Introduction
Lymphangioma is an uncommon malformation of the
lymphatic system (1). The majority of
lymphangiomas usually occur in the head, neck regions and axillary
areas, but rarely in the colon (2–4). Colonic
lymphangioma used to be considered an extremely rare disease, but
recently, along with the increasing prevalence of endoscopy and
endoscopic ultrasound (EUS), it has become more commonly
encountered and has been reported more frequently (5,6). There are
several reports on the use of EUS for the diagnosis of colonic
lymphangioma (4,6). The present study reports a case of
colonic lymphangiomatosis manifested as recurrent bowel bleeding,
which was diagnosed by EUS and treated with laparoscopic segmental
sigmoid colon resection. In addition, the relevant medical
literature on colonic lymphangiomatosis is reviewed.
Case report
A 79-year-old Chinese man, who had no personal or
familial history of any specific disease, presented to the People's
Hospital of Hu County (Xi'an, China) in 2012 with intermittent
attacks of bowel bleeding and abdominal discomforts for 3 months.
Physical examination and complete blood cell count were
unremarkable, with the exception of anemia. Fecal occult blood test
was positive. The biochemical tests were all normal, and the levels
of carcinoembryonic antigen, carbohydrate antigen (CA)125 (11.41
U/ml; normal range, 0.00–35.00 U/ml) and CA19-9 (14.34 U/ml; normal
range, 0.00–39.00 U/ml) were within the normal limits. Upper
abdominal ultrasound and chest X-ray displayed no specific
findings. Colonoscopy revealed multiple cystic masses with a
translucent and smooth surface, ranging from 4 to 8 mm in diameter,
located in the sigmoid colon. The color and surface characteristics
of the lesions had no difference with those of the surrounding
normal mucosa, and no ulcerations or erosions were present
(Fig. 1). By EUS, these cystic masses
were confirmed to be echo-free and to exhibit septal walls in the
submucosal layer (Fig. 2).
Based on the colonoscopy and EUS findings, the
lesions were diagnosed as lymphangiomatosis of the sigmoid colon.
Considering the repeated bleeding, laparoscopy-assisted partial
sigmoid colon resection was performed. Surgical findings were
multiple bulges on the serosal surface of the sigmoid colon by
laparoscopy. The excised specimens were multiple masses of ~1 cm in
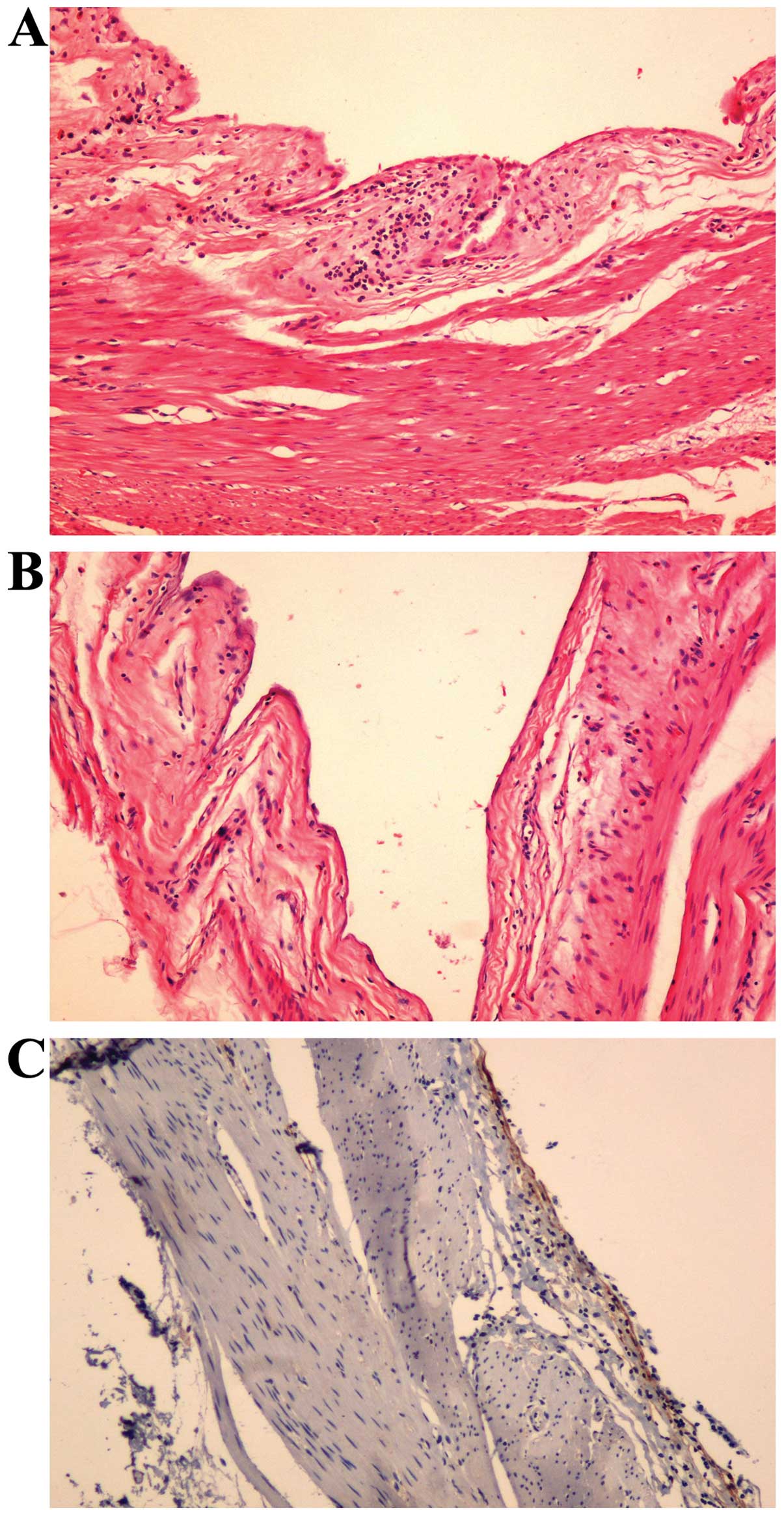
diameter, which were vesicular and soft. Histological examination
revealed that the cysts were located in the submucosal layer and
were surrounded by flat endothelial cells. Immunohistochemistry was
positive for D2-40 (ready-to-use anti-D2-40; MAB-0567; MXB, Fuzhou,
China), a specific lymphatic endothelial marker (Fig. 3). The pathological diagnosis was
submucosal cystic lymphangioma of the sigmoid colon. In the 2-year
follow-up after the operation, no bleeding or other complications
were noticed.
Discussion
Lymphangiomas are usually detected in the head, neck
and axillary areas, which account for 50–75% of all cases, while
only 5% of cases occur in the abdomen (2–4).
Intra-abdominal lymphangiomas frequently locate in the mesentery,
greater omentum and retroperitoneum, but rarely in the colon
(7). In 1932, Chisholm and Hillkowitz
reported the first case of rectal lymphangioma (8). For a long time, colonic lymphangioma has
been regarded as an exceptionally rare illness (5).
With the widespread use of colonoscopy, an increased
number of colonic lymphangiomas have been detected (5). Although the specific mechanisms
contributing to colonic lymphangioma are unclear, the acknowledged
main cause is a congenital malformation of the lymphatic system,
which results in an abnormal dilatation and proliferation of the
lymphatic channel, leading to the formation of cystic masses
(9,10). The reasons leading to secondary
colonic lymphangioma include abdominal trauma, partial lymphatic
obstruction and inflammation (11–13).
According to Matsuda et al (5), the age distribution of the patients with
colonic lymphangioma is 1–83 years, and the incidence is higher in
males, with a gender ratio of 2–2.5:1 in Japan. Regarding site, the
majority of colonic lymphangioma cases occur in the transverse
colon (5). In the present case, the
patient was a 79-year-old male, and the lesions were discovered in
the sigmoid colon.
The clinical signs and symptoms of colonic
lymphangioma vary depending on the size and position of the lesion,
and include abdominal discomfort such as abdominal pain and
abdominal distension, colonic bleeding, acute abdomen and
protein-losing enteropathy (5,14,15). The current patient presented with
intermittent attacks of colonic bleeding. The diagnosis of colonic
lymphangioma depends on X-ray and endoscopic examination, and EUS
provides a novel and powerful method for the diagnosis of colonic
lymphangioma (5). EUS aids to display
the entire hierarchy of the colon wall and the association between
the lesion and the colon wall; in addition, it aids to ascertain
whether the lesion is separated, and to determine the nature of the
lesion according to the echo characteristics (16). The majority of colonic lymphangiomas
are isoechoic or hypoechoic, and pathological examination is the
gold standard for diagnosis (5,16,17). The lesions detected in colonic
lymphangioma cases result from malformations of the lymphatic
tissue, causing an abnormal dilatation and mass-like proliferation
of lymphatic channels, which enables to observe the submucosal
lymphatic cysts and fiber-like separation by hematoxylin and eosin
staining (16,17). A history of recurrent bowel bleeding
and anemia is an important clue that should alert clinicians about
the possibility of colonic lymphangioma.
Regarding therapy, since colonic lymphangioma is
benign, ~10% of cases ease naturally (2,18), Lee
et al (19) recommended
regular follow-ups rather than treatment for those asymptomatic
colonic lymphangiomas. Positive treatments include surgery,
endoscopic resection and drug injection. Complete surgical
resection is the first choice for large lymphangiomas accompanied
with complications such as bowel obstruction, bleeding, volvulus or
intussusception (5). With the
development of endoscopy, endoscopic resection is preferred for
pedunculated and semipedunculated lesions of <2 cm (20). In the present case report, the patient
exhibited recurrent bowel bleeding, and his lesions measured ≤10
cm; thus, laparoscopy-assisted surgical resection was conducted.
During 2 years of follow-up after the operation, no bleeding or
other complications were noticed.
Colonic lymphangioma is a rare disease. The present
study reports a case of colonic lymphangioma that presented with
recurrent bowel bleeding and anemia, which revealed that
lymphangiomas may be a cause of gastrointestinal bleeding.
References
|
1
|
Watanabe T, Kato K, Sugitani M, Hasunuma
O, Sawada T, Hoshino N, Kaneda N, Kawamura F, Arakawa Y and Hirota
T: A case of multiple lymphangiomas of the colon suggesting colonic
lymphangiomatosis. Gastrointest Endosc. 52:781–784. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alqahtani A, Nguyen L, Flageole H, Shaw K
and Laberge JM: 25 years' experience with lymphangiomas in
children. J Pediatr Surg. 34:1164–1168. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung JH, Suh YL, Park IA, Jang JJ, Chi
JG, Kim YI and Kim WH: A pathologic study of abdominal
lymphangiomas. J Korean Med Sci. 14:257–262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhuo CH, Shi DB, Ying MG, Cheng YF, Wang
YW, Zhang WM, Cai SJ and Li XX: Laparoscopic segmental colectomy
for colonic lymphangiomas: A definitive, minimally invasive
surgical option. World J Gastroenterol. 20:8745–8750. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsuda T, Matsutani T, Tsuchiya Y,
Okihama Y, Egami K, Yoshioka M, Maeda S and Onda M: A clinical
evaluation of lymphangioma of the large intestine: A case
presentation of lymphangioma of the descending colon and a review
of 279 Japanese cases. J Nippon Med Sch. 68:262–265. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung SW, Cha JM, Lee JI, Joo KR, Choe JW,
Shin HP and Kim KY: A case report with lymphangiomatosis of the
colon. J Korean Med Sci. 25:155–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roisman I, Manny J, Fields S and Shiloni
E: Intra-abdominal lymphangioma. Br J Surg. 76:485–489. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chisholm AJ and Hillkowitz P: Lymphangioma
of the rectum. The American Journal of Surgery. 17:281–282. 1932.
View Article : Google Scholar
|
|
9
|
de Perrot M, Rostan O, Morel P and Le
Coultre C: Abdominal lymphangioma in adults and children. Br J
Surg. 85:395–397. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weeda VB, Booij KA and Aronson DC:
Mesenteric cystic lymphangioma: A congenital and an acquired
anomaly? Two cases and a review of the literature. J Pediatr Surg.
43:1206–1208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JH, Ryu WS, Min BW, Song TJ, Son GS,
Kim SJ, Kim YS and Um JW: Acquired omental cystic lymphangioma
after subtotal gastrectomy: A case report. J Korean Med Sci.
24:1212–1215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tezuka K, Ogawa Y, Satake K, Ohira M,
Yamada S, Uno H, Wakasa K and Hirakawa K: Lymphangioma of the
lesser omentum associated with abdominal esophageal carcinoma:
Report of a case. Surg Today. 32:362–366. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fisher D and Hiller N: Case report: Giant
tuberculous cystic lymphangioma of posterior mediastinum,
retroperitoneum and groin. Clin Radiol. 49:215–216. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuba Y, Mizuiri H, Murata T and Niimi
K: Adult intussusception due to lymphangioma of the colon. J
Gastroenterol. 38:181–185. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim J, Han D, Hong CH, Lee HL, Kim JP,
Sohn JH and Hahm JS: Colonic lymphangiomatosis associated with
protein-losing enteropathy. Dig Dis Sci. 50:1747–1753. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Black T, Guy CD and Burbridge RA:
Retroperitoneal cystic lymphangioma diagnosed by endoscopic
ultrasound-guided fine needle aspiration. Clin Endosc. 46:595–597.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gottlieb K and Elkharwily A: Endoscopic
ultrasound evaluation of a cystic lymphangioma of the colon. J
Ultrasound Med. 26:1803–1804. 2007.PubMed/NCBI
|
|
18
|
Steyaert H, Guitard J, Moscovici J,
Juricic M, Vaysse P and Juskiewenski S: Abdominal cystic
lymphangioma in children: Benign lesions that can have a
proliferative course. J Pediatr Surg. 31:677–680. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JM, Chung WC, Lee KM, Paik CN, Kim YJ,
Lee BI, Cho YS and Choi HJ: Spontaneous resolution of multiple
lymphangiomas of the colon: A case report. World J Gastroenterol.
17:1515–1518. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato K, Maekawa T, Yabuki K, Tomita N,
Eguchi M, Matsumoto M and Sugiyama N: Cystic lymphangiomas of the
colon. J Gastroenterol. 34:520–524. 1999. View Article : Google Scholar : PubMed/NCBI
|