physical examination reported a blood pressure reading of 100/80
mmHg (nomal range, 139–90/99–60 mmHg) and a heart rate of 88
beats/min (normal range, 60–100 beats/min). An electrocardiogram
(ECG) identified oblique type ST segment elevation in leads V1-V3.
Troponin was elevated to 0.85 ng/ml (normal range, 0–0.04 ng/ml),
and other myocardial biomarker levels were normal.
The initial suspected diagnosis was acute coronary
syndrome, primarily due to the characteristic changes observed in
the ECG and the markedly elevated myocardial biomarker level.
Emergency coronary angiography showed normal coronary arteries. The
possibility of pulmonary embolism or aortic dissection was not
excluded; however, the patient had no history of hypertension and
the current blood pressure was within normal limits, thereby
reducing the likelihood of aortic dissection.
Pulmonary embolism-associated laboratory and
auxiliary examinations were subsequently performed and the results
were as follows: D-dimer, 4,162 ng/ml (normal range, 0–400 ng/ml);
blood gas analysis: pH, 7.42 (normal range, 7.35–7.45);
pCO2, 35 mmHg (normal range, 35–45 mmHg);
pO2, 72 mmHg (normal range, 80–100 mmHg); and base
excess, 2.5 mmol/l (−3.0 to 3.0 mmol/l). Pulmonary
ventilation/perfusion scintigraphy identified multiple pulmonary
emboli in each lung (Fig. 1).
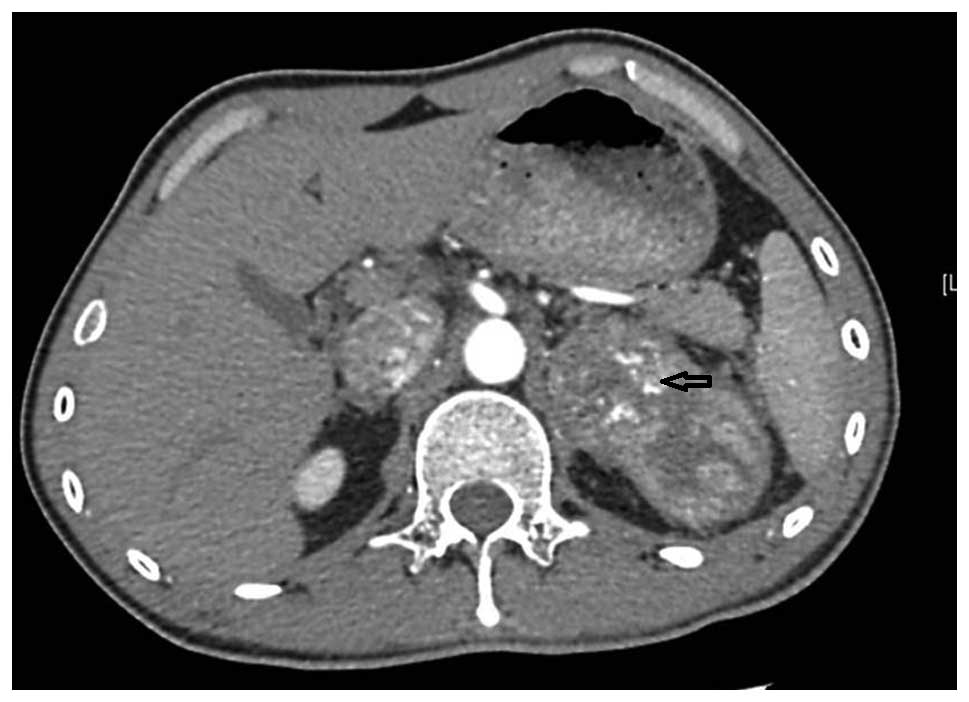
Furthermore, computed tomography angiography (CTA) revealed
pulmonary emboli over each lung field and pulmonary emphysema of
the left lung (Fig. 2), prompting a
diagnosis of pulmonary embolism.
Generally, 70% of pulmonary emboli occur as a result
of lower limb deep vein thrombosis (6,7). However,
the patient in the present case had normal lower limb
ultrasonography results. Routine abdominal ultrasonography
examination identified a substantial, heterogeneous, hypoechoic
lesion, and subsequent enhanced magnetic resonance imaging (MRI) of
the kidney revealed a massive shadow (3.9×5.1×4.2 cm) in the upper
pole of the left kidney (Fig. 3) with
a lower density, patchy, necrotic area in the center. In addition,
MRI identified that the inferior vena cava and proximal region of
the left kidney vein were significantly dilated with multiple
filling defects within the cavities. The renopuncture tissues were
used for SP staining and immunohistochemical analysis with
monoclonal mouse anti-human Ki-67 antigen (clone MIB-1; Agilent
Technologies Co. Ltd., Beijing, China). The result showed that the
pathological change was consistent with RCC, cytonecrosis and worse
degeneration. There was a Ki-67 index of 20%. When combined with
the other test results, a diagnosis of stage III (T3bN0M0) RCC
(8) was formed. The patient did not
undergo surgery due to the risk of bleeding from large vessels
present near the tumor, and was instead treated with oral warfarin
(1.25 mg; once a day for 1 month) and subcutaneous injections of
low molecular weight heparin (40 mg; twice daily for 7 days), which
significantly alleviated symptoms in comparison with
pretreatment.
In addition, sunitinib targeted therapy was
initiated for 1 month, and regular chemotherapy with interleukin-2
was administered, consisting of 600,000 intravenous units per day
for 10 days and 2 million units via aerosol inhalation per day for
10 days every 3 months. At 12 months after the initial treatment,
the patient began to experience left abdominal pain and the maximal
diameter of the left renal mass had increased by 7 cm (12.1 cm in
diameter) on renal CT images. Furthermore, multiple small lymph
nodes were identified in the abdomen (Fig. 4). At this time the patient had poor
general health, and exhibited shortness of breath and chest
congestion during limited physical activity that required frequent
oxygen inhalation. Since surgery could not be performed and
targeted therapy using sunitinib was not desired, the prognosis of
the patient is poor. A follow-up was planned every 6 months.
Written informed consent was obtained from the
patient for the publication of the present study.
Discussion
In the present case, a 43-year-old male presented
with severe chest pain, and a diagnosis of acute coronary syndrome
was initially suspected due to the excruciating chest pain,
ischemic ECG changes and an elevated myocardial biomarker. However,
negative coronary angiogram results excluded coronary artery
abnormalities. With regards to early clinical manifestation and
auxiliary examination results, one-third of patients with an acute
pulmonary embolism demonstrate uncharacteristic features that may
mimic acute coronary syndrome, resulting in a false diagnosis
(6). It is widely known that up to
70% of pulmonary emboli occur as a complication of lower limb deep
vein thrombosis, and ultrasonography examination is the first
choice modality for screening venous thrombi. However, abdominal
ultrasonography can also be useful for screening venous thrombi
(9,10). In the present case, the asymptomatic,
large, renal tumor was clearly identified by abdominal
ultrasonography examination as a space-occupying phenomenon in the
left renal area.
The early symptoms of RCC are uncharacteristic and
vary among individuals, which often leads to false and/or
misdiagnosis (10). Only 10% of
patients with RCC present with the three characteristic clinical
symptoms observed during early stages, including hematuria,
abdominal pain and an abdominal mass, whereas >40% of patients
do not present with these symptoms (10). In the current case, the patient lacked
the typical symptoms of RCC and primarily presented with symptoms
of pulmonary embolism.
Cancer is one of the leading causes of pulmonary
embolism, however, asymptomatic malignant tumors are not always
found in the clinic (11). Previous
studies have demonstrated that 10% of patients with idiopathic or
unexplained pulmonary emboli were subsequently diagnosed with
malignant tumors at 5–10 year follow-ups, indicating the
requirement for greater attention to secondary pulmonary emboli
caused by asymptomatic tumors (11–13).
Thromboembolism may occur in patients with cancer as
a result of hypercoagulable states and is closely associated with
degree of malignancy, tumor location, pathological type and tumor
staging (14). In the present case,
the renal tumor was solid, highly malignant and may have
metastasized to distant organs through the blood stream, which is
an important risk factor for the occurrence of pulmonary embolism
from thrombosis. In addition, the RCC invaded the inferior vena
cava and the proximal region of the left kidney vein, resulting in
vascular injuries, inflammatory response and vascular stenosis,
thereby promoting thrombogenesis.
The pulmonary ventilation/perfusion scintigraphy and
pulmonary CTA showed highly consistent results when compared with
the elevated D-dimer results, but the pulmonary
ventilation/perfusion scintigraphy was more meaningful for the
diagnosis of pulmonary embolism below subsegmental levels. In
comparison with ventilation/perfusion scintigraphy, pulmonary CTA
has a higher diagnostic sensitivity and specificity, and a higher
accuracy in identifying the attached embolismic sites (3). A total of 92–96% of positive diagnostic
results have been diagnosed following CTA in patients with an
intermediate or highly suspected pulmonary embolism (3).
In the current case, dyspnea and markedly restricted
physical activities reoccurred at the 1 year follow-up, indicating
recurrence of the pulmonary embolism, which most likely resulted
from insufficient anticoagulation intensity and duration. However,
the possibility of cancer-associated emboli could not be excluded.
Progressive targeted therapies for the treatment of RCC and other
comprehensive supporting strategies should be administered in
combination with long-term anticoagulants for concurrent pulmonary
embolism. Such therapy aims to improve the patient prognosis and
enhance the quality of life.
In conclusion, RCC is a common malignant tumor that
involves the urinary system and is associated with a high mortality
rate. Given that only 10% of patients present with the three
characteristic clinical symptoms, it is difficult to accurately
diagnose the disease in the early stages. Therefore, clinicians
should pay close attention to patients presenting with
uncharacteristic symptoms and strengthen the early screening
process for RCC based on ultrasonography or CT examinations.
Acknowledgements
This study was supported by a grant from the
National Science Foundation entitled ‘function and mechanism of the
activin A/FS system imbalance in the myocardial cell apoptosis of
heart failure’ (grant no. 81270315).
References
|
1
|
Hamm CW, Bassand JP, Agewall S, Bax J,
Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, et al: ESC
Committee for Practice Guidelines: ESC Guidelines for the
management of acute coronary syndromes in patients presenting
without persistent ST-segment elevation: The Task Force for the
management of acute coronary syndromes (ACS) in patients presenting
without persistent ST-segment elevation of the European Society of
Cardiology (ESC). Eur Heart J. 32:2999–3054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Task Force on the management of ST-segment
elevation acute myocardial infarction of the European Society of
Cardiology (ESC), ; Steg PG, James SK, Atar D, Badano LP,
Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq
G, Fernandez-Aviles F, et al: ESC Guidelines for the management of
acute myocardial infarction in patients presenting with ST-segment
elevation. Eur Heart J. 33:2569–2619. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torbicki A, Perrier A, Konstantinides S,
Agnelli G, Galiè N, Pruszczyk P, Bengel F, Brady AJ, Ferreira D,
Janssens U, et al: ESC Committee for Practice Guidelines (CPG):
Guidelines on the diagnosis and management of acute pulmonary
embolism: The task force for the diagnosis and management of acute
pulmonary embolism of the European society of cardiology (ESC). Eur
Heart J. 29:2276–2315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nienaber CA and Powell JT: Management of
acute aortic syndromes. Eur Heart J. 33:26–35b. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amerian Cancer Society, . Cancer Facts and
Figures 2009. American Cancer Society; Atlanta, USA: 2009
|
|
6
|
Kukla P, Długopolski R, Krupa E, Furtak R,
Mirek-Bryniarska E, Szełemej R, Jastrzębski M, Nowak J, Kulak L,
Hybel J, et al: How often pulmonary embolism mimics acute coronary
syndrome? Kardiol Pol. 69:235–240. 2011.PubMed/NCBI
|
|
7
|
Dalen JE: Pulmonary embolism: What have we
learned since Virchow? Natural history, pathophysiology, and
diagnosis. Chest. 122:1440–1456. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Decastro GJ and McKiernan JM:
Epidemiology, clinical staging, and presentation of renal cell
carcinoma. Urol Clin North Am. 35:581–592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kearon C: Natural history of venous
thromboembolism. Circulation. 107:(23 Suppl 1). I22–I30. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gibbons RP, Monte JE, Correa RJ Jr and
Mason JT: Manifestations of renal cell carcinoma. Urology.
8:201–206. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Monreal M, Fernandez-Llamazares J,
Perandreu J, Urrutia A, Sahuquillo JC and Contel E: Occult cancer
in patients with venous thromboembolism: Which patients, which
cancers. Thromb Haemost. 78:1316–1318. 1997.PubMed/NCBI
|
|
12
|
Hettiarachchi RJ, Lok J, Prins MH, Büller
HR and Prandoni P: Undiagnosed malignancy in patients with deep
vein thrombosis: Incidence, risk indicators, and diagnosis. Cancer.
83:180–185. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schulman S and Lindmarker P: Incidence of
cancer after prophylaxis with warfarin against recurrent venous
thromboembolism. Duration of Anticoagulation Trial. N Engl J Med.
342:1953–1958. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Connolly GC and Francis CW:
Cancer-associated thrombosis. Hematology Am Soc Hematol Educ
Program. 2013:684–691. 2013.PubMed/NCBI
|