Introduction
Epidemiological studies have suggested that gastric
cancer is predominant in males, and that the ratio of morbidity of
gastric cancer between male and female patients is 2:1–3:1
(1–3).
The differences between the genders become negligible when female
patients reach the menopause, and the morbidity associated with
gastric cancer was reported to decrease in men who had been treated
with estrogen for prostate carcinoma (1–3). These
findings suggested that estrogen has a positive association with
gastric cancer, although the underlying reasons are unclear.
Estrogen acts by binding to its ligand (4,5). Previous
studies reported that estrogen receptors (ERs) were expressed in
the tumors of estrogen-independent organs, including the stomach,
which indicates that, in these organs, the occurrence and
development of a tumor is associated with estrogen (6–10).
ER-α36 is a novel ER variant identified by Professor
Zhaoyi Wang (4), and whose molecular
weight is 35.7 kDa. The difference between ER-α36 and the
traditional ER is that ER-α36 lacks intrinsic transcriptional
activity due to the loss of the activation function (AF) 1 and AF2
domains (4,5). Previous studies have demonstrated that
ER-α36 is located at the cytomembrane, where is involved in various
biological processes, including cell differentiation, proliferation
and apoptosis, by mediating rapid signal transduction (5). There are few reports regarding the
function, mechanism and clinical significance of ER-α36 in gastric
cancer, although previous studies suggested that ER-α36 has a
central role in balancing the proliferation and apoptosis of
gastric cancer cells (11–14). 17β-estradiol, which is a type of
agonist of the ER, has a critical role in physiological processes
by binding to its ligand to mediate the expression of various genes
(15–17). Conversely, tamoxifen, which is a
non-steroidal triphenylethylene, affects the proliferation and
apoptosis of cells by selectively competing with estrogen for the
ER binding site, thus altering the expression levels of various
cytokines (18–20). The present study aimed to investigate
the effects of 17β-estradiol and tamoxifen on the proliferation and
apoptosis of gastric cancer cell lines cultured in vitro. In
addition, the role of ER-α36 in the proliferation and apoptosis of
gastric cancer cells was evaluated by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Materials and methods
Reagents
The BGC823 human gastric cancer and MCF-7 human
breast adenocarcinoma cell lines were purchased from the Institute
of Basic Medical Sciences at the Chinese Academy of Medical
Sciences (Beijing, China). The MKN45 and SGC7901 human gastric
cancer cell lines were donated by the Department of Immunology at
Huazhong University of Science and Technology (Wuhan, China).
Gibco® RPMI-1640 medium and fetal bovine serum (FBS)
were purchased from Thermo Fisher Scientific, Inc., (Waltham, MA,
USA). Trypsin was purchased from Sangon Biotech, Co., Ltd.,
(Shanghai, China) and glutamine was obtained from Ameresco, LLC
(Solon, OH, USA). 17β-estradiol and tamoxifen were purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). The
water-soluble tetrazolium (WST)-1 kit was purchased from Beyotime
Institute of Biotechnology (Haimen, China). The RT-PCR kit and PCR
primers were purchased from Sangon Biotech, Co., Ltd. The
THUNDERBIRD® SYBR® qPCR Mix was purchased
from Toyobo Co., Ltd. (Osaka, Japan). The Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) Apoptosis Detection kit
was purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing,
China).
Cell culture
BGC823, SGC7901, MKN45 and MCF-7 cells were cultured
in RPMI-1640 medium supplemented with 10% FBS at 5% CO2
and 37°C. The cells were digested using trypsin and passaged upon
reaching 70–80% confluence, followed by passaging every 2–3 days.
BGC823 cells were digested and plated at a density of
1×105 cells/well onto Costar® 6-well plates
in RPMI-1640 medium containing 10% FBS at 37°C. After 24 h, the
culture medium was removed, and the cells were washed twice with
phosphate-buffered saline, followed by culturing in phenol red-free
RPMI-1640 medium containing 1% charcoal-stripped FBS for 6 h at
37°C. Subsequently, BGC823 cells were cultured in the same medium
containing various concentrations of 17β-estradiol or tamoxifen for
24 or 48 h at 37°C. The MCF-7 control cells were cultured in medium
containing 1:1,000 absolute ethyl alcohol at 37°C. 17β-estradiol
and tamoxifen, which were dissolved in absolute ethyl alcohol and
stored at a concentration of 10−5 and 10−2
mol/l, respectively, at −20°C, were diluted prior to use. BGC823
cells were treated with 10−12, 10−11 or
10−10 mol/l 17β-estradiol, or with 5×10−6 or
1×10−5 mol/l tamoxifen, for 24 or 48 h.
Nested RT-PCR
Total RNA was extracted from BGC823 cells upon
reaching 80% confluence, using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol,
and was reverse transcribed into complementary (c)DNA using an
RT-PCR kit. The RT system (25 µl) consisted of the forward and
reverse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers,
and the forward and reverse ER-α36 first and second nested primers.
The primer sequences were as follows: GAPDH (452 bp) forward,
5′-ACCACAGTCCATGCCATCAC-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3;
ER-α36 first nested primer (290 bp) forward,
5′-CAAGTGGTTTCCTCGTGTCTAAAG-3′ and reverse,
5′-TGTTGAGTGTTGGTTGCCAGG-3′; and ER-α36 second nested primer (219
bp) forward, 5′-TGGTTTCCTCGTGTCTAA-3′ and reverse,
5′-CAAAGTTTGTGGGTAGCT-3′. The first nested PCR system consisted of
2 µl cDNA, 2 µl first nested primer, 1.0 µl MgCl2, 2 µl
10X PCR buffer, 0.2 µl Taq polymerase (5 U/µl) and 13.2 µl double
distilled (dd)H2O in a total volume of 20 µl. The
cycling conditions were an initial denaturation for 1 min at 94°C,
followed by 20 cycles consisting of denaturation at 94°C for 45
sec, an annealing step at 53°C for 45 sec and an extension step at
72°C for 60 sec. This was followed by a final extension at 72°C for
10 min. The second nested PCR system consisted of 2 µl cDNA, 2 µl
each of the forward and reverse GAPDH primers, 1.5 µl
MgCl2, 2 µl 10X PCR buffer (15 mmol/l), 0.1 µl Taq
polymerase (5 U/µl) and 12.4 µl sterile ddH2O. The
cycling conditions for this reaction system were an initial
denaturation for 1 min at 94°C, followed by 15 cycles consisting of
denaturation at 94°C for 45 sec, an annealing step at 55°C for 45
sec and an extension step at 72°C for 60 sec. This was followed by
a final extension at 72°C for 10 min. PCR products were separated
by 1.5% agarose gel electrophoresis and visualized by ethidium
bromide staining under ultraviolet illumination.
WST-1 assay for assessment of cell
proliferation
Exponential phase BGC823 cells were digested using
trypsin and plated at a density of 3×103 cells/well onto
96-well plates. Each group included five parallel wells. To each
well, 20 µl WST-1 solution was added and, after 24 or 48 h, the
absorbance at 450 nm was measured using a microplate reader after
culturing for a further 2 h in an incubator at 5% CO2
and 37°C.
RNA extraction and quantitative
(q)PCR
Total RNA was extracted from gastric cancer cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Total
RNA was reverse transcribed into cDNA using an RT-PCR kit. qPCR was
performed on a StepOnePlus Real-Time PCR System (Thermo Fisher
Scientific, Inc.) using the THUNDERBIRD®
SYBR® qPCR Mix. The qPCR consisted of 35 cycles of 94°C
for 30 sec, 56°C for 30 sec and 72°C for 30 sec. ER-α36 and β-actin
primers were designed using Primer Premier 5.0 software (Premier
Biosoft International, Palo Alto, CA, USA), and had the following
sequences: ER-α36 forward, 5′-ACAAGTGGTTTCCTCGTGTCTAA-3′ and
reverse, 5′-GGGTGTTGAGTGTTGGTTGC-3′; and β-actin forward,
5′-ATGATGATATCGCCGCGCTC-3′; and reverse,
5′-GTACATGGCTGGGGTGTTGA-3′. β-actin was used as an internal
invariant endogenous control for qPCR. Expression levels were
determined using the relative 2(−∆∆C(T)) method. All experiments
were performed at least three times to ensure the reproducibility
of the results.
Apoptosis assay
The cells were stained using the Annexin V-FITC/PI
Apoptosis Detection kit, according to the manufacturer's protocol,
and apoptotic cells, including early apoptotic cells (Annexin
V+/PI−) and necrotic or late apoptotic cells
(Annexin V+/PI+), were measured by flow
cytometry. Briefly, BGC823 cells were treated with 17β-estradiol or
tamoxifen for 24 or 48 h, and subsequently, the cells were
collected and resuspended in phenol red-free RPMI-1640 medium with
1% charcoal-stripped FBS at a density of 1×106 cells/ml.
Next, the cells were stained with 5 µl Annexin V-FITC and 5 µl PI
in 300 µl binding buffer (10 mmol/l
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4, 140
mmol/l NaOH and 2.5 mmol/l CaCl2) for 15 min at room
temperature in the dark. Quantification of apoptotic cells was
performed using a flow cytometer (FACScan; Beckman Coulter, Inc.,
Brea, CA, USA).
Statistical analysis
Statistical analyses were performed using SPSS 12.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard deviation of three replicate samples, and
differences were compared using the Student's t-test or one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference. All experiments were
performed at least three times to ensure the reproducibility of the
results.
Results
Expression of ER-α36 in various human
gastric cancer cell lines
The expression of ER-α36 messenger (m)RNA in BGC823,
MKN45 and SGC7901 human gastric cancer cell lines was determined by
semiquantitative nested RT-PCR, using the MCF-7 cell line as a
positive control. Notably, ER-α36 mRNA expression was positive in
the gastric cancer cell lines, and there was no detection of gene
amplification (Fig. 1). These results
suggest that ER-α36 is highly expressed in gastric cancer
cells.
WST-1-based detection of cell
proliferation in gastric cancer cells treated with an ER-α36
agonist or inhibitor
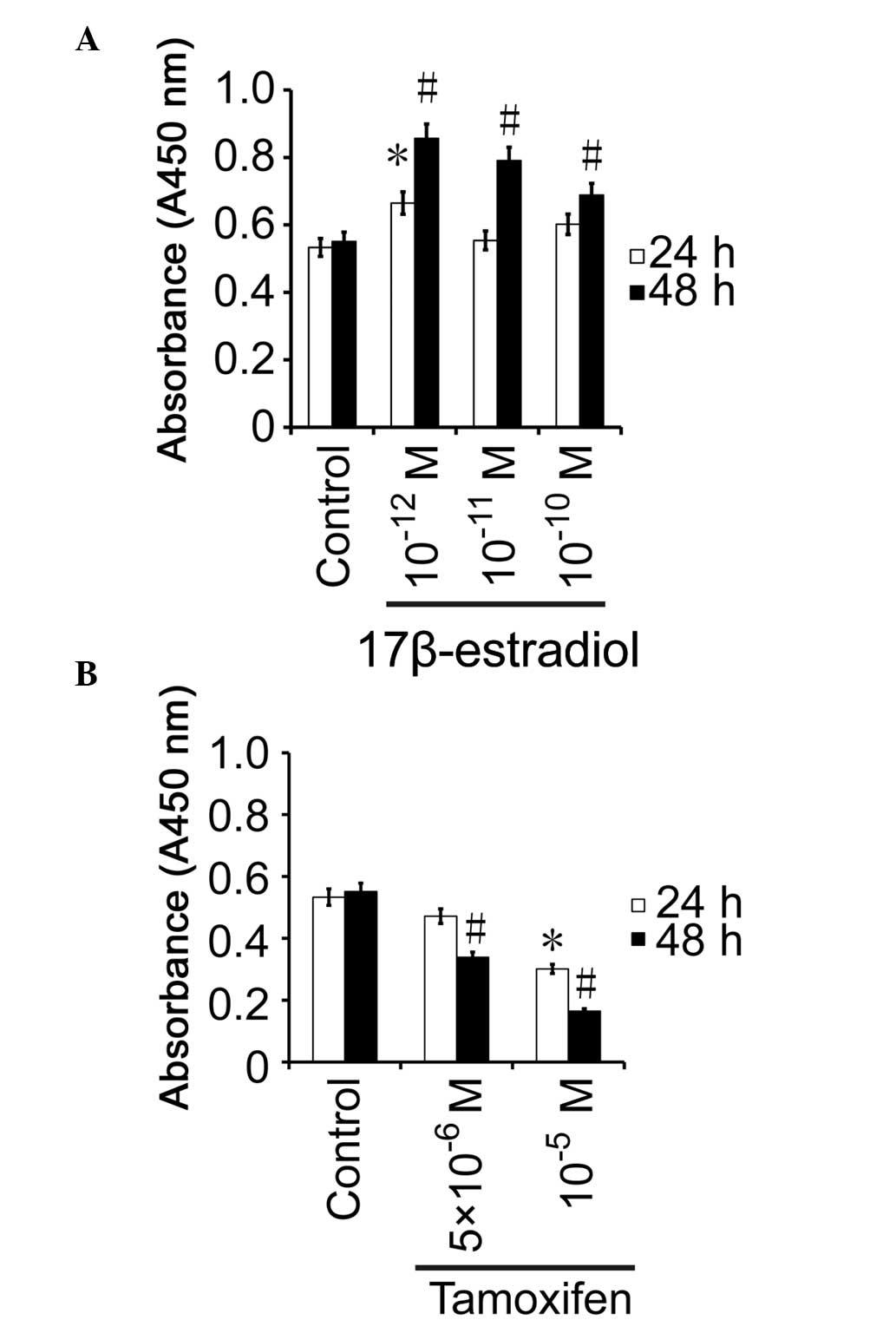
BGC823 cells were stimulated with various
concentrations of 17β-estradiol or tamoxifen for 24 or 48 h, and
WST-1 assays were then performed to assess cell proliferation. In
the cells treated with 10−12 mol/l 17β-estradiol for 24
h, proliferation was increased by 13.2% compared with the control
group, and the difference was significant (P=0.013; Fig. 2A). Conversely, the proliferation rates
of the cells treated with 10−11 mol/l (P=0.0841) or
10−10 mol/l (P=0.0735) 17β-estradiol for 24 h were not
significantly different from those exhibited by the control
(P>0.05; Fig. 2A). After 48 h, the
cell growth activity was increased by 30.5, 23.9 and 13.8% in the
10−12 mol/l (P=0.0015), 10−11
mol/l (P=0.0178) and 10−10 mol/l (P=0.0245)
17β-estradiol-treated groups, respectively, as compared with the
control group, and the difference was significant (Fig. 2A). These results suggest that
17β-estradiol promotes the proliferation of gastric cancer cells
in vitro.
There was no significant difference in the cell
proliferation rate between BGC823 cells treated with
5×10−6 mol/l tamoxifen and the control group after 24 h
(P=0.0724; Fig. 2B). After 24 h, the
activity of the BGC823 cells treated with 1×10−5 mol/l
tamoxifen was 56.4% of that displayed by the control group, which
was significantly different (P=0.0233; Fig. 2B). The activity of the BGC823 cells
treated with 5×10−6 mol/l was 61.5% of that of the
control group after 48 h (P=0.0021), and the cell activity of the
1×10−5 mol/l group was 29.9% of that of the control
group after 48 h (P=0.0059), which were significantly different
(Fig. 2B). These results suggest that
tamoxifen inhibits the growth of gastric cancer cells in
vitro.
Gastric cancer cell apoptosis
following treatment with an ER-α36 agonist or inhibitor
The apoptosis rate was significantly reduced in the
BGC823 cells treated with 17β-estradiol for 24 h (10−12
mol/l, P=0.013; 10−11 mol/l, P=0.023; and
10−10 mol/l, P=0.017) and for 48 h (10−12
mol/l, P=0.002; 10−11 mol/l, P=0.011 and
10−10 mol/l, P=0.033) (Fig.3). Conversely, the rates of the
apoptosis were significantly increased in the BGC823 cells as the
tamoxifen concentration increased after 24 h (5×10−6
mol/l, P=0.002; and 5×10−5 mol/l, P=0.001) and after 48
h (5×10−6 mol/l, P=0.014; and 10−5 mol/l,
P=0.0021), as compared with the control group. These results
indicate that tamoxifen inhibits the growth of BGC823 cells,
potentially by promoting gastric cancer cell apoptosis.
Alterations in the expression levels
of ER-α36 following treatment of gastric cancer cells with
17β-estradiol or tamoxifen
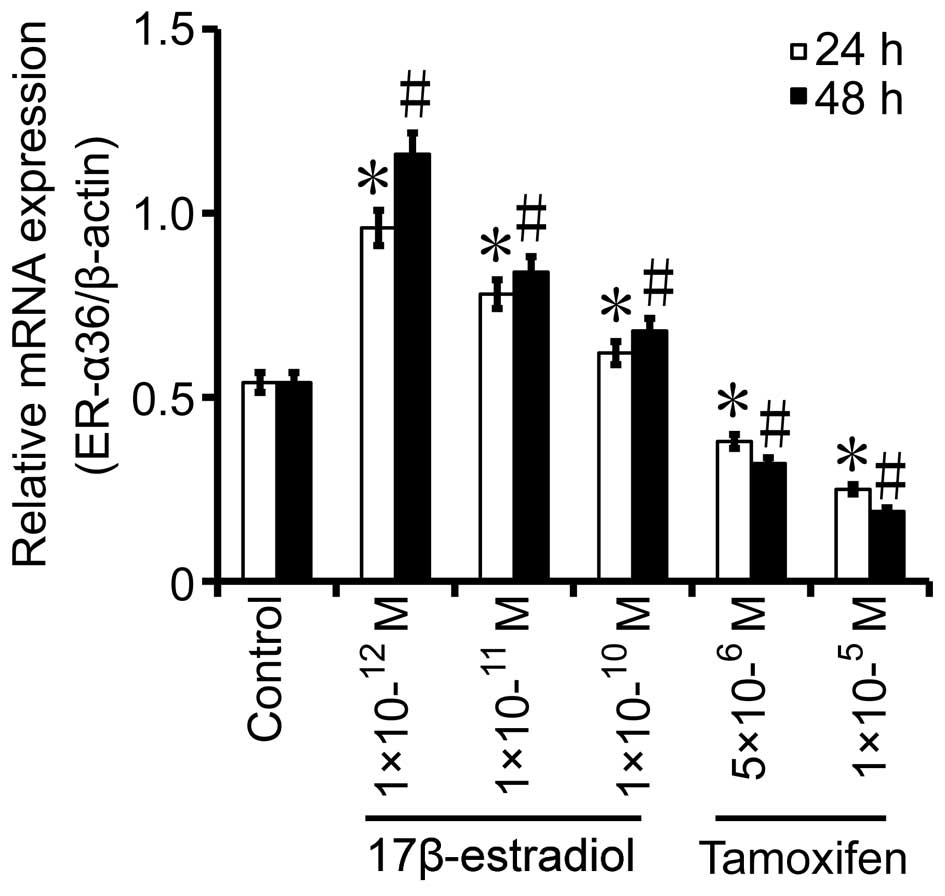
BGC823 cells were treated with various
concentrations of 17β-estradiol or tamoxifen for 24 or 48 h, and
subsequently, the mRNA expression levels of ER-α36 were determined
by RT-qPCR. After 24 h, the mRNA expression levels of ER-α36 in the
10−12 mol/l (P=0.024), 10−11 mol/l
(P=0.0113) and 10−10 mol/l (P=0.0037)
17β-estradiol-treated groups exhibited a fold-change of 1.78, 1.44
and 1.15, respectively, as compared with those in the control
(Fig. 4). After 48 h, the mRNA
expression levels of ER-α36 in the 10−12 mol/l
(P=0.0164), 10−11 mol/l (P=0.0342) and
10−10 mol/l (P=0.0198) 17β-estradiol-treated groups
displayed a fold-change of 2.15, 1.56 and 1.26, respectively, as
compared with those in the control (Fig.
4). Conversely, the mRNA expression levels of ER-α36 were
decreased by 29.6% after 24 h in the 5×10−6 mol/l
tamoxifen-treated group (P=0.0233), as compared with the mRNA
levels observed in the control group, while those in the
1×10−5 mol/l tamoxifen-treated group (P=0.007) were
decreased by 53.7%, as compared with the mRNA levels detected in
the control group (Fig. 4). In the
5×10−6 mol/l tamoxifen-treated group (P=0.001), the mRNA
expression levels of ER-α36 were decreased by 40.7% after 48 h, as
compared with the levels measured in the control group, and those
in the 1×10−5 mol/l tamoxifen-treated group (P=0.0153)
were decreased by 64.8%, as compared with the levels displayed by
the control group (Fig. 4). These
results suggest that tamoxifen downregulates the expression of
ER-α36 in gastric cancer cells.
Discussion
Gastric cancer is a type of gastrointestinal cancer
and, as compared to other gastrointestinal cancers, there is no
fundamental difference in its diagnosis and treatment (21). Recurrence is commonly observed in
patients with advanced gastric cancer who have missed the
opportunity for surgical resection and have instead undergone
non-radical surgery. Although novel methods for the targeted
treatment of gastric cancer are abundant, reports regarding the
effects of these treatments have been less than satisfactory
(21). It is not a coincidence that
the incidence of gastric cancer is higher in males than in females
(1–3).
The present study aimed to investigate the role of estrogen in
gastric cancer, in order to aid the development of better
prophylactic and therapeutic strategies for gastric cancer and to
improve the understanding of its ontogenesis.
Estrogen performs its biological functions by
binding to the ER, which belongs to a family of receptors
consisting of α and β subtypes. The ER exerts its function via the
estrogen response element (ERE) and activating protein-1 in its
target genes. It is considered that the ER is a ligand-dependent
transcriptional activator (4,5). It has been demonstrated that
membrane-bound ER quickly activates an intracellular second
messenger to exert its biological functions in numerous cell types
(5,22–24).
ER-α36 is a novel subtype of ER that was discovered
and cloned by Professor Zhaoyi Wang, and whose molecular weight is
35.7 kDa. As compared with the traditional ER, ER-α36 does not
participate in the activation of the ERE due to loss of the AF1 and
AF2 domains (4,5). However, ER-α36 possesses three
myristoylation sites, including amino acids 25–30 [glycine
(Gly)-valine-tryptophan-serine-cysteine-glutamate (Glu)], 76–81
[Gly-methionine (Met)-Met-lysine-Gly-Gly] and 171–176 [Glu-leucine
(Leu)-Leu-threonine-asparagine-Leu], which are associated with the
receptor's location at the membrane (5). Wang et al (4) reported that the phosphorylation of
extracellular signal-regulated kinase (ERK) 1/2 was increased
following stimulation of HEK-293 cells overexpressing ER-α36 with
17β-estradiol for 5 min. The level of ERK1/2 phosphorylation peaked
at 30 min, and then commenced to decline, which may suggest that
ER-α36 promotes the proliferation of cells by activating the
mitogen-activated protein kinase (MAPK)/ERK signaling pathway
(15).
The present study demonstrated that a low
concentration of 17β-estradiol was able to promote the
proliferation of gastric cancer cells in vitro, and that the
proliferation of these cells was negatively correlated with the
concentration of 17β-estradiol. Low-dose 17β-estradiol displayed an
enhanced ability to promote the proliferation of gastric cancer
cells, as compared with high concentrations of 17β-estradiol. These
results were consistent with the findings of previous
epidemiological studies, in which lower levels of estrogen in males
were associated with higher morbidity of gastric cancer in males
compared with females (1). In
addition, in the present study, the proliferation of gastric cancer
cells was inhibited by tamoxifen in a concentration- and
time-dependent manner. These results suggested that gastric cancer
cells were sensitive to estrogen, and that gastric cancer tumors
are estrogen-responsive. Furthermore, the rate of apoptosis was
increased in gastric cancer cells treated with tamoxifen, thus
indicating that tamoxifen induces gastric cancer cell apoptosis
in vitro.
The present study demonstrated that the
proliferation of gastric cancer cells was increased to a greater
extent following stimulation with lower concentrations of
17β-estradiol than using higher concentrations of this molecules.
In addition, the current study demonstrated that the mRNA
expression levels of ER-α36 were increased in the
17β-estradiol-treated group compared with the control group at all
times, particularly when 17β-estradiol was obviously promoting cell
proliferation. Tamoxifen was observed to induce gastric cancer cell
apoptosis in vitro, and its concentration was negatively
correlated with the expression of ER-α36. The apoptosis of gastric
cancer cells was more obvious, and their mRNA expression levels of
ER-α36 were decreased to a greater extent, which indicated that
ER-α36 is important in the balance between proliferation and
apoptosis of gastric cancer cells.
Since the ER is located at the cell membrane, ER-α36
may activate members of the MAPK family. MAPK is the main
transducer of information from the cell surface to the nucleus
(4). In eukaryotic cells, at least
four types of MAPK signal transducers have been reported, including
ERK, c-Jun N-terminal kinase (JNK), P38 and ERK5 (24). It has been hypothesized that JNK
mediates the apoptosis of cells in the emergency response, thus
inhibiting apoptosis and promoting proliferation when the ERK
cascade is dominant, while initiating apoptosis when the JNK
cascade is dominant (25–33).
In conclusion, the present study demonstrated that
low concentrations of 17β-estradiol were able to promote ER-α36
expression in gastric cancer cells, which in turn led to their
increased proliferation, potentially via activation of the MAPK
signaling pathway. Conversely, high concentrations of tamoxifen
downregulated ER-α36 expression, which led to the apoptosis of
gastric cancer cells. The aforementioned results indicated that a
high concentration of tamoxifen could be important in the curative
treatment of stomach cancer. Further studies are required to
validate the results of the present study.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant nos.
81470110, 81272754 and 30870981), the National Natural Science
foundation of Guangxi (grant no. 0848014) and the Science
Foundation of the Health Office of Hubei Province (Wuhan, China;
grant no. WJ2015Z059).
References
|
1
|
Chandanos E and Lagergren J: The mystery
of male dominance in oesophageal cancer and the potential
protective role of oestrogen. Eur J Cancer. 45:3149–3155. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung HW, Noh SH and Lim JB: Analysis of
demographic characteristics in 3242 young age gastric cancer
patients in Korea. World J Gastroenterol. 16:256–263. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chandanos E and Lagergren J: Oestrogen and
the enigmatic male predominance of gastric cancer. Eur J Cancer.
44:2397–2403. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, Zhang X, Shen P, Loggie BW, Chang
Y and Deuel TF: Identification, cloning, and expression of human
estrogen receptor-alpha36, a novel variant of human estrogen
receptor-alpha66. Biochem Biophys Res Commun. 336:1023–1027. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Z, Zhang X, Shen P, Loggie BW, Chang
Y and Deuel TF: A variant of estrogen receptor-{alpha},
hER-{alpha}36: Transduction of estrogen- and antiestrogen-dependent
membrane-initiated mitogenic signaling. Proc Natl Acad Sci USA.
103:9063–9068. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saqui-Salces M, Neri-Gomez T,
Gamboa-Dominguez A, Ruiz-Palacios G and Camacho-Arroyo I: Estrogen
and progesterone receptor isoforms expression in the stomach of
Mongolian gerbils. World J Gastroenterol. 14:5701–5706. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuyama S, Ohkura Y, Eguchi H, Kobayashi
Y, Akagi K, Uchida K, Nakachi K, Gustafsson JA and Hayashi S:
Estrogen receptor beta is expressed in human stomach
adenocarcinoma. J Cancer Res Clin Oncol. 128:319–324. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang M, Pan JY, Song GR, Chen HB, An LJ
and Qu SX: Altered expression of estrogen receptor alpha and beta
in advanced gastric adenocarcinoma: Correlation with prothymosin
alpha and clinicopathological parameters. Eur J Surg Oncol.
33:195–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kameda C, Nakamura M, Tanaka H, Yamasaki
A, Kubo M, Tanaka M, Onishi H and Katano M: Oestrogen
receptor-alpha contributes to the regulation of the hedgehog
signalling pathway in ERalpha-positive gastric cancer. Br J Cancer.
102:738–747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motohashi M, Wakui S, Muto T, Suzuki Y,
Shirai M, Takahashi H and Hano H: Cyclin D1/cdk4, estrogen
receptors alpha and β, in
N-methyl-N'-nitro-N-nitrosoguanidine-induced rat gastric
carcinogenesis: Immunohistochemical study. J Toxicol Sci.
36:373–378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Deng H, Zou F, Fu Z, Chen Y, Wang
Z and Liu L: ER-α36-mediated gastric cancer cell proliferation via
the c-Src pathway. Oncol Lett. 6:329–335. 2013.PubMed/NCBI
|
|
12
|
Fu Z, Deng H, Wang X, Yang X, Wang Z and
Liu L: Involvement of ER-α36 in the malignant growth of gastric
carcinoma cells is associated with GRP94 overexpression.
Histopathology. 63:325–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Huang X, Fu Z, Zou F, Li Y, Wang Z
and Liu L: Biphasic ER-α36-mediated estrogen signaling regulates
growth of gastric cancer cells. Int J Oncol. 45:2325–2330.
2014.PubMed/NCBI
|
|
14
|
Deng H, Huang X, Fan J, Wang L, Xia Q,
Yang X, Wang Z and Liu L: A variant of estrogen receptor-alpha,
ER-alpha36 is expressed in human gastric cancer and is highly
correlated with lymph node metastasis. Oncol Rep. 24:171–176.
2010.PubMed/NCBI
|
|
15
|
Nethrapalli IS, Tinnikov AA, Krishnan V,
Lei CD and Toran-Allerand CD: Estrogen activates mitogen-activated
protein kinase in native, nontransfected CHO-K1, COS-7, and RAT2
fibroblast cell lines. Endocrinology. 146:56–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zappa L, Savady R and Sugarbaker PH:
Gastric perforation following cytoreductive surgery with
perioperative intraperitoneal chemotherapy. J Surg Oncol.
101:634–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Acconcia F and Marino M: The Effects of 17
β-estradiol in cancer are mediated by estrogen receptor signaling
at the plasma membrane. Front Physiol. 2:302011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou L, Cai B, Bao W, He YY, Chen XY, Yang
YX, Liu XL and Wan XP: Crosstalk between estrogen receptor and
mitogen-activated protein kinase signaling in the development and
progression of endometrial cancer. Int J Gynecol Cancer.
21:1357–1365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anbalagan M, Carrier L, Glodowski S,
Hangauer D, Shan B and Rowan BG: KX-01, a novel Src kinase
inhibitor directed toward the peptide substrate site, synergizes
with tamoxifen in estrogen receptor α positive breast cancer.
Breast Cancer Res Treat. 132:391–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mabuchi S, Ohmichi M, Kimura A, Ikebuchi
Y, Hisamoto K, Arimoto-Ishida E, Nishio Y, Takahashi K, Tasaka K
and Murata Y: Tamoxifen inhibits cell proliferation via
mitogen-activated protein kinase cascades in human ovarian cancer
cell lines in a manner not dependent on the expression of estrogen
receptor or the sensitivity to cisplatin. Endocrinology.
145:1302–1313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK,
Jeon TJ, Kim JM, Kim YI, Ryu KW, Kong SH, et al: Clinical practice
guidelines for gastric cancer in Korea: An evidence-based approach.
J Gastric Cancer. 14:87–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fox EM, Andrade J and Shupnik MA: Novel
actions of estrogen to promote proliferation: Integration of
cytoplasmic and nuclear pathways. Steroids. 74:622–627. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong CW, McNally C, Nickbarg E, Komm BS
and Cheskis BJ: Estrogen receptor-interacting protein that
modulates its nongenomic activity-crosstalk with Src/Erk
phosphorylation cascade. Proc Natl Acad Sci USA. 99:14783–14788.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lei YY, Wang WJ, Mei JH and Wang CL:
Mitogen-activated protein kinase signal transduction in solid
tumors. Asian Pac J Cancer Prev. 15:8539–8548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shlevkov E and Morata G: A
dp53/JNK-dependant feedback amplification loop is essential for the
apoptotic response to stress in Drosophila. Cell Death Differ.
19:451–460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu HC, Yang CY, Hung DZ, Su CC, Chen KL,
Yen CC, Ho TJ, Su YC, Huang CF, Chen CH, et al: Nickel(II) induced
JNK activation-regulated mitochondria-dependent apoptotic pathway
leading to cultured rat pancreatic β-cell death. Toxicology.
289:103–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pedram A, Razandi M, Sainson RC, Kim JK,
Hughes CC and Levin ER: A conserved mechanism for steroid receptor
translocation to the plasma membrane. J Biol Chem. 282:22278–22288.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qu JL, Qu XJ, Zhao MF, Teng YE, Zhang Y,
Hou KZ, Jiang YH, Yang XH and Liu YP: Gastric cancer exosomes
promote tumour cell proliferation through PI3K/Akt and MAPK/ERK
activation. Dig Liver Dis. 41:875–880. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Razandi M, Pedram A, Merchenthaler I,
Greene GL and Levin ER: Plasma membrane estrogen receptors exist
and functions as dimers. Mol Endocrinol. 18:2854–2865. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lau WS, Chen WF, Chan RY, Guo DA and Wong
MS: Mitogen-activated protein kinase (MAPK) pathway mediates the
oestrogen-like activities of ginsenoside Rg1 in human breast cancer
(MCF-7) cells. Br J Pharmacol. 156:1136–1146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu RW, Yow CM, Wong CK and Lam YH:
Photodynamic therapy (PDT)-Initiation of apoptosis via activation
of stress-activated p38 MAPK and JNK signal pathway in H460 cell
lines. Photodiagnosis Photodyn Ther. 8:254–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu WK, Cho CH, Lee CW, Wu YC, Yu L, Li ZJ,
Wong CC, Li HT, Zhang L, Ren SX, et al: Macroautophagy and ERK
phosphorylation counteract the antiproliferative effect of
proteasome inhibitor in gastric cancer cells. Autophagy. 6:228–238.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chuang SM, Wang IC and Yang JL: Roles of
JNK, p38 and ERK mitogen-activated protein kinases in the growth
inhibition and apoptosis induced by cadmium. Carcinogenesis.
21:1423–1432. 2000. View Article : Google Scholar : PubMed/NCBI
|