Introduction
Renal cell carcinoma (RCC) is one of the most lethal
types of cancer within the urinary system, and up to one-third of
patients with RCC already present with primary metastases at the
time of diagnosis (1). RCC is
categorized into four major subtypes according to the Heidelberg
classification system (2): i) Clear
cell RCC (CC-RCC); ii) papillary RCC: iii) chromophobe RCC; and iv)
renal oncocytoma. CC-RCC is the most prevalent subtype of RCC,
accounting for ~80% of cases (3).
Specific molecular mechanisms have been identified in RCC
tumorigenesis, including von Hipple-Lindau gene mutation (4). Somatic mutation of this gene has been
associated with the development of ~60% of sporadic CC-RCC
(5). However, no current theory is
able to explain all cases of CC-RCC development. To understand the
pathogenesis of CC-RCC in detail, further research is
warranted.
A number of epidemiological studies have connected
the occurrence of renal cancer to obesity (6–8). A
specific association between obesity and RCC has been established,
however, the mechanism by which obesity increases the risk of
cancer remains unclear (9). Recently,
certain obesity-associated biomarkers have been identified and are
considered as a possible link between the two features (10). Among these biomarkers, the adipokines
are of particular note. Adipokines, including adiponectin, leptin,
resistin, visfatin and apelin, are peptide hormones secreted
primarily by adipose tissue and are associated with metabolic
syndrome (11). Studies have
identified that adipokines affect various pro-neoplastic
mechanisms, including inflammation, cell growth and proliferation
(12,13). Studies have also demonstrated that
adipokines are promising predictors of risk and progression in
various types of cancer (14,15). Such evidence suggests that adipokines
contribute to the initiation of carcinogenesis and tumor
progression.
Microarray technology provides a wide range of
information regarding molecules that have been associated with
disease pathogenesis, and subsequently aids the elucidation of the
underlying molecular mechanisms of disease. To gain insights into
the pathogenesis of CC-RCC, several gene expression profiling
studies have been conducted analyzing differences between tumor and
normal tissue (16,17). Such studies have identified a number
of differentially-expressed genes, including the adipokine genes.
In the present study, to investigate the association between
adipokine genes and the molecular mechanisms of RCC, gene
expression profiles of 10 CC-RCC and 10 adjacent normal tissue
controls were downloaded from the Gene Expression Omnibus (GEO)
database, and the relevant adipokine gene data was analyzed. To
verify the results of mRNA microarray, the expression of various
adipokine genes were analyzed in 77 CC-RCC patients using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). The
present study aimed to identify the underlying mechanisms of
obesity that may result in an increased risk of CC-RCC.
Materials and methods
Patients
In the current study, a total of 77 patients were
enrolled, who had been histologically diagnosed with CC-RCC between
December 2005 and September 2012. The patients consisted of 14
females and 63 males, with ages ranging from 17 to 85 years (mean ±
standard deviation, 56.22±12.27 years).
All protocols were approved by the ethics committee
of Wuxi People's Hospital Affiliated to Nanjing Medical University
(Wuxi, China) prior to the initiation of the study, and the
protocols conformed to the ethical guidelines of the 1975 Helsinki
Declaration. Informed consent was obtained from each patient prior
to surgery.
RNA extraction
Tumor tissue samples and corresponding adjacent
normal tissues were obtained from resection surgical specimens, and
were immediately dissociated into single cells using the
Medimachine system (BD Biosciences, Franklin Lakes, NJ, USA). The
cells were then dissolved in 1 ml Trizol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
stored at −80°C. Total cellular RNA was extracted using the Trizol
reagent following the manufacturer's protocols. The quantity and
purity of RNA were determined with the Beckman DU-640
Spectrophotometer (Beckman Coulter, Inc., Brea, CA, USA).
Reverse transcription reaction
Total RNA (2 µg) and 200 ng random primer (Sangon
Biotech Co., Ltd., Shanghai, China) were added to 15 µl
diethylpyrocarbonate (DEPC) treated with H2O; this was
subsequently incubated at 65°C for 5 min and then cooled rapidly on
ice. Following this, 2.0 µl DEPC H2O, 5.0 µl 5X RT
buffer (containing 375 mM/l KCl, 250 mM/l Tris-HCl, 3 mM/l
MgCl2 and 50 mM/l dithiothreitol), 1.25 µl 10 mM/l
deoxynucleoside triphosphate (dNTP; Sangon Biotech Co., Ltd.), 0.75
µl 40 U/µl RNAsin (Sangon Biotech Co., Ltd.) and 1.0µl 200 U/µl
MMLV Reverse Transcriptase (Promega Corporation, Madison, WI, USA)
were added, centrifuged at 1,000 × g for 30 sec at 4°C and
incubated at 37°C for 60 min, and finally stored at −20°C.
Gene expression analysis using
RT-qPCR
RT-qPCR was performed using the
LightCycler® 480 Real-Time PCR system (Roche
Diagnostics, Basel, Switzerland). The primers utilized during PCR
are presented in Table I. PCR was
performed using a final volume of 20 µl, which contained 2 µl 25
mM/l MgCl2, 10 mM/µl sense and antisense primers (1.0 µl
each), 0.4 µl 10 mmol/l dNTP, 1.0 µl EvaGreen® dye
(Biotium Inc., Hayward, CA, USA), 2.0 µl 5X PCR buffer (Promega
Corporation), 2.0 µl complementary DNA, 0.5 units Taq DNA
Polymerase buffer (Promega Corporation) and up to 20 µl
H2O. Following initial denaturation at 94°C for 2 min, a
total of 40 cycles were performed. Each cycle consisted of
denaturation at 94°C for 5 sec, annealing at 58°C for 20 sec and
elongation at 72°C for 20 sec, followed by a single fluorescence
measurement. Melting curve analyses of the amplified products were
performed to confirm the specificity of qPCR assay. Samples were
normalized using the housekeeping gene GAPDH.
 | Table I.Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction analysis of
the adipokine genes and the GAPDH gene. |
Table I.
Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction analysis of
the adipokine genes and the GAPDH gene.
| Gene | Orientation | Primer sequence
(5′-3′) |
|---|
| GAPDH | Forward |
CAACTTTGGTATCGTGGAAGGACTC |
|
| Reverse | AGG
GATGATGTTCTGGAGAGCC |
| Leptin | Forward |
CGTTAAGGGAAGGAACTCTGG |
|
| Reverse |
TGGCTTAGAGGAGTCAGGGA |
| Resistin | Forward |
GTGTGCCGGATTTGGTTAGC |
|
| Reverse |
AGGAGGAGGAGACAGAGAGC |
| Visfatin | Forward |
CTTCGGTTCTGGTGGAGGTT |
|
| Reverse |
ATCGGCCCTTTTTGGACCTT |
| Apelin | Forward |
GCTGACAGTTCGCCCTTACT |
|
| Reverse |
ATATGTGGGCATGGGGACAC |
| Adiponectin | Forward |
ATGGCCCCTGCACTACTCTA |
|
| Reverse |
CAGGGATGAGTTCGGCACTT |
Affymetrix microarray analysis
The gene expression profile of GSE6344 was
downloaded from the National Center for Biotechnology Information
GEO database, which is based on the Affymetrix Human Genome U133A
Array platform (Affymetrix, Inc., Santa Clara, CA, USA). A total of
20 samples, including 10 CC-RCC and 10 adjacent normal tissue
specimens, were available for GSE6344 microarray analysis. The
original mRNA expression profile was standardized using rank value,
and the relative expression levels of adipokine genes were
analyzed.
Statistical analysis
Changes in adipokine genes expression between CC-RCC
and adjacent normal tissues were determined by the comparative ΔΔCq
method (18), using GAPDH as an
internal reference. ΔΔCq = ΔCq (CC-RCC group) - ΔCq (normal group)
for RNA samples. Quantification cycle (Cq) is defined as an index
of the number of cycles required for the fluorescent signal to
cross the threshold. ΔCq represents the difference in Cq values
derived from the target gene (in each sample assayed) and the GAPDH
gene, while ΔΔCq represents the difference between the paired
samples. The n-fold differential ratio was expressed as
2-ΔΔCq. Comparisons between the samples (CC-RCC vs.
adjacent normal tissue) were performed using a one-way analysis of
variance. All statistical data were analyzed using SPSS software
v15.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was used to
indicate a statistically significant difference. As compared with
the adjacent normal tissue, genes in the CC-RCC tissue with
statistically significant differences at P<0.05 and a fold
change of ≥1.5 were considered as upregulated, whereas those with
P<0.05 with a fold change of ≤0.75 were considered as
downregulated. Comparisons between the samples (CC-RCC vs. adjacent
normal tissue) in the GSE6344 dataset were performed using a
non-parametric Mann-Whitney U test.
Results
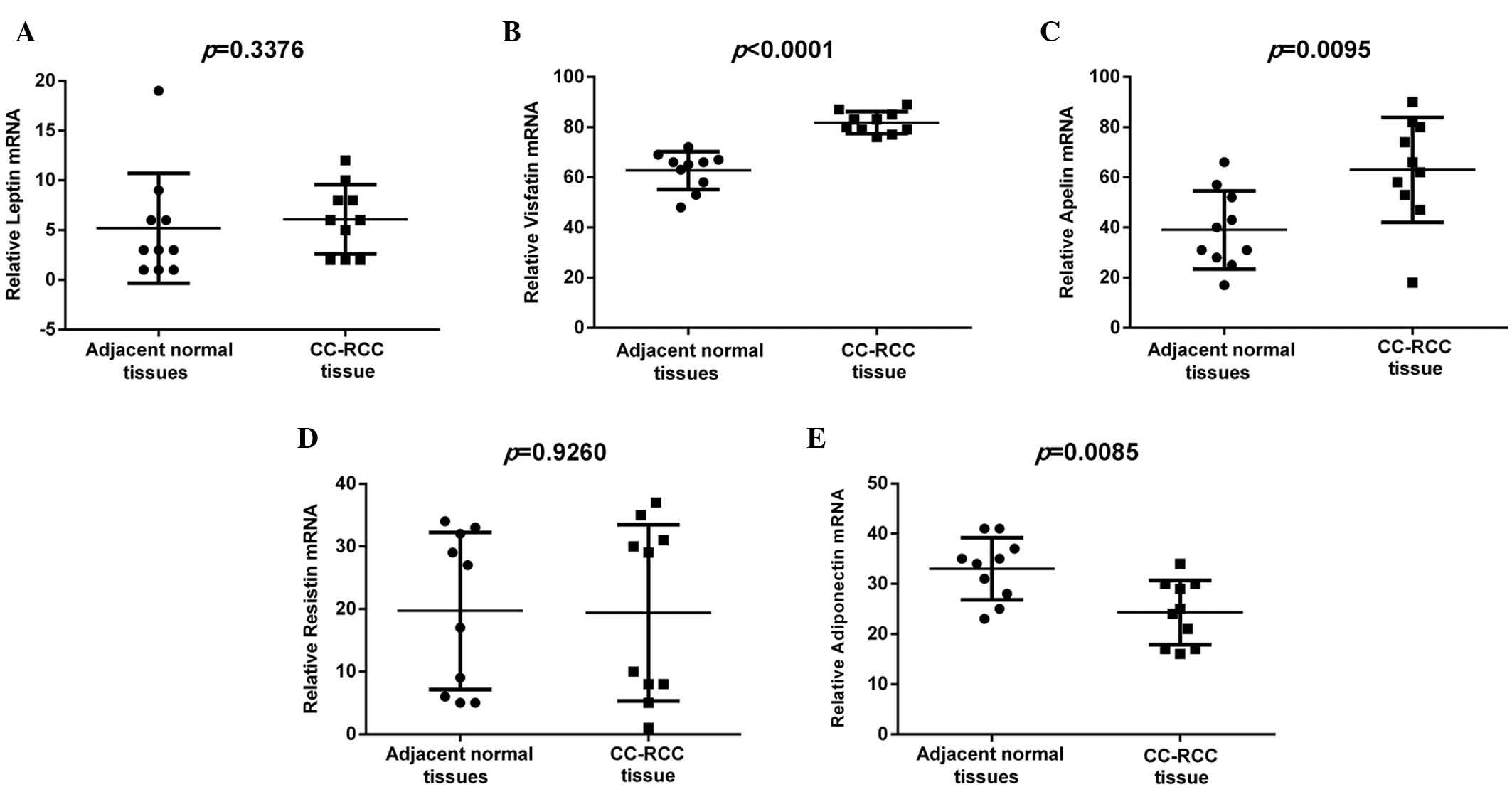
Expression of leptin, visfatin,
apelin, resistin and adiponectin mRNA in CC-RCC with Affymetrix
microarray analysis
Analysis of leptin, visfatin, apelin, resistin and
adiponectin relative expression was performed between the CC-RCC
and adjacent normal tissues from the GSE6344 microarray dataset
(Fig. 1). The GSE6344 dataset
demonstrated that the expression of visfatin and apelin was
upregulated (P<0.0001 and P<0.01, respectively), whilst
adiponectin was downregulated (P<0.001) in the CC-RCC tissues
compared with the adjacent normal tissues, relative to GAPDH.
However, the expression of leptin and resistin exhibited no
significant difference between the CC-RCC and adjacent normal
tissues (P>0.05).
Expression of leptin, visfatin,
apelin, resistin and adiponectin mRNA in CC-RCC and adjacent normal
tissue samples with RT-qPCR
RT-qPCR was used to confirm the results of gene
microarray for the selected adipokine genes. Table II presents the mRNA expression of
leptin, visfatin, apelin, resistin and adiponectin between the
CC-RCC and adjacent normal tissues. The data demonstrated that
visfatin and resistin gene expression was upregulated in the CC-RCC
tissues (P<0.01 and P<0.05, respectively). However, the mRNA
expression level of leptin and adiponectin in adjacent normal
tissue was higher than that in the cancer tissue (P<0.01). The
data also indicated that the expression of apelin was not
significantly different between the CC-RCC and adjacent normal
tissues (P>0.05).
 | Table II.mRNA expression profiles of adipokine
in 77 patients with CC-RCC, acquired through reverse
transcription-quantitative polymerase chain reaction. |
Table II.
mRNA expression profiles of adipokine
in 77 patients with CC-RCC, acquired through reverse
transcription-quantitative polymerase chain reaction.
| Gene | CC-RCC tissue, mean
± SD | Adjacent normal
tissue, mean ± SD |
2−ΔΔCq |
|---|
| Leptina | 30.61±1.62 | 30.63±2.46 | 0.37 |
|
Resistinb | 30.76±2.05 | 32.90±1.31 | 1.62 |
|
Visfatina | 22.73±0.88 | 25.24±1.07 | 2.09 |
| Ampelin | 28.31±1.61 | 30.02±2.02 | 1.20 |
|
Adiponectina | 31.57±1.99 | 31.55±2.64 | 0.37 |
| GAPDH | 20.48±1.18 | 21.92±0.80 |
|
Discussion
Although obesity is recognized as a potent risk
factor for RCC development (19), the
mechanism through which it functions to increase RCC risk is
unclear. Recently, adipokines have been thoroughly investigated as
novel biomarkers that may indicate cancer risk. Adipokines, derived
from adipose tissue, serve roles in lipid and glucose metabolism,
regulation of energy balance and inflammatory processes (20). Adipokines are understood to be
involved in certain obesity-associated forms of cancer, and serve a
key role in tumorigenesis and prognosis (21,22). To
the best of our knowledge, the current study is the first to
identify a difference in visfatin and apelin expression levels in
CC-RCC and control tissues. Furthermore, several studies have
reported a difference in leptin, resistin and adiponectin serum
levels in CC-RCC tissues (23,24).
However, the physiological role of leptin and adiponectin in CC-RCC
remains controversial. Therefore, in the present study, the mRNA
expression variation of visfatin, resistin, apelin, leptin and
adiponectin was investigated in CC-RCC.
Leptin is a multifunctional peptide hormone that
regulates energy expenditure (25),
promotes proliferation (26) and
angiogenesis (27), and inhibits cell
apoptosis (28). Studies have
demonstrated that leptin levels are higher in lung cancer (15), breast cancer (29) and RCC (30), whilst other studies have reported that
serum leptin levels are inversely associated with RCC risk
(23) or are not significantly
associated with RCC (24,31). These contradictory results, and the
serum level features, suggest that further investigation is
required. The microarray analysis from the present study
demonstrated that the expression of leptin exhibited no significant
difference between the CC-RCC and adjacent normal tissues. However,
it was also identified that the mRNA expression level of leptin in
the CC-RCC tissues was significantly lower than that in the
adjacent normal tissues with RT-qPCR.
Visfatin is an adipokine associated with obesity and
glucose regulation. Visfatin was originally identified as a
cytokine and was termed pre-B cell-enhancing factor, also known as
Nampt (32). The adipokine possesses
nicotinamide adenine dinucleotide biosynthetic activity and
regulates mammalian cell growth and apoptosis (33); it is also known to promote novel blood
vessel formation and migration (34).
The function of visfatin in carcinogenesis and as a
chemotherapeutic target has gained the increasing attention of
researchers. Studies have reported that the expression of visfatin
is correlated with various types of cancer, including breast
(35), colorectal (36) and gastric (37) cancer. However, to the best of our
knowledge, the association between visfatin and CC-RCC has not been
confirmed until now. In the present study, the GSE6344 dataset
demonstrated that the expression of visfatin was upregulated in the
CC-RCC tissues compared to the adjacent normal tissues. The RT-qPCR
data of 77 CC-RCC patients also indicated that visfatin gene
expression was upregulated.
Apelin, a recently described adipokine, is a
mitogenic factor expressed in endothelial cells (38). Apelin is involved in the process of
cell proliferation (39) and
stimulates cancer angiogenesis (40).
A case-control study observed that serum apelin levels increased
significantly in patients with gastroesophageal cancer (41). However, there is no epidemiological
data regarding the association between apelin and CC-RCC risk. The
GSE6344 dataset demonstrates that the expression of apelin was
upregulated in the CC-RCC tissues compared to the adjacent normal
tissues. However, the RT-qPCR data indicated that the expression of
apelin was not significantly different between the CC-RCC and
adjacent normal tissues (P>0.05).
Resistin is a member of the recently identified
family of cysteine-rich proteins; it is the primary product of
macrophages that infiltrates into adipose tissue (42), and has been associated with adiposity,
inflammation and insulin resistance (43). Resistin serum concentrations have been
observed to be significantly higher in patients with cancer than in
controls (41,44). However, one study has reported that
circulating levels of resistin are not associated with RCC
(24). In the present study, the
GSE6344 dataset demonstrated that the expression of resistin
exhibited no significant difference between the CC-RCC and adjacent
normal tissues. However, the RT-qPCR data indicated that the mRNA
expression level of resistin in the CC-RCC tissues was
significantly higher than that in the adjacent normal tissues.
Adiponectin is a circulating adipokine secreted by
mature adipocytes. The circulating level of adiponectin is
inversely associated with insulin resistance and obesity (45). Abundant evidence indicates that
adiponectin suppresses the growth of cancer cells and reduces the
risk of cancer (14,46). Studies have reported that serum
adiponectin levels are inversely associated with RCC, and breast
and colon cancer (47,48). While the preponderance of evidence
suggests that an inverse association exists between adiponectin and
malignancy, another study reported that higher levels of serum
adiponectin were associated with RCC risk, specifically among
African-American males (31).
Enhanced adiponectin serum concentrations appear to indicate
inflammatory status and advanced stages of malignancy (49,50).
However, all aforementioned studies were confined to studying
adiponectin levels within the circulatory system. In the present
study, the RT-qPCR data indicated that the mRNA expression level of
adiponectin in the CC-RCC tissues was significantly lower than that
of the adjacent normal tissues, which was consistent with the
GSE6344 dataset.
Microarray technology provides a wide range of
information regarding molecules that are associated with disease
pathogenesis. However, the high cost of detection limits the
application in larger samples, which may lead to reduced
sensitivity. RT-qPCR is the gold standard for verification of
microarray data. In the present study, the data regarding visfatin
and adiponectin is consistent between microarray and RT-qPCR.
However, the data concerning leptin, resistin and apelin from
RT-qPCR cannot verify the results from the GSE6344 microarray
dataset. This may be attributed to the small sample size of the
GSE6344 microarray. Furthermore, it was demonstrated in another
study that the expression level of leptin varies in different
ethnicities (31), which may lead to
the contradictory results.
In conclusion, such findings suggest that visfatin
and resistin are high-risk factors for the development of CC-RCC.
By contrast, leptin and adiponectin are inversely associated with
CC-RCC risk. The present study may provide novel information
concerning the role of adipokines in CC-RCC risk. Due to the
relatively small number of patients and contradictory results from
microarray data, further studies are required to investigate the
etiological significance of adipokine levels in CC-RCC risk.
Acknowledgements
The study was funded by a grant from the Wuxi
Hospital Management Center (no. YGZ1102).
References
|
1
|
Fujioka T and Obara W: Committee for
Establishment of the Clinical Practice Guideline for the Management
of Renal Cell Carcinoma and the Japanese Urological Association:
Evidence-based clinical practice guideline for renal cell
carcinoma: The Japanese Urological Association 2011 update. Int J
Urol. 19:496–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kovacs G, Akhtar M, Beckwith BJ, Bugert P,
Cooper CS, Delahunt B, Eble JN, Fleming S, Ljungberg B, Medeiros
LJ, et al: The Heidelberg classification of renal cell tumours. J
Pathol. 183:131–133. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shenoy N and Pagliaro L: Sequential
pathogenesis of metastatic VHL mutant clear cell renal cell
carcinoma: Putting it together with a translational perspective.
Ann Oncol. 27:1685–1695. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Razafinjatovo C, Bihr S, Mischo A, Vogl U,
Schmidinger M, Moch H and Schraml P: Characterization of VHL
missense mutations in sporadic clear cell renal cell carcinoma:
Hotspots, affected binding domains, functional impact on pVHL and
therapeutic relevance. BMC Cancer. 16:6382016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arjumand W and Sultana S: Role of VHL gene
mutation in human renal cell carcinoma. Tumour Biol. 33:9–16. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adams KF, Leitzmann MF, Albanes D, Kipnis
V, Moore SC, Schatzkin A and Chow WH: Body size and renal cell
cancer incidence in a large US cohort study. Am J Epidemiol.
168:268–277. 2008.PubMed/NCBI
|
|
7
|
Luo J, Margolis KL, Adami HO, Lopez AM,
Lessin L and Ye W: Women's Health Initiative Investigators: Body
size, weight cycling, and risk of renal cell carcinoma among
postmenopausal women: The Women's Health Initiative (United
States). Am J Epidemiol. 166:752–759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lipworth L, Tarone RE, Lund L and
McLaughlin JK: Epidemiologic characteristics and risk factors for
renal cell cancer. Clin Epidemiol. 1:33–43. 2009.PubMed/NCBI
|
|
9
|
Calle EE and Kaaks R: Overweight, obesity
and cancer: Epidemiological evidence and proposed mechanisms. Nat
Rev Cancer. 4:579–591. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fischer-Posovszky P, Wabitsch M and
Hochberg Z: Endocrinology of adipose tissue - an update. Horm Metab
Res. 39:314–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raucci R, Rusolo F, Sharma A, Colonna G,
Castello G and Costantini S: Functional and structural features of
adipokine family. Cytokine. 61:1–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balistreri CR, Caruso C and Candore G: The
role of adipose tissue and adipokines in obesity-related
inflammatory diseases. Mediators Inflamm. 2010:8020782010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riondino S, Roselli M, Palmirotta R,
Della-Morte D, Ferroni P and Guadagni F: Obesity and colorectal
cancer: Role of adipokines in tumor initiation and progression.
World J Gastroenterol. 20:5177–5190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye J, Jia J, Dong S, Zhang C, Yu S, Li L,
Mao C, Wang D, Chen J and Yuan G: Circulating adiponectin levels
and the risk of breast cancer: A meta-analysis. Eur J Cancer Prev.
23:158–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song CH, Liao J, Deng ZH, Zhang JY, Xue H,
Li YM, Liang C, Han M, Zhang K and Yan GT: Is leptin a predictive
factor in patients with lung cancer? Clin Biochem. 47:230–232.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tun HW, Marlow LA, von Roemeling CA,
Cooper SJ, Kreinest P, Wu K, Luxon BA, Sinha M, Anastasiadis PZ and
Copland JA: Pathway signature and cellular differentiation in clear
cell renal cell carcinoma. PLoS One. 5:e106962010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lenburg ME, Liou LS, Gerry NP, Frampton
GM, Cohen HT and Christman MF: Previously unidentified changes in
renal cell carcinoma gene expression identified by parametric
analysis of microarray data. BMC Cancer. 3:312003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gati A, Kouidhi S, Marrakchi R, El Gaaied
A, Kourda N, Derouiche A, Chebil M, Caignard A and Perier A:
Obesity and renal cancer: Role of adipokines in the tumor-immune
system conflict. OncoImmunology. 3:e278102014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gulen ST, Karadag F, Karul AB, Kilicarslan
N, Ceylan E, Kuman NK and Cildag O: Adipokines and systemic
inflammation in weight-losing lung cancer patients. Lung.
190:327–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ntikoudi E, Kiagia M, Boura Pand and
Syrigos KN: Hormones of adipose tissue and their biologic role in
lung cancer. Cancer Treat Rev. 40:22–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gonullu G, Kahraman H, Bedir A, Bektas A
and Yücel I: Association between adiponectin, resistin, insulin
resistance, and colorectal tumors. Int J Colorectal Dis.
25:205–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spyridopoulos TN, Petridou ET, Dessypris
N, Terzidis A, Skalkidou A, Deliveliotis C and Chrousos GP: Obesity
and Cancer Oncology Group: Inverse association of leptin levels
with renal cell carcinoma: Results from a case-control study.
Hormones (Athens). 8:39–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liao LM, Weinstein SJ, Pollak M, Li Z,
Virtamo J, Albanes D, Chow WH and Purdue MP: Prediagnostic
circulating adipokine concentrations and risk of renal cell
carcinoma in male smokers. Carcinogenesis. 34:109–112. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimizu H and Mori M: Role of leptin and
its receptor in the regulation of appetite and body fat. Nihon
Rinsho. 59:421–426. 2001.(In Japanese). PubMed/NCBI
|
|
26
|
Yuan HJ, Sun KW and Yu K: Leptin promotes
the proliferation and migration of human breast cancer through the
extracellular-signal regulated kinase pathway. Mol Med Rep.
9:350–354. 2014.PubMed/NCBI
|
|
27
|
Zhou W, Guo S and Gonzalez-Perez RR:
Leptin pro-angiogenic signature in breast cancer is linked to IL-1
signalling. Br J Cancer. 104:128–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen C, Chang YC, Lan MS and Breslin M:
Leptin stimulates ovarian cancer cell growth and inhibits apoptosis
by increasing cyclin D1 and Mcl-1 expression via the activation of
the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol.
42:1113–1119. 2013.PubMed/NCBI
|
|
29
|
Mohammadzadeh G, Ghaffari MA, Bafandeh A
and Hosseini SM: Association of serum soluble leptin receptor and
leptin levels with breast cancer. J Res Med Sci. 19:433–438.
2014.PubMed/NCBI
|
|
30
|
Horiguchi A, Sumitomo M, Asakuma J, Asano
T, Zheng R, Asano T, Nanus DM and Hayakawa M: Increased serum
leptin levels and over expression of leptin receptors are
associated with the invasion and progression of renal cell
carcinoma. J Urol. 176:1631–1635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liao LM, Schwartz K, Pollak M, Graubard
BI, Li Z, Ruterbusch J, Rothman N, Davis F, Wacholder S, Colt J, et
al: Serum leptin and adiponectin levels and risk of renal cell
carcinoma. Obesity (Silver Spring). 21:1478–1485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stastny J, Bienertova-Vasku J and Vasku A:
Visfatin and its role in obesity development. Diabetes Metab Syndr.
6:120–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bi TQ and Che XM: Nampt/PBEF/visfatin and
cancer. Cancer Biol Ther. 10:119–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Li X, Liu KR, Zhang JN, Liu Y and
Zhu Y: Visfatin derived from ascites promotes ovarian cancer cell
migration through Rho/ROCK signaling-mediated actin polymerization.
Eur J Cancer Prev. 24:231–239. 2014. View Article : Google Scholar
|
|
35
|
Li XY, Tang SH, Zhou XC, Ye YH, Xu XQ and
Li RZ: Preoperative serum visfatin levels and prognosis of breast
cancer among Chinese women. Peptides. 51:86–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakajima TE, Yamada Y, Hamano T, Furuta K,
Matsuda T, Fujita S, Kato K, Hamaguchi T and Shimada Y:
Adipocytokines as new promising markers of colorectal tumors:
Adiponectin for colorectal adenoma, and resistin and visfatin for
colorectal cancer. Cancer Sci. 101:1286–1291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu GW, Wang QJ, Xia MM and Qian J:
Elevated plasma visfatin levels correlate with poor prognosis of
gastric cancer patients. Peptides. 58:60–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sorli SC, Le Gonidec S, Knibiehler B and
Audigier Y: Apelin is a potent activator of tumour neoangiogenesis.
Oncogene. 26:7692–7699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang L, Su T, Lv D, Xie F, Liu W, Cao J,
Sheikh IA, Qin X, Li L and Chen L: ERK1/2 mediates lung
adenocarcinoma cell proliferation and autophagy induced by
apelin-13. Acta Biochim Biophys Sin (Shanghai). 46:100–111. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heo K, Kim YH, Sung HJ, Li HY, Yoo CW, Kim
JY, Park JY, Lee UL, Nam BH, Kim EO, et al: Hypoxia-induced
up-regulation of apelin is associated with a poor prognosis in oral
squamous cell carcinoma patients. Oral Oncol. 48:500–506. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Diakowska D, Markocka-Mączka K,
Szelachowski P and Grabowski K: Serum levels of resistin,
adiponectin, and apelin in gastroesophageal cancer patients.
Disease Markers. 2014:6196492014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Curat CA, Wegner V, Sengenès C, Miranville
A, Tonus C, Busse R and Bouloumié A: Macrophages in human visceral
adipose tissue: Increased accumulation in obesity and a source of
resistin and visfatin. Diabetologia. 49:744–747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hivert MF, Sullivan LM, Fox CS, Nathan DM,
D'Agostino RB Sr, Wilson PW and Meigs JB: Associations of
adiponectin, resistin, and tumor necrosis factor-alpha with insulin
resistance. J Clin Endocrinol Metab. 93:3165–3172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dalamaga M, Sotiropoulos G, Karmaniolas K,
Pelekanos N, Papadavid E and Lekka A: Serum resistin: A biomarker
of breast cancer in postmenopausal women? Association with
clinicopathological characteristics, tumor markers, inflammatory
and metabolic parameters. Clin Biochem. 46:584–590. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yildiz Y, Ozaksit G, Unlu B Serdar, Ozgu
E, Energin H, Kaba M and Ugur M: Serum adiponectin level and
clinical, metabolic, and hormonal markers in patients with
polycystic ovary syndrome. Int J Fertil Steril. 7:331–336.
2014.PubMed/NCBI
|
|
46
|
Hebbard L and Ranscht B: Multifaceted
roles of adiponectin in cancer. Best Pract Res Clin Endocrinol
Metab. 28:59–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Spyridopoulos TN, Petridou ET, Skalkidou
A, Dessypris N, Chrousos GP and Mantzoros CS: Obesity and Cancer
Oncology Group: Low adiponectin levels are associated with renal
cell carcinoma: A case-control study. Int J Cancer. 120:1573–1578.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gulcelik MA, Colakoglu K, Dincer H, Dogan
L, Yenidogan E and Gulcelik NE: Associations between adiponectin
and two different cancers: Breast and colon. Asian Pac J Cancer
Prev. 13:395–398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dalamaga M, Diakopoulos KN and Mantzoros
CS: The role of adiponectin in cancer: A review of current
evidence. Endocr Rev. 33:547–594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Izadi V, Farabad E and Azadbakht L: Serum
adiponectin level and different kinds of cancer: A review of recent
evidence. ISRN Oncol. 2012:9827692012.PubMed/NCBI
|