Introduction
Ovarian cancer is the fourth leading cause of
cancer-associated mortality in women in western countries (1). The incidence of epithelial ovarian
cancer (EOC)-associated mortality in the United States is estimated
to be 15,280 cases per year, with 22,430 newly diagnosed cases of
EOC (1). Surgical removal and
chemotherapy are the mainstays of treatment for ovarian cancer.
However, despite initial responses, the majority of patients
eventually develop relapsed disease (2). The pathogenesis of ovarian cancer is a
complex process, which involves interactions among inflammatory
cells, the environment and hereditary factors. It is also clear
that the body's immune system is important in protecting the host
from ovarian cancer (3–7). The interaction between ovarian cancer
and the immune system is complex, including mechanisms of immune
suppression and immune activation (8).
Cancer-associated inflammation is associated with
several aspects of malignancy, including the survival and
proliferation of malignant cells, tumor angiogenesis and metastasis
(9). In the tumor microenvironment,
the most abundant immune cell population is that of the
tumor-associated macrophages (TAMs) (10). TAMs are derived from monocytes
circulating in the blood, and can be recruited to the ovarian tumor
site by certain molecules, including transforming growth factor-β,
vascular endothelial growth factor (VEGF) and C-C motif chemokine
ligand 5, (11). TAMs affect certain
aspects of tumor biology and resemble M2-polaized macrophages in
the tumor microenvironment. TAMs are known to have an
immunosuppressive role in ovarian cancer, which is associated with
poor outcomes (4).
TAMs comprise a large group of the immune cells in
the ovarian cancer microenvironment and are capable of regulating T
cell differentiation (12,13). However, the mechanisms affecting TAMs
and regulatory T cells (Treg cells) remain to be elucidated in
ovarian cancer. Treg cells are characterized by a
CD4+CD25+ forkhead box P3 (Foxp3+)
phenotype (14). T helper cell 17
(Th17 cell) is a CD4+ T helper lymphocyte, which
secretes interleukin (IL)-17 (15).
Treg cells and Th17 cells share a relevant differentiation pathway
from CD4+ precursors. The balance between Treg cells and
Th17 cells is important to the maintenance of immune homeostasis
(16). Increased numbers of Treg
cells have been reported in several tumors, including those of
colorectal cancer, gastric cancer, pancreatic cancer, lung cancer
and ovarian cancer (7,17,18).
Furthermore, it has been reported that depleting Treg cells can
result in antitumor immunity and reduce tumor growth (19). Thus, Treg cells and Th17 cells may
interact to shape the immune environment in ovarian cancer.
The aim of the present study was to evaluate the
distribution of TAMs, Treg cells and Th17 cells, the ratio of
Treg/Th17 cells and the microvessel density (MVD) in tissues from
patients with benign ovarian tumors and EOC, and to determine their
association with the clinical pathology of EOC.
Materials and methods
Patients and tissue specimens
The present study included tissue specimens from 126
patients with EOC (mean age, 51.40 years) and tissue specimens from
26 patients with benign ovarian tumors (mean age, 52.15 years).
Formalin-fixed and frozen sections of tissue specimens of all cases
were obtained from Shanghai First Maternity and Infant Hospital,
Tongji University (Shanghai, China). The tissue samples were
obtained during surgical resection from January 2009 to December
2014. The major clinical and pathological characteristics of the
152 patients are listed in Table I.
Only tissue samples of the central areas of EOC were used, and
metastases were excluded. The present study was approved by the
Institutional Review Board of the First Maternity and Infant
Hospital Affiliated to Tongji University. Written informed consent
was obtained from all patients.
 | Table I.Clinical and pathological
characteristics of the 152 patients. |
Table I.
Clinical and pathological
characteristics of the 152 patients.
| Characteristic | N | % |
|---|
| Diagnosis |
|
|
| Benign
tumor | 26 | 17.11 |
|
Invasive carcinoma | 126 | 82.89 |
| Tumor grade |
|
|
| I | 12 |
9.52 |
| II | 37 | 29.37 |
|
III | 77 | 61.11 |
| Clinical stage |
|
|
| I | 34 | 26.98 |
| II | 30 | 23.81 |
|
III | 61 | 48.41 |
| IV |
1 |
0.79 |
| Histological
type |
|
|
|
Serous | 81 | 64.29 |
|
Mucinous | 14 | 11.11 |
|
Endometrioid | 11 |
8.73 |
| Clear
cell | 20 | 15.87 |
Immunofluorescence confocal
microscopy
The tissue samples were obtained during surgical
resection, approved by the Shanghai First Maternity and Infant
Hospital. The frequency of TAMs was evaluated using the F4/80
marker. The frequency of Treg cells was evaluated using CD4 and
Foxp3 markers, and the frequency of Th17 cells was evaluated using
CD4 and IL-17 markers. The frequencies of TAMs, Treg cells, Th17
cells were calculated, and the ratio of Treg/Th17 was determined in
five randomly selected high power fields per tumor tissue (original
magnification, ×400). The tissue samples were embedded in O.C.T,
and 10 µm sections were prepared. The slides were fixed with 4%
paraformaldehyde and treated with 0.2% Triton X-100 (Shenggong
Biotech, Shanghai, China) for 5 min at room temperature. The slides
were blocked with 10% goat serum (Amresco, LLC, Solon, OH, USA) and
then incubated with the following antibodies: Rat anti-human F4/80
(1:50; Abcam, Cambridge, MA, USA), mouse anti-human CD4 (1:200; EMD
Millipore, Billerica, MA, USA), rat anti-human Foxp3 (1:100; Abcam)
and rabbit anti-human IL-17A (1:500; Abcam), followed by incubation
with Cy3-conjugated goat anti-rat IgG for F4/80 and Foxp3 detection
(1:250; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA,
USA), Alexa Flour 488-conjugated goat anti-mouse IgG for the
detection of CD4 (1:250; Jackson, ImmunoResearch Laboratories,
Inc), and Alexa Flour 647-conjugated goat anti-rabbit IgG for the
detection of IL-17A (1:250; Jackson ImmunoResearch Laboratories,
Inc.). For the primary antibodies, the tissues were incubated in
PBS overnight at 4°C. For the secondary antibodies, the tissues
were incubated in PBS for 60 min at 37°C. The cell nuclei were
stained with DAPI (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) for 10 min at 37°C. Images were captured with a ZEISS LSM
510 scanning confocal microscope (Zeiss AG, Oberkochen,
Germany).
Analysis of MVD via
immunohistochemical staining for CD31
For immunohistochemical analysis of the tumor
samples from patients with benign ovarian tumors and EOC, samples
were collected and embedded in O.C.T for frozen section analysis.
Sections of 10 µm were prepared, and stained with hematoxylin and
eosin. The detection of MVD was performed using mouse anti-human
monoclonal CD31 (1:100; Abcam). The tumors tissues were fixed with
4% paraformaldehyde. Sections of 10 µm were prepared, and the
slides were fixed in cold acetone for 20 min. Following PBS washes,
endogenous peroxide was blocked with 3% H2O2
for 10 min at room temperature. The slides were blocked with 10%
normal goat serum for 90 min at room temperature, followed by
incubation with mouse anti-human monoclonal CD31 (1:100; Abcam) in
PBS overnight at 4°C. Biotin-SP-conjugated affinipure goat
anti-mouse IgG (1:600; Jackson ImmunoResearch Laboratories, Inc.)
was added for 30 min at 37°C, and horseradish peroxidase (1:800;
Jackson ImmunoResearch Laboratories) was added for 45 min at 37°C.
Subsequently, the samples were detected using 3,3′-diaminobenzidine
(Sigma-Aldrich; Merck Millipore) as a substrate for 3 min, followed
by counterstaining with hematoxylin (Sigma-Aldrich; Merck
Millipore). The MVD was calculated in five randomly selected high
power fields per tumor tissue (original magnification, ×200).
Vessels with a linear vessel shape or well-defined lumen were
considered to be a blood microvessel. A negative control was also
included by replacing CD31 with PBS. The same conditions were used
as those used for the mouse anti-human monoclonal CD31
antibody.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 22.0; IBM SPSS, Armonk, NY, USA). The data
obtained from the patients with EOC were compared with data from
the patients with benign ovarian tumors. P<0.05 was considered
to indicate a statistically significant difference using the
Mann-Whitney nonparametric test. The frequency of TAMs, ratio of
Treg/Th17 cells and MVD in the EOC tissues of different tumor
grades and histological types were also calculated using the
Mann-Whitney U test. Data are expressed as the mean ± standard
deviation. Graphs were prepared using GraphPad Prism 6 (GraphPad,
Software Inc., La Jolla, CA, USA) and continuous variables in
figures are expressed as the mean ± standard error of the mean.
Results
Frequency of TAMs is high in patients
with EOC
To confirm the frequency of TAMs, tissue samples
from 126 patients with malignant EOC and 26 patients with benign
tumors were analyzed using triple color immunofluorescence confocal
microscopy (Fig. 1A and B). Compared
with the benign tumor tissues (2.05±4.12), the frequency of TAMs,
which were defined as F4/80+ cells, was significantly
higher in the EOC tissues (8.48±10.81), as determined using a
Mann-Whitney U test (P<0.001; Fig.
1C). This result showed that TAMs may be significant in the
progression of EOC.
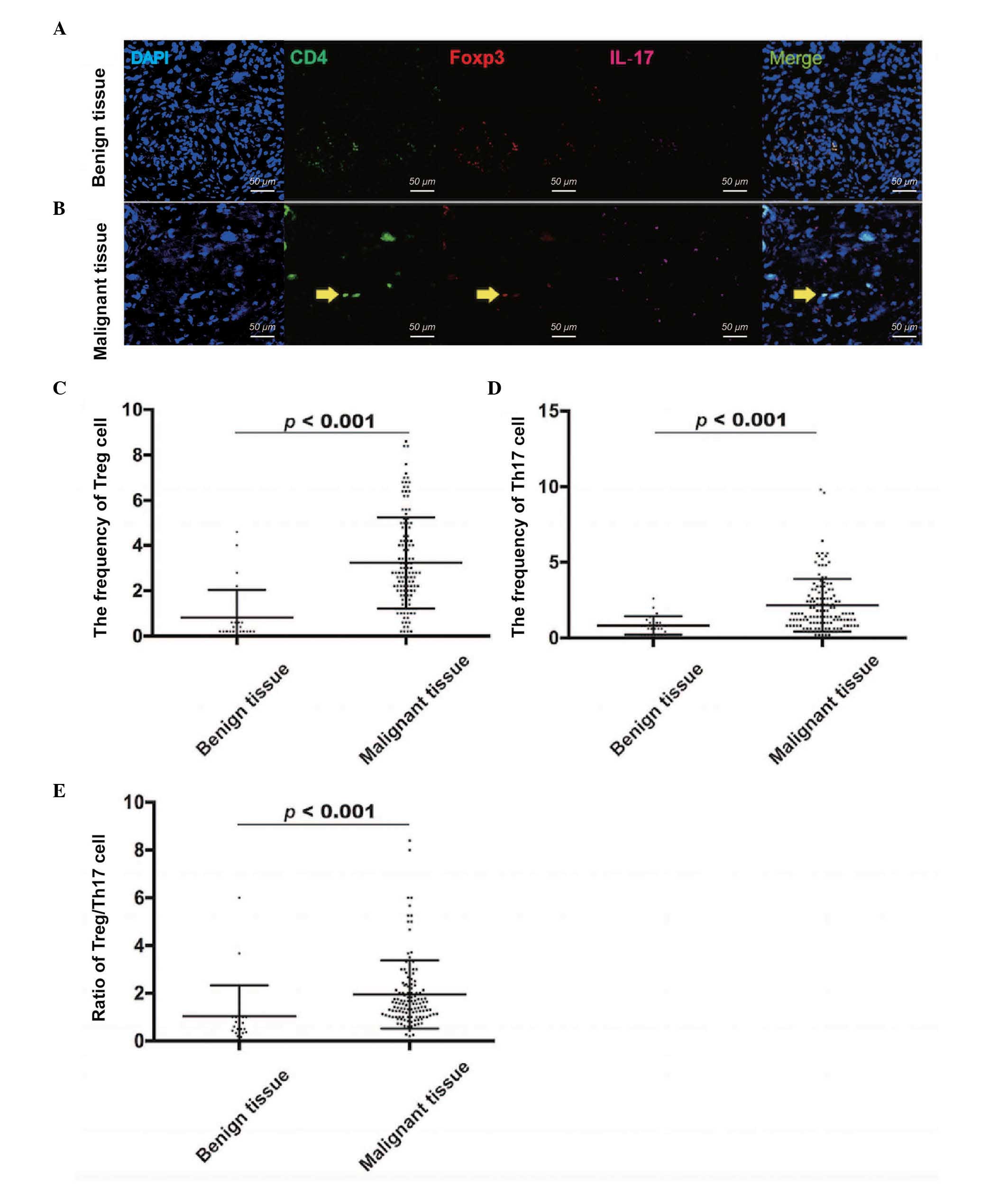
Frequencies of Treg cells and Th17
cells, and the ratio of Treg/Th17 cells are high in patients with
EOC
Tissue samples from the 126 patients with malignant
EOC and 26 patients with benign tumors were also used to confirm
the frequencies of Treg cells and Th17 cells. The frequency of Treg
cells was evaluated using CD4 and Foxp3 markers (Fig. 2A and B), and the frequency of Th17
cells was evaluated using CD4 and IL-17 markers (Fig. 2A and B). Compared with the benign
tumor tissues (0.82±1.21), the frequency of Treg cells was
significantly higher in the EOC tissues (3.23±2.02), as determined
using a Mann-Whitney U test (P<0.001; Fig. 2C). In addition, the frequency of Th17
cells was significantly higher in the EOC tissues (2.15±1.74),
compared with the benign tumor tissues (0.82±0.61; Mann-Whitney U
test; P<0.001; Fig. 2D).
Similarly, the ratio of Treg/Th17 cells was higher in the EOC
tissues (1.95±1.43), compared with the benign tumor tissues
(1.04±1.29; Mann-Whitney U Test; P<0.001; Fig. 2E). Therefore, the results showed that
the distribution of Treg cells and Th17 cells, and the ratio of
Treg/Th17 cells were increased in the EOC microenvironment,
compared with benign tumor microenvironment.
Distribution of TAMs and ratio of Treg/Th17 cells
differ between EOC tumor grades. As mentioned above, the
frequencies of TAMs, Treg cells and Th17 cells, and the ratio of
Treg/Th17 cells in benign tumor tissues and EOC tissues were
evaluated using immunofluorescence. The frequency of TAMs was
significantly higher in tissues of grade III (11.29±12.75; n=77)
tumors, compared with those of grade II (4.95±3.92; n=37) and grade
I (1.33±1.06; n=12) tumors, determined using a Mann-Whitney U test
(P<0.001 for grades I and II; P<0.001 for grades I and III;
P=0.001 for grades II and III; Fig.
3A). Furthermore, the ratio of Treg/Th17 cells was also higher
in grade III tumor tissues (1.81±1.43), compared with that in grade
II tumor tissues (2.24±1.51), determined using the Mann-Whitney U
test (P=0.025; Fig. 3B). However, no
significant difference was found in the ratio of Treg/Th17 cells
between grade I (1.98±1.11) and grade II EOC tissues (P>0.05).
These results suggested that their expression correlated with
ovarian carcinoma formation.
Frequency of TAMs between histological
types of EOC
In the EOC tissues, the frequency of TAMs was higher
in clear cell ovarian cancer (12.17±10.75; n=20), compared with
that in endometrimoid ovarian cancer (5.13±5.68; n=11; Mann-Whitney
U test, P=0.012). The frequency of TAMs was also higher in clear
cell ovarian cancer, compared with mucinous ovarian cancer
(3.47±2.73; n=14; Mann-Whitney U test, P=0.001). The frequency of
TAMs was higher in serous ovarian cancer (8.89±11.85; n=81;
Mann-Whitney U test, P=0.014) compared with that in mucinous
ovarian cancer (Fig. 4). However, no
significant differences in the frequency of Treg cells, Th17 cells
or ratio of Treg/Th17 were found among these subtypes. In addition,
no significant differences (P>0.05) were found in the frequency
of TAMs or ratio of Treg/Th17 cells between early stages
[International Federation of Gynecology and Obstetrics (FIGO)] I
and II] and late stages (FIGO III and IV) (20). These results demonstrated that TAMs
may have a substantial effect on the grade of EOC.
MVD in benign tumor and EOC
tissues
To investigate the angiogenesis in benign tumor
tissues and EOC tissues, the MVDs were evaluated using
immunohistochemistry (Fig. 5A and B).
The MVD in the EOC patient tissues (6.06±5.06) was significantly
higher, compared with that in the benign tumor patient tissues
(3.08±2.85; Mann-Whitney U test, P<0.001; Fig. 5C). In addition, the MVDs were higher
in grade III (6.74±5.65; n=77) tumors, compared with grade I
(3.77±2.94, n=12) tumors (Mann-Whitney U test, P=0.033; Fig. 5D). However, no significant difference
in MVDs were found between grade II (5.39±3.93; n=37) and grade III
EOC patient tissues (P>0.05). Thus, it was hypothesized that
TAMs may promote the progression of ovarian tumor through
angiogenesis.
Discussion
Ovarian cancer is one of the leading causes of
cancer-associated mortality in women with gynecological oncology.
Although surgery and chemotherapy are the mainstays of treatment
for ovarian cancer, the five-year-survival rate of patients with
ovarian cancer is ~40% (21).
Therefore, novel targets require improvement for the treatment of
ovarian cancer. The tumor microenvironment in ovarian cancer may be
a potential therapeutic target and contains several types of immune
cell, which result in tumor progression. TAMs are the most abundant
type of immune cell in the tumor microenvironment. A study by
Colvin (2014) reported that TAMs can create an immunosuppressive
microenvironment and lead to tumor cells evading immune detection
(22). However, which cells TAMs
interact with and the mechanism by which TAMs promote ovarian
cancer remain to be elucidated
Treg cells, which were evaluated using the
CD4+Foxp3+ marker in the present study, are a
specific population of T cells, which mediate homeostatic
peripheral tolerance (23,24) and function as suppressors of
autoimmune reactions (25,26). Treg cells can infiltrate into ovarian
cancer cells and suppress the tumor specific T cell immune
response, which may result in tumor growth. There is evidence that
TAMs and Treg cells mediate the invasiveness of several types of
cancer, including endometrial cancer, breast cancer, prostate
cancer and colorectal cancer (18,27,28). In
addition, studies have reported that TAMs and T cells may be
important in the progression of EOC (28–30). The
present study provided a clinical data that high frequencies of
TAMs, Treg cells, Th17 cells and ratio of Treg/Th17 were
infiltrated into EOC tissues, compared with benign tumor tissues.
These results indicated potential associations among TAMs, Treg
cells, Th17 cells and the ratio of Treg/Th17 cells with the
progression of EOC. Consistent with these findings, previous
studies have reported similar results, reporting an increased
number of TAMs in ovarian cancer, compared with benign tumors
(12,31,32), and
others have reported that Treg cells can be a predictive factor for
prolonged survival rates, with a marked reduction in the mortality
rates of patients with ovarian carcinoma (30).
TAMs and Treg cells have synergistic effects in
promoting ovarian cancer proliferation, tumor angiogenesis and
metastasis (33,34). Therefore, TAMs and Treg cells may be
potential targets for the immunotherapy of EOC. On consideration of
the association between TAMs and Treg cells, the present study
hypothesized that TAMs may be correlated with Treg cells or the
ratio of Treg/Th17 cells in EOC. However, no significant
correlation was found among TAMs, Treg cells, Th17 cells or the
ratio of Treg/Th17 cells in EOC. Therefore, further examination of
the mechanism underlying their function in EOC is required, which
was a limitation of the present study. Furthermore, the frequency
of TAMs and the ratio of Treg/Th17 cells in grade III tumor tissues
were higher, compared with those in low grade EOC tissues. In
accordance, a previous study reported that Treg cells exhibited
increased expression in high grade EOC, compared with low grade EOC
(35).
Previous studies have also reported a correlation
between TAMs and MVD in malignant human tumors (36–39).
Therefore, the present study investigated the trend towards a
higher MVD in EOC tissues, compared with benign tumor tissues.
Similar to the result described above revealing the high expression
of TAMs in the EOC tissues, it was demonstrated and that TAMs
contributed to oncogenesis and neoplasm growth through tumor
angiogenesis. Previous studies have also reported that Treg and
Th17 cells may lead to tumor angiogenesis, by Th17 cells secreting
IL-17 and Treg cells affecting the expression of VEGF (40,41). Thus,
the present study also analyzed the association between the ratio
of Treg/Th17 and MVD. No significant association was found between
the ratio of Treg/Th17 and MVD. This result may be due to
differences in sample size between the EOC and benign ovarian
tumors, and requires further investigation.
Taken together, the present study showed that the
immune system was involved in the progression of ovarian cancer.
Higher frequencies of TAMs, Treg cells, Th17 cells, ratio of
Treg/Th17 cells and MVDs in malignant tissues may be significant in
tumor growth. Further experiments on TAMs, Treg cells and Th17
cells are required in the future for revealing their potential as
immune therapeutic targets in ovarian carcinoma.
Acknowledgements
This study was supported by grants from the National
Science Foundation of China (grant nos. 81372787 and 81072136), and
the Top 100 Medical Elite in Shanghai (grade no. XBR 2011065).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yap TA, Carden CP and Kaye SB: Beyond
chemotherapy: Targeted therapies in ovarian cancer. Nat Rev Cancer.
9:167–181. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dunn GP, Old LJ and Schreiber RD: The
immunobiology of cancer immunosurveillance and immunoediting.
Immunity. 21:137–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah CA, Allison KH, Garcia RL, Gray HJ,
Goff BA and Swisher EM: Intratumoral T cells, tumor-associated
macrophages, and regulatory T cells: Association with p53
mutations, circulating tumor DNA and survival in women with ovarian
cancer. Gynecol Oncol. 109:215–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Milne K, Köbel M, Kalloger SE, Barnes RO,
Gao D, Gilks CB, Watson PH and Nelson BH: Systematic analysis of
immune infiltrates in high-grade serous ovarian cancer reveals
CD20, FoxP3 and TIA-1 as positive prognostic factors. PloS One.
4:e64122009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adams SF, Levine DA, Cadungog MG, Hammond
R, Facciabene A, Olvera N, Rubin SC, Boyd J, Gimotty PA and Coukos
G: Intraepithelial T cells and tumor proliferation: Impact on the
benefit from surgical cytoreduction in advanced serous ovarian
cancer. Cancer. 115:2891–2902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kryczek I, Wei S, Zhu G, Myers L, Mottram
P, Cheng P, Chen L, Coukos G and Zou W: Relationship between B7-H4,
regulatory T cells, and patient outcome in human ovarian carcinoma.
Cancer Res. 67:8900–8905. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuzaki J, Gnjatic S, Mhawech-Fauceglia
P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant
P, et al: Tumor-infiltrating NY-ESO-1-specific CD8+ T
cells are negatively regulated by LAG-3 and PD-1 in human ovarian
cancer. Proc Natl Acad Sci USA. 107:7875–7880. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Witz IP: The tumor microenvironment: The
making of a paradigm. Cancer Microenviron 2 Suppl. 1:9–17. 2009.
View Article : Google Scholar
|
|
10
|
Allavena P, Sica A, Solinas G, Porta C and
Mantovani A: The inflammatory micro-environment in tumor
progression: The role of tumor-associated macrophages. Crit Rev
Oncol Hematol. 66:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yaal-Hahoshen N, Shina S, Leider-Trejo L,
Barnea I, Shabtai EL, Azenshtein E, Greenberg I, Keydar I and
Ben-Baruch A: The chemokine CCL5 as a potential prognostic factor
predicting disease progression in stage II breast cancer patients.
Clin Cancer Res. 12:4474–4480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Deavers M, Patenia R, Bassett RL
Jr, Mueller P, Ma Q, Wang E and Freedman RS: Monocyte/macrophage
and T-cell infiltrates in peritoneum of patients with ovarian
cancer or benign pelvic disease. J Transl Med. 4:302006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mantovani A and Allavena P: The
interaction of anticancer therapies with tumor-associated
macrophages. J Exp Med. 212:435–445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fontenot JD, Gavin MA and Rudensky AY:
Foxp3 programs the development and function of
CD4+CD25+ regulatory T cells. Nat Immunol.
4:330–336. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ouyang W, Kolls JK and Zheng Y: The
biological functions of T helper 17 cell effector cytokines in
inflammation. Immunity. 28:454–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Z, Ding J, Pang N, Du R, Meng W, Zhu
Y, Zhang Y, Ma C and Ding Y: The Th17/Treg balance and the
expression of related cytokines in Uygur cervical cancer patients.
Diagn Pathol. 8:612013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Charbonneau B, Moysich KB, Kalli KR, Oberg
AL, Vierkant RA, Fogarty ZC, Block MS, Maurer MJ, Goergen KM,
Fridley BL, et al: Large-scale evaluation of common variation in
regulatory T cell-related genes and ovarian cancer outcome. Cancer
Immunol Res. 2:332–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng
Q and Wang H, Chen J and Wang H: Tumor-associated macrophages
recruit CCR6+ regulatory T cells and promote the
development of colorectal cancer via enhancing CCL20 production in
mice. PloS One. 6:e194952011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zou W: Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiaodan Meng, Volkmar Müller, Karin
Milde-Langosch, Fabian Trillsch, Klaus Pantel and Heidi
Schwarzenbach: Circulating cell-free mir-373, mir-200a, mir-200b
and mir-200c in patients with epithelial ovarian cancer. Adv Exp
Med Biol. 924:3–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Winter WE III, Maxwell GL, Tian C, Carlson
JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F and
McGuire WP: Gynecologic Oncology Group Study: Prognostic factors
for stage III epithelial ovarian cancer: A Gynecologic Oncology
Group Study. J Clin Oncol. 25:3621–3627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colvin EK: Tumor-associated macrophages
contribute to tumor progression in ovarian cancer. Front Oncol.
4:1372014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salvi S, Segalla F, Rao S, Arienti F,
Sartori M, Bratina G, Caronni E, Anichini A, Clemente C, Parmiani
G, et al: Overexpression of the T-cell receptor beta-chain variable
region TCRBV14 in HLA-A2-matched primary human melanomas. Cancer
Res. 55:3374–3379. 1995.PubMed/NCBI
|
|
24
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barnett B, Kryczek I, Cheng P, Zou W and
Curiel TJ: Regulatory T cells in ovarian cancer: Biology and
therapeutic potential. Am J Reprod Immunol. 54:369–377. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dietl J, Engel JB and Wischhusen J: The
role of regulatory T cells in ovarian cancer. Int J Gynecol Cancer.
17:764–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eljaszewicz A, Wiese M, Helmin-Basa A,
Jankowski M, Gackowska L, Kubiszewska I, Kaszewski W, Michalkiewicz
J and Zegarski W: Collaborating with the enemy: Function of
macrophages in the development of neoplastic disease. Mediators
Inflamm. 2013:8313872013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sasaki A, Tanaka F, Mimori K, Inoue H, Kai
S, Shibata K, Ohta M, Kitano S and Mori M: Prognostic value of
tumor-infiltrating FOXP3+ regulatory T cells in patients
with hepatocellular carcinoma. Eur J Surg Oncol. 34:173–179. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mhawech-Fauceglia P, Wang D, Ali L, Lele
S, Huba MA, Liu S and Odunsi K: Intraepithelial T cells and
tumor-associated macrophages in ovarian cancer patients. Cancer
Immun. 13:12013.PubMed/NCBI
|
|
30
|
Sato E, Olson SH, Ahn J, Bundy B,
Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone
C, et al: Intraepithelial CD8+ tumor-infiltrating
lymphocytes and a high CD8+/regulatory T cell ratio are
associated with favorable prognosis in ovarian cancer. Proc Natl
Acad Sci USA. 102:18538–18543. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Klimp AH, Hollema H, Kempinga C, van der
Zee AG, de Vries EG and Daemen T: Expression of cyclooxygenase-2
and inducible nitric oxide synthase in human ovarian tumors and
tumor-associated macrophages. Cancer Res. 61:7305–7309.
2001.PubMed/NCBI
|
|
32
|
Kawamura K, Komohara Y, Takaishi K,
Katabuchi H and Takeya M: Detection of M2 macrophages and
colony-stimulating factor 1 expression in serous and mucinous
ovarian epithelial tumors. Pathol Int. 59:300–305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pittet MJ: Behavior of immune players in
the tumor microenvironment. Curr Opin Oncol. 21:53–59. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zamarron BF and Chen W: Dual roles of
immune cells and their factors in cancer development and
progression. Int J Biol Sci. 7:651–658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leffers N, Gooden MJ, de Jong RA,
Hoogeboom BN, ten Hoor KA, Hollema H, Boezen HM, van der Zee AG,
Daemen T and Nijman HW: Prognostic significance of
tumor-infiltrating T-lymphocytes in primary and metastatic lesions
of advanced stage ovarian cancer. Cancer Immunol Immunother.
58:449–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao
YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, et al:
Tumor-associated macrophage-induced invasion and angiogenesis of
human basal cell carcinoma cells by cyclooxygenase-2 induction. J
Invest Dermatol. 129:1016–1025. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shieh YS, Hung YJ, Hsieh CB, Chen JS, Chou
KC and Liu SY: Tumor-associated macrophage correlated with
angiogenesis and progression of mucoepidermoid carcinoma of
salivary glands. Ann Surg Oncol. 16:751–760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Toge H, Inagaki T, Kojimoto Y, Shinka T
and Hara I: Angiogenesis in renal cell carcinoma: The role of
tumor-associated macrophages. Int J Urol. 16:801–807. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Soeda S, Nakamura N, Ozeki T, Nishiyama H,
Hojo H, Yamada H, Abe M and Sato A: Tumor-associated macrophages
correlate with vascular space invasion and myometrial invasion in
endometrial carcinoma. Gynecol Oncol. 109:122–128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gnerlich JL, Mitchem JB, Weir JS, Sankpal
NV, Kashiwagi H, Belt BA, Porembka MR, Herndon JM, Eberlein TJ,
Goedegebuure P and Linehan DC: Induction of Th17 cells in the tumor
microenvironment improves survival in a murine model of pancreatic
cancer. J Immunol. 185:4063–4071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hou F, Li Z, Ma D, Zhang W, Zhang Y, Zhang
T, Kong B and Cui B: Distribution of Th17 cells and
Foxp3-expressing T cells in tumor-infiltrating lymphocytes in
patients with uterine cervical cancer. Clin Chim Acta.
413:1848–1854. 2012. View Article : Google Scholar : PubMed/NCBI
|