Introduction
The level of morbidity and mortality in lung cancer
is among the highest of all cancers in the world (1). Therefore, lung cancer poses a serious
threat to human health, so it is important to conduct research
regarding the possible mechanisms of the development and metastasis
of lung cancer in order to improve the treatment of this disease.
Glucocorticoids, which are secreted by the adrenal glands, are
steroid hormones that can regulate the growth, development and
differentiation of the organism and stabilize the internal
environment. For almost all cells glucocorticoids play a regulatory
role in growth and differentiation. Dexamethasone is a synthetic
glucocorticoid that is widely used clinically in order to improve
the general condition of cancer patients and reduce adverse
reactions to chemotherapy (2).
Previous studies have found that dexamethasone may inhibit the
expression of vascular endothelial growth factor (VEGF) mRNA in
induced rat glioma cells (3) and
human vascular smooth muscle cells (4) in vitro. It has been suggested
that dexamethasone has certain anti-angiogenic and anti-tumor
effects (5,6). In addition, dexamethasone may also
inhibit proliferation and induce differentiation in numerous
tissue-derived carcinoma cells, such as gynecological and digestive
system tumor cells (7–9). However, little research has been
performed on the inhibitory effect of dexamethasone on lung
carcinoma cells, particularly for patients who received palliative
surgery.
The aim of the present study was to investigate the
inhibitory effect of dexamethasone on angiogenesis, cell
proliferation and tumor growth in residual Lewis lung cancer cells
in mice subsequent to palliative surgery by establishing a C57BL/6
mouse xenograft model using Lewis lung carcinoma cells. The present
study aims to expand current treatments of lung cancer and provide
a new basis for hormone therapy for patients, particularly those
who received palliative surgery.
Materials and methods
Animal models
The use of 18 male athymic BALB/c nude mice,
weighing 20±6 g each, aged between 6 and 8 weeks, was approved by
Animal Experiment Center of Shandong University (Jinan, China).
This study was performed strictly in accordance with the
recommendations in the Guide for the Care and use of Laboratory
Animals of the National Institutes of Health. All experimental
protocols described in the present study were approved by the
Committee on Animal Investigation of Shandong Medical College of
Shandong University (Jinan, China).
Materials
Lewis lung carcinoma cells were obtained from
Shandong Academy of Medical Sciences (Jinan, China). Cisplatin was
purchased from Qilu Pharmaceutical Co., Ltd., (Jinan, China) and
was dissolved in 0.2 ml normal saline to give a 2 mg/ml stock
solution at 4°C, mice in the cisplatin treatment group were
administered 2 mg/kg cisplatin. Bicinchoninic acid (BCA) protein
assay kit was obtained from Pierce Biotechnology, Inc., (Rockford,
IL, USA). Polyvinylidene difluoride (PVDF) membranes were from Pall
Life Sciences (EMD Millipore, Billerica, MA, USA). Rabbit
monoclonal anti-human hypoxia inducible factor 1α (HIF-1α) antibody
(cat. no. ab51608), rabbit polyclonal anti-cluster of
differentiation 31 (CD31) antibody (cat. no. ab28364), rabbit
monoclonal anti-human VEGF antibody (cat. no. ab32562) and rabbit
polyclonal anti-human proliferating cell nuclear antigen (PCNA)
antibody (cat. no. ab29) were purchased from Abcam (Cambridge,
UK).
Animal groups and processing
Lewis lung carcinoma cells (Shandong Academy of
Medical Sciences) were cultivated in plastic tissue culture flasks
(Corning Incorporated, Corning, NY, USA) with complete Dulbecco's
modified Eagle's medium-F12 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin-streptomycin (HyClone;
GE Healthcare Life Sciences, Logan, UT, USA). The cells were then
placed in a 37°C culture incubator with a 5% CO2
atmosphere. The cell suspension was collected, which contained
logarithmic growth phase cells, and the cell density was adjusted
to 1×107 cells/ml with phosphate-buffered saline. A 0.2
ml suspension containing 2×106 cells was inoculated into
the right axilla of each mouse, and they were then fed under a
specific pathogen-free environment.
The tumors of mice were observed as subcutaneous
nodules in the right axilla, 7 days following inoculation. The rate
of tumor formation in mice was 100%. When the tumor diameter
reached 0.5 cm, 2 weeks later, palliative surgery was performed
leaving ~1 mm3 tumor tissue in the right axilla of the
mice. The mice were then divided equally into 3 groups at random
(control group; cisplatin group; and dexamethasone group).
Postoperatively, all the mice were administered with different
treatments for 10 consecutive days. The mice of the control group
were intraperitoneally administered with saline. The cisplatin
group was intraperitoneally administered with 2 mg/kg cisplatin
dissolved in 0.2 ml saline on days 1, 2 and 3, and 0.2 ml saline
alone was administered on the other days. The mice of the second
group were intraperitoneally administered with 5 mg/kg
dexamethasone (Shandong Xinhua Pharmaceutical Co., Ltd., Shandong,
China) dissolved in 0.2 ml saline once each day. Each group
received treatment for 10 consecutive days. During the period of
treatment subsequent to palliative surgery, the mental state,
eating habits, activities and defecation of the mice were observed.
The subcutaneous tumor nodules of each mouse were measured on days
4, 5, 6, 7, 8, 9 and 10 post-surgery using vernier calipers
(accuracy of 0.1 mm) and calculated the volume of the tumors as
follows: V (mm3) = 0.52ab2 (a, longest
diameter of the nodule; b, shortest diameter). The average of each
group was used to plot the tumor growth curve. All mice were
sacrificed on day 11 postoperatively; the whole body and the local
tumors were weighed immediately. The inhibitory rate of the tumor
was calculated as follows: Inhibitory rate (IR) (%) = [control
group average tumor weight (g) - treatment group average tumor
weight (g)] / control group average tumor weight (g) × 100. The
tumors were put in 4% paraformaldehyde for IHC immediately.
Portions of each tumor were immediately put in liquid nitrogen and
stored at −80°C for RT-quantitative polymerase chain reaction
(RT-PCR) and western blotting.
IHC
Antibodies against HIF-1α, VEGF and PCNA were used
for IHC analysis of tumors, which was performed according to the
manufacturer's protocol. Results may be judged according to the
staining intensity of positive cells and the percentage of positive
cells. Each 3-µm-thick section was observed by microscopy, with 10
random high-power field measurements taken at a ×200 magnification.
A reference method of scoring was used as previously described by
Mamori et al (10). The
staining intensity was scored as follows: No staining, 0; slight
staining, 1; moderate staining, 2; and deep staining, 3. The
criteria for scoring the percentage of positive cells was as
follows: No positive cells, 0; <25% positive cells, 1; 25–50%
positive cells, 2; and >50% positive cells, 3. The 2 scores were
then added and the judgment standard of results was as follows:
Strong positive (++), 9–12; positive (+), 5–8; and negative (−),
0–4. Assessment of vascularity used the average of the microvessel
density (MVD), which was judged by assessing IHC staining for CD31
as reported by Vermeulen et al (11). The slice was scanned to identify the
most intense vascularization areas under a microscope at a low
magnification (x100). Subsequently, these areas were counted by
observing the slice at a ×400 magnification in 10 chosen fields of
view. A single cluster of endothelial cells or a branch structure
of vascular stem cells that were stained positive for CD31 were
regarded as one blood vessel. The average vessel number of MVD in
10 randomly assigned fields was considered to be the number of
microvessels for each case.
RNA isolation and RT-qPCR assay
RT-qPCR was utilized for HIF-1α, VEGF and PCNA
expression analysis. Total RNA was isolated from tumor tissue using
the RNA simple total RNA kit (Tiangen Biotech Co., Ltd., Beijing,
China), according to the manufacturer's protocol. The concentration
of total RNA was detected by spectrophotometry (SPECTRA MAX190;
Molecular Devices, LLC, Sunnyvale, CA, USA). Complementary DNA
(cDNA) was synthesized from 2 µg total RNA using the fastquant RT
kit (Tiangen Biotech Co., Ltd.), according to the manufacturer's
protocol. qPCR was performed in an ABI ViiA7 Dx instrument (Thermo
Fisher Scientific, Ltd., Waltham, MA, USA) using the SYBR green PCR
Master Mix (Tiangen Biotech Co., Ltd.) following the manufacturer's
protocol. Rat β-actin gene was used as the control. Gene-specific
primers were designed in GenBank (Accession number, NM_02306;
National Center for Biotechnology Information, Bethesda, MD, USA)
Primer sequences for HIF-1α, VEGF, PCNA and β-actin are described
as follows: HIF-1α forward, 5′-TGTGTTTGATTTTACTCATCCATGT-3′ and,
reverse, 5′-CTCCGCTGTGTGTTTAGTTCTT-3′; VEGF forward,
5′-AAAGGGAAAGGGTCAAAAACGAA-3′ and reverse,
5′-AGGAACATTTACACGTCTGCGG-3′; PCNA forward,
5′-ATCGTGAATCGGGGGACCTTG−3′ and reverse,
5′-CTTTGAGAGCCTCCAGCACCTTC-3′; β-actin forward,
5′-GACCACACCTTCTACAATGAG-3′ and reverse,
5′-GCATACCCCTCGTAGATGGG-3′.
PCR results were quantified using the ΔCq method
following the formula: Ratio = 2-ΔCq, where ΔCq = Cq target gene -
Cq endogenous control gene (β-actin) (12).
Western blotting
The expression of HIF-1α, VEGF and PCNA was
determined by western blot analysis. Tumor tissue was lysed in
lysis buffer (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
and centrifuged at 12,000 × g for 30 min at 4°C. The
supernatant was collected following centrifugation. The protein
concentration was determined using a BCA protein assay kit (Pierce
Biotechnology, Inc.). The samples were boiled, sheared and
clarified by centrifugation. Equal quantities (20 µg) of protein
were loaded onto and separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to a
PVDF membrane (EMD Millipore). The membranes were blocked with 5%
skim milk in Tris-buffered saline and Tween-20 (TBST) buffer for
1.5 h with agitation, and the membranes were incubated with primary
antibodies against HIF-1α (dilution, 1:1,000), VEGF (dilution,
1:800) and PCNA (dilution, 1:1,000) overnight at 4°C. The membranes
were subsequently rinsed 3 times with TBST and incubated with
polyclonal sheep anti-rat IgG conjugated to horseradish peroxidase
(dilution, 1:1,000; Sigma-Aldrich; Merck Millipore; cat. no.
BA1050). The visualization of immunosignals was performed using the
protein detector 5-bromo-4-chloro-3-indolyl-phosphate/nitro blue
tetrazolium western blotting kit (Beyotime Institute of
Biotechnology, Haimen, China) was in accordance with the
manufacturer's protocol. ECL (EMD Millipore) images were captured
with a FluorChem E instrument (Cell Biosciences, Inc., Santa Clara,
CA, USA). The present study used ImageQuant 5.2 software (GE
Healthcare, Little Chalfont, UK) to conduct the quantification of
each sample. A separate membrane was prepared using the same
methods, and was probed with mouse polyclonal anti-human GAPDH
(dilution, 1:1,000; cat. no. ab8245; Abcam) antibodies.
Statistical analysis
The data was analyzed using the SPSS 13.0
statistical software (SPSS, Inc., Chicago, IL, USA). Values are
expressed as the mean ± standard error of the mean. Significance
was tested by one-way analysis of variance with Dunnett's test.
P<0.05 was considered to indicate a statistically significant
difference. The association between HIF-1α and VEGF expression and
MVD was analyzed using Spearman's rank correlation coefficient.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of dexamethasone on residual
Lewis lung cancer cells growth subsequent to palliative resection
surgery
Tumors in the normal saline group grew significantly
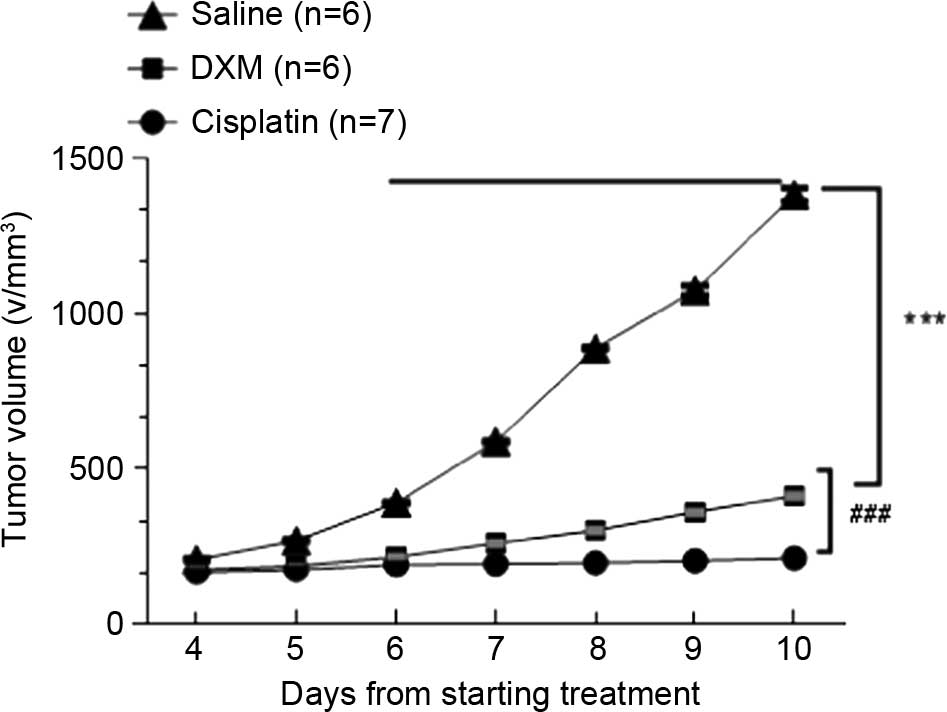
faster than those in the other groups. The growth curve (Fig. 1) showed tumor growth in the treated
groups gradually slowed down. Compared to controls, treatments with
2 mg/kg cisplatin, and 5 mg/kg dexamethasone were found to
apparently inhibit the tumor growth, and the inhibitory rate was
39.4 and 72.24%, respectively (Table
I). The inhibitory effect was evident in the dexamethasone
groups and the cisplatin groups (P<0.001) compared with the
control group. The mice in dexamethasone groups and the cisplatin
groups were in good condition and increased in body weight
following the experiment (Table I).
This suggests that dexamethasone not only inhibited the growth of
residual Lewis lung cancer cells subsequent to palliative surgery,
but also reduced the tumor's consumption of resources.
 | Table I.Body weight, tumor size and tumor IR
of the residual Lewis lung cancer cells in the 3 groups. |
Table I.
Body weight, tumor size and tumor IR
of the residual Lewis lung cancer cells in the 3 groups.
|
| Body weight, g |
|
|
|---|
|
|
|
|
|
|---|
| Group | Prior to
treatment | Subsequent to
treatment | Tumor weight, g | IR, % |
|---|
| Normal saline | 22.44±0.24 | 24.57±0.18 | 0.66±0.08 |
|
| Dexamethasone | 22.72±0.28 | 24.92±0.14 |
0.40±0.06a | 39.4 |
| Cisplatin | 22.54±0.25 | 25.49±0.17 |
0.17±0.05a,b | 72.24 |
HIF-1α, VEGF, PCNA expression and MVD
in tumors
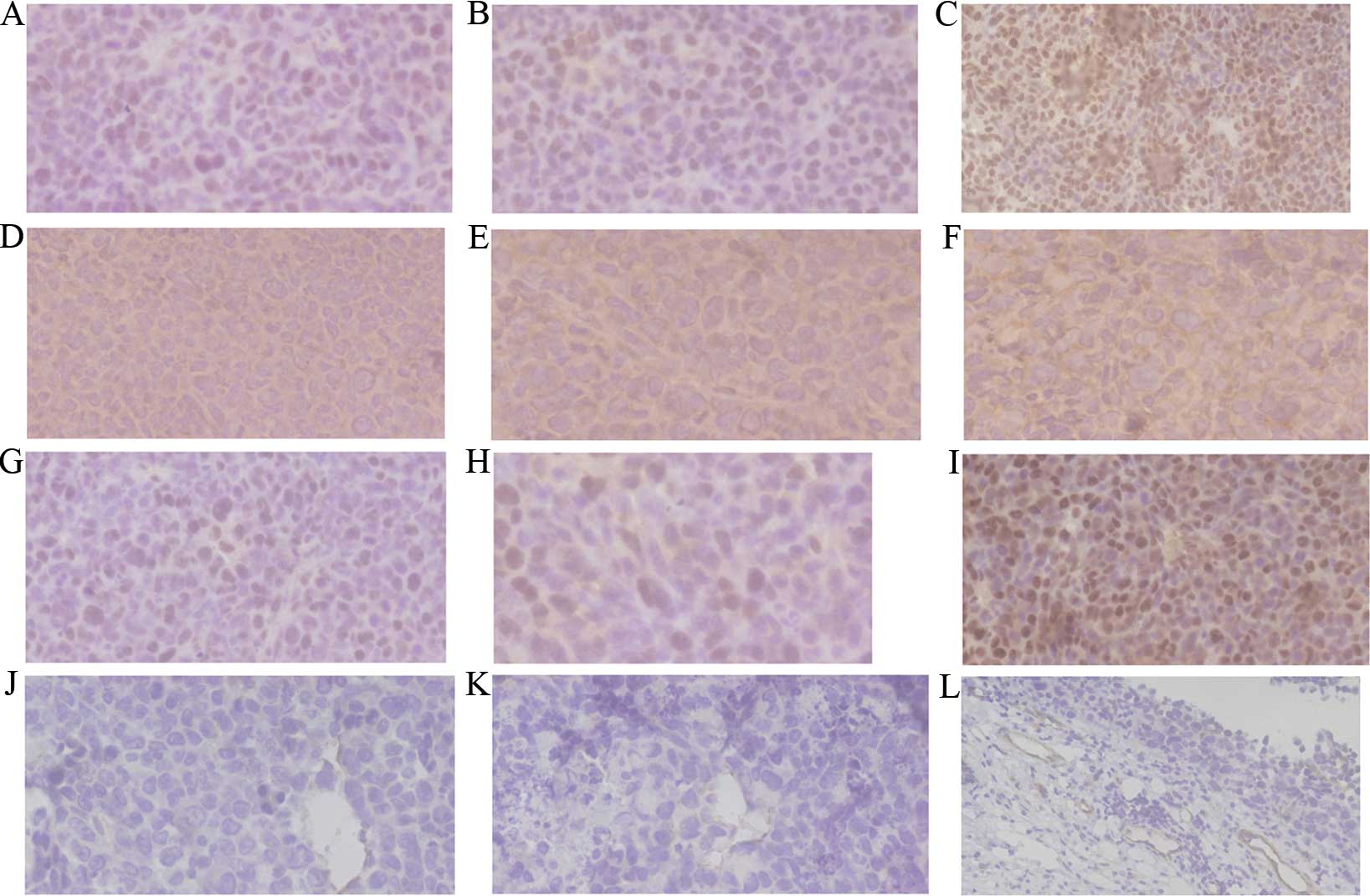
VEGF was expressed in the cytoplasm or membrane of
tumor cells based on IHC staining of tumors. HIF-1α and PCNA
protein showed mainly positive staining in the nucleus of tumor
cells. VEGF protein showed positive staining mainly in the
cytoplasm of the tumor cells. Positive staining of HIF-1α, VEGF and
PCNA was characterized by brown and finely granular staining at the
original ×400 magnification in tumors (Fig. 2A-I). Cells positive for CD31 were
stained brown in the cytoplasm. Microvessel distribution is shown
at the original ×400 magnification (Fig.
2J-L). In the present results, the expression of HIF-1α, VEGF
and PCNA in the normal saline group was higher than that in the
other treatment groups. Grayscale intensity variants of HIF-1α,
VEGF immunoreactivity were assessed by Image-Pro Plus software
(Media Cybernetics, Inc., Rockville, MD, USA). Compared with the
control groups, HIF-1α (P=0.0013), VEGF (P=0.0025) and PCNA
(P=0.0038) expression scores, and MVD counts (P=0.0018), decreased
significantly in the cisplatin and dexamethasone groups. In each
comparison, there was a significant difference (Table II).
 | Table II.Expression scores of HIF-1α, VEGF,
PCNA and MVD in each group. |
Table II.
Expression scores of HIF-1α, VEGF,
PCNA and MVD in each group.
| Group | Cases, n | HIF-1α score | VEGF score | PCNN score | MVD score |
|---|
| Normal saline | 6 | 4.21±0.35 | 4.47±0.34 | 4.76±0.31 | 29.75±5.64 |
| Dexamethasone | 6 |
2.67±0.43a |
2.74±0.35a |
2.91±0.24a |
17.01±3.24a |
| Cisplatin | 6 |
1.39±0.25a,b |
1.60±2.35a,b |
1.67±0.43a,b |
12.09±2.96a,b |
Expression levels of HIF-1α, VEGF and
PCNA mRNA in the control, cisplatin and dexamethasone groups were
quantified by qPCR
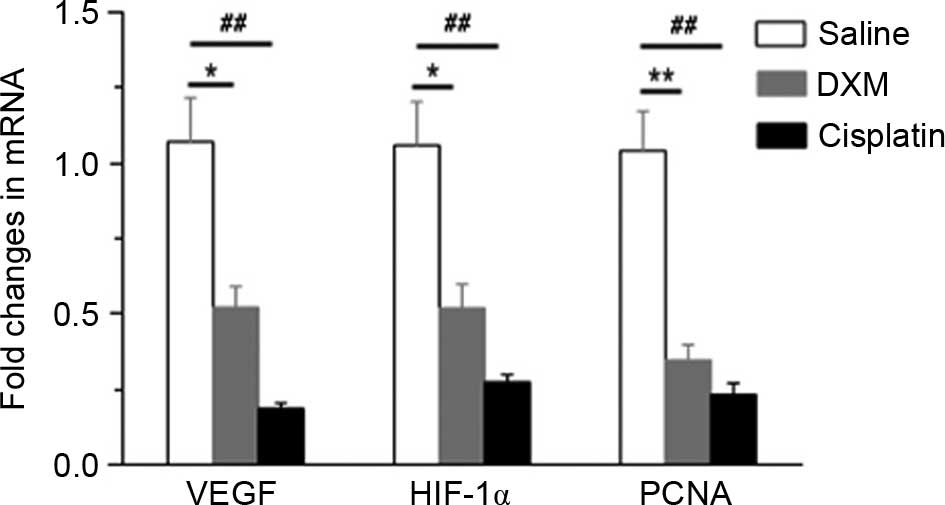
HIF-1α, VEGF and PCNA mRNA were quantified in the
control, cisplatin and dexamethasone groups by qPCR. As shown in
Fig. 3, the expression levels of
HIF-1α (P=0.018), VEGF (P=0.012) and PCNA (P=0.0018) mRNA in the
dexamethasone group were significantly reduced compared with the
control group, which suggests that dexamethasone can inhibit the
expression levels of HIF-1α, VEGF and PCNA mRNA. The results
indirectly suggest that dexamethasone may inhibit angiogenesis.
Effect of dexamethasone treatment on
HIF-1α, VEGF and PCNA protein expression as assessed by western
blot analysis
HIF-1α, VEGF and PCNA expression was normalized to
glyceraldehyde 3-phosphate dehydrogenase expression by band
intensity (Fig. 4). Band intensities
were analyzed by ImageJ software (National Institutes of Health,
Bethesda, MD, USA). As shown in Fig.
5, HIF-1α (P=0.007), VEGF (P=0.019) and PCNA (P=0.0094)
expression decreased significantly in the tissue treated with
dexamethasone.
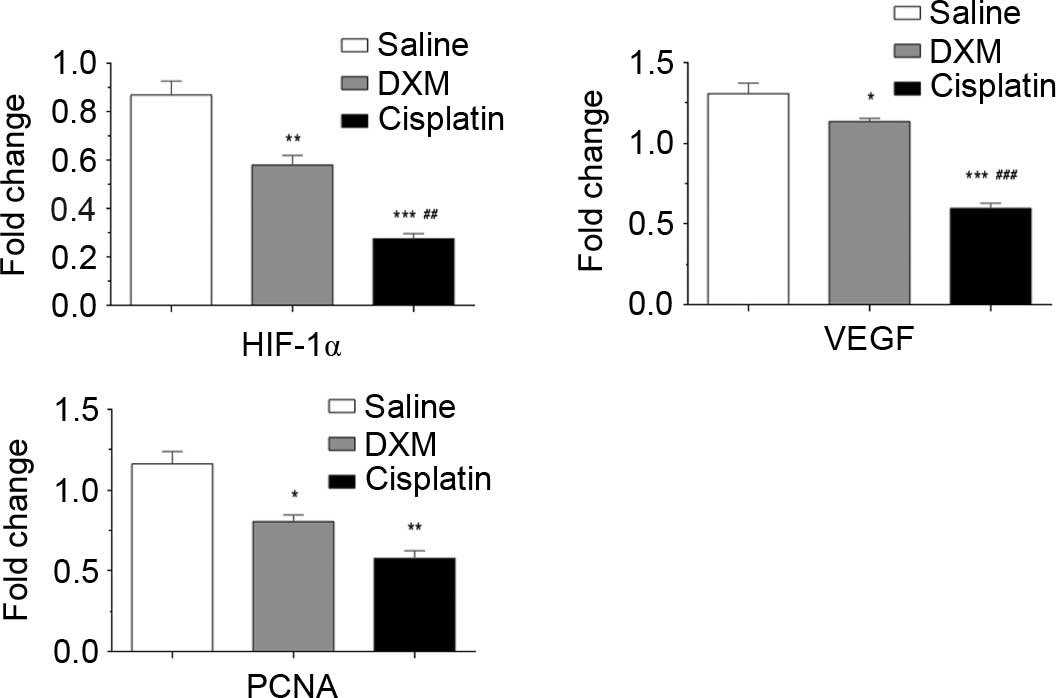 | Figure 5.Western blot analysis of HIF-1α, VEGF
and PCNA protein expression in the control group, dexamethasone
group, and cisplatin group. Protein expression was significantly
decreased in the cisplatin and dexamethasone treatment groups.
Values are expressed as the mean ± standard error of the mean.
Significance was tested by one-way analysis of variance with
Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference. *P<0.05, **P<0.01,
***P<0.001 vs. normal saline group; ##P<0.01,
###P<0.01 vs. dexamethasone group. HIF-1α, hypoxia
inducible factor 1α; VEGF, vascular endothelial growth factor;
PCNA, proliferating cell nuclear antigen; DXM, dexamethasone. |
Discussion
Tumors cannot grow beyond 0.2 mm from vessels,
suggesting that angiogenesis is necessary for the progression and
metastasis of tumors (13). Numerous
malignant tumors, including lung cancers, overexpress angiogenic
factors (14). HIF-1α is one major
regulator of the neovascularization process. HIF-1α was first
identified by Wang and Semenza in 1992 (15) when they studied hypoxia-inducible gene
expression. HIF-1α is the most important factor associated with
cell responses under hypoxic conditions for cell survival and
angiogenesis (16). HIF-1α activates
the transcription of numerous genes by binding to the hypoxia
response element (HRE) in the promoter, such as VEGF, and it plays
an important role in the angiogenesis, survival, invasion and
metastasis of tumors. In the process of tumor formation and
development, VEGF, which has a variety of functions, is the major
factor for inducing angiogenesis. VEGF plays an important role in
mitogenic and chemotactic properties of vascular endothelial cells,
and stimulates the endothelial cell proliferation for increasing
vascular permeability and promoting angiogenesis by integrating
with insulin-specific receptors (17). Excessive expression of the VEGF gene
promotes angiogenesis and tumor growth, and leads to unlimited
amplification of the tumor vasculature. In addition, VEGF
accelerates the invasion and transfer of tumors through autocrine
invasion. The process of angiogenesis is tightly regulated by a
series of protein and anti-angiogenic molecules in normal
physiology (18,19). In addition, HIF-1α and VEGF are the
most important factors of angiogenesis. They facilitate the
development of malignant cells by increasing vascular permeability
(20–23). The excessive expression of HIF-1α and
VEGF is closely associated with the progression and angiogenesis of
the tumor. Previous studies demonstrated that hypoxia leads to
overexpression of VEGF, which promotes angiogenesis through HIF-1α
in the development of the tumor in vivo (24,25). The
unlimited proliferation of mesenchymal cells is regulated by tumor
cells through an intercellular signaling system, leading to the
tumor microenvironment that promotes the proliferation and
development of tumor cells. In general, the microenvironment of the
tumor appears to be associated with extremely vigorous tumor
angiogenesis (26). Malignant tumor
growth and metastasis depends on angiogenesis, the extent of which
determines the tumor microenvironment and thus the degree of tumor
metastasis.
At present, accumulating evidence has already
demonstrated that HIF-1α and VEGF play an important role in
promoting angiogenesis, which is well recognized as a pivotal step
for cancer growth, invasion and metastasis in solid tumors
(27). Proliferation signals from
outside of the cell are involved in PCNA in order to promote DNA
synthesis (28), so that PCNA can
mediate cell survival and proliferation. Studies have confirmed
that PCNA protein synthesis may indirectly reflect the rate of DNA
synthesis and cell proliferation during the cell cycle. As a
result, during the development of tumors, PCNA is a reliable factor
of the proliferative phase, providing a marker for the
proliferation of tumor cells (29).
Lung cancer is rich in blood vessels, and previous research
(30) has identified excessive
expression of HIF-1α and VEGF in patients. Therefore, a method of
therapy that inhibits angiogenesis may be identified as one of the
most promising and hopeful strategies to restrain the development
of lung cancer and prolong the lives of patients who receive
palliative surgery. Therefore, theoretically, tumor treatments may
consist of not only cytotoxic agents against tumor cells, but also
agents targeted to inhibit the proliferation of or kill
interstitial cells, such as vascular endothelial cells, which play
a critical role in tumor growth.
The study by Folkman (31) demonstrated that the inhibition of
tumor angiogenesis is able to slow down the development of tumors
or reduce tumor size and burden. The importance of angiogenesis to
tumor formation and development is gradually being recognized
(32).
In order to demonstrate how dexamethasone inhibited
the overexpression of VEGF in remaining tumor cells subsequent to
palliative surgery, the present study divided subjects into 3
groups (cisplatin, dexamethasone and normal saline groups) to
analyze the expression of associated proteins, such as HIF-1α, VEGF
and PCNA by IHC, western blotting and RT-qPCR. The present study
showed that the protein expression of HIF-1α, VEGF and PCNA in
tumor tissues of the dexamethasone group, was significantly lower
than those in the control group.
However, MVD is also an important factor of
angiogenesis. Tumor angiogenesis was evaluated by microvessel
density (MVD). Tumor immunostaining for CD31 in endothelial cells
was performed to detect MVD. It was found that MVD in the tumor
tissues of the dexamethasone group was also significantly lower
than those in the control tumor tissues. The decreased expression
of HIF-1α, VEGF and PCNA in the present study suggested that
dexamethasone had an inhibitory effect on angiogenesis, which was
in accordance with previous experimental results that tumor
progression and metastasis can be inhibited by various
antiangiogenic therapies (33,34). The
present results show that tumor growth was significantly inhibited
in mice receiving dexamethasone. Not only was tumor growth
significantly inhibited in the dexamethasone group, the MVD of
tumor tissue in this group was also significantly lower than that
in the control group. Expression levels of HIF-1α and VEGF were
lower than those in the control group, and the expression of the
two markers showed a significant positive correlation (35–37).
Therefore, dexamethasone indirectly inhibits the generation of
angiogenic factors, such as VEGF, by decreasing the expression of
HIF-1α, thereby inhibiting tumor angiogenesis. This is an important
role of dexamethasone in anti-angiogenic effects and suppression of
tumor growth. PCNA, a cofactor for the DNA polymerase δ, is a type
of nucleoprotein that is necessary for DNA synthesis within the
nucleus. The present results suggested that dexamethasone
significantly reduced PCNA expression.
In summary, dexamethasone can effectively inhibit
the growth and angiogenic properties of residual Lewis lung
carcinoma subsequent to palliative surgery in mice and also can
reduce the adverse reaction to chemotherapy. Dexamethasone can
serve as the basis for tumor hormone therapy, which provides a new
method of postoperative adjuvant therapy for patients, particularly
those who received palliative surgery.
References
|
1
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnes PJ: Anti-inflammatory actions of
glucocorticoids: Molecular mechanisms. Clin Sci. 94:557–572. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Machein MR, Kullmer J, Ronicke V, Machein
U, Krieg M, Damert A, Breier G, Risau W and Plate KH: Differential
downregulation of vascular endothelial growth factor by
dexamethasone in normoxic and hypoxic rat glioma cells. Neuropathol
Appl Neurobiol. 25:104–112. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nauck M, Karakiulakis G, Perruchoud AP,
Papakonstantinou E and Roth M: Corticosteroids inhibit the
expression of the vascular endothelial growth factor gene in human
vascular smooth muscle cells. Eur J Pharmacol. 341:309–315. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rutz HP: Effects of corticosteroid use on
treatment of solid tumours. Lancet. 360:1969–1970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang H, Wang Y, Rayburn ER, Hill DL,
Rinehart JJ and Zhang R: Dexcamethasone as a chemosensitizer for
breast cancer chemotherapy: Potentiation of the antitumor activity
of adriamycin, modulation of cytokine expression, and
pharmacokinetics. Int J Oncol. 30:947–953. 2007.PubMed/NCBI
|
|
7
|
Arafa HM, Abdel-Hamid MA, El-Khouly AA,
Elmazar MM and Osman AM: Enhancement by dexamethasone of the
therapeutic benefits of cisplatin via regulation of tumor
angiogenesis and cell cycle kinetics in a murine tumor paradigm.
Toxicology. 222:103–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yemelyanov A, Czwornog J, Gera L, Joshi S,
Chatterton RT Jr and Budunova I: Novel steroid receptor
phyto-modulator compound a inhibits growth and survival of prostate
cancer cells. Cancer Res. 68:4763–4773. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moghaddam SJ, Haghighi EN, Samiee S,
Shahid N, Keramati AR, Dadgar S and Zali MR: Immunohistochemical
analysis of p53, cyclinD1, RB1, c-fos and N-ras gene expression in
hepatocellular carcinoma in Iran. World J Gastroenterol.
13:588–593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mamori S, Nagatsuma K, Matsuura T, Ohkawa
K, Hano H, Fukunaga M, Matsushima M, Masui Y, Fushiya N, Onoda H,
et al: Useful detection of CD147 (EMMPRIN) for pathological
diagnosis of early hepatocellular carcinoma in needle biopsy
samples. World J Gastroenterol. 13:2913–2917. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vermeulen PB, Gasparini G, Fox SB, Toi M,
Martin L, McCulloch P, Pezzella F, Viale G, Weidner N, Harris AL
and Dirix LY: Quantification of angiogenesis in solid human
tumours: An international consensus on the methodology and criteria
of evaluation. Eur J Cancer 32A. 2474–2484. 1996. View Article : Google Scholar
|
|
12
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yano T, Tanikawa S, Fujie T, Masutani M
and Horie T: Vascular endothelial growth factor expression and
neovascularisation in non-small cell lung cancer. Eur J Cancer.
36:601–609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang GL and Semenza GL: Characterization
of hypoxia-inducible factor l and regulation of DNA binding
activity by hypoxia. J Biol Chem. 268:21513–21518. 1993.PubMed/NCBI
|
|
16
|
Ferenci P, Fried M, Labrecque D, Bruix J,
Sherman M, Omata M, Heathcote J, Piratsivuth T, Kew M, Otegbayo JA,
et al: Hepatocellular carcinoma (HCC): A global perspective. J Clin
Gastroenterol. 44:239–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Da MX, Wu XT, Wang J, Guo TK, Zhao ZG, Luo
T, Zhang MM and Qian K: Expression of cyclooxygenase-2 and vascular
endothelial growth factor-C correlates with lymphangiogenesis and
lymphatic invasion in human gastric cancer. Arch Med Res. 39:92–99.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martínez A: A new family of angiogenic
factors. Cancer Lett. 236:157–163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jakob C, Sterz J, Zavrski I, Heider U,
Kleeberg L, Fleissner C, Kaiser M and Sezer O: Angiogenesis in
multiple myeloma. Eur J Cancer. 42:1581–1590. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pang RW, Joh JW, Johnson PJ, Monden M,
Pawlik TM and Poon RT: Biology of hepatocellular carcinoma. Ann
SurgOncol. 15:962–971. 2008.
|
|
21
|
Barr MP, Bouchier-Hayes DJ and Harmey JJ:
Vascular endothelial growth factor is an autocrine survival factor
for breast tumour cells under hypoxia. Int J Oncol. 32:41–48.
2008.PubMed/NCBI
|
|
22
|
Tzao C, Lee SC, Tung HJ, Hsu HS, Hsu WH,
Sun GH, Yu CP, Jin JS and Cheng YL: Expression of hypoxia-inducible
factor (HIF)-1alpha and vascular endothelial growth factor (VEGF)-D
as outcome predictors in resected esophageal squamous cell
carcinoma. Dis Markers. 25:141–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin C, McGough R, Aswad B, Block JA and
Terek R: Hypoxia induces HIF-1alpha and VEGF expression in
chondrosarcoma cells and chondrocytes. J Orthop Res. 22:1175–1181.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaelin WG Jr: How oxygen makes its
presence felt. Genes Dev. 16:1441–1445. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Cox SR, Morita T and Kourembanas S:
Hypoxia regulates vascular endothelial growth factor gene
expression in endothelial cells. Identification of a 5′ enhancer.
Circ Res. 77:638–643. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukumura D and Jain RK: Tumor
microvasculature and microenvironment: Targets for
anti-angiogenesis and normalization. Microvasc Res. 74:72–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu S, Yu M, He Y, Xiao L, Wang F, Song C,
Sun S, Ling C and Xu Z: Melittin prevents liver cancer cell
metastasis through inhibition of the Rac1-dependent pathway.
Hepatology. 47:1964–1973. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Y, Li XJ, Sui X, Tang XJ, Qin H and
Ren H: Expression and significance of PCNA and Caspase-3 in the
tissue of lung cancer. Xi Bao Yu Fen ZiMian Yi XueZa Zhi.
26:154–156. 2010.(In Chinese).
|
|
29
|
Yang XH and Zou L: Dual functions of DNA
replication forks in checkpoint signaling and PCNA ubiquitination.
Cell Cycle. 8:191–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen H, Feng G, Cui J, Du Q, Qin Y, Cai J,
Shen L and Zhu Y: Clinical implications of serum hypoxia inducible
factor-1á and vascular endothelial growth factor inlung cancer.
Tumori. 101:404–411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Folkman J: Angiogenesis: An organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ng KT, Guo DY, Cheng Q, Geng W, Ling CC,
Li CX, Liu XB, Ma YY, Lo CM, Poon RT, et al: A garlic derivative,
S-allylcysteine (SAC), suppresses proliferation and metastasis of
hepatocellular carcinoma. PLoS ONE. 7:e316552012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Tang ZY, Qin LX, Wu XF, Sun HC,
Xue Q and Ye SL: High-dose and long-term therapy with
interferon-alfa inhibits tumor growth and recurrence in nude mice
bearing human hepatocellular carcinoma xenografts with high
metastatic potential. Hepatology. 32:43–48. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bremnes RM, Camps C and Sirera R:
Angiogenesis in non-small cell lung cancer: The prognostic impact
of neoangiogenesis and the cytokines VEGF and bFGF in tumours and
blood. Lung Cancer. 51:143–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liao M, Wang H, Lin Z, Feng J and Zhu D:
Vascular endothelial growth factor and other biological predictors
related to the postoperative survival rate on non-small cell lung
cancer. Lung Cancer. 33:125–132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ushijima C, Tsukamoto S, Yamazaki K,
Yoshino I, Sugio K and Sugimachi K: High vascularity in the
peripheral region of non-small cell lung cancer tissue is
associated with tumor progression. Lung Cancer. 34:233–241. 2001.
View Article : Google Scholar : PubMed/NCBI
|