Introduction
Colon cancer is one of the most prevalent cancers
throughout the world, and particularly in Western countries
(1). There has been an increase in
the number of studies aiming to identify novel sources of bioactive
compounds that prevent colon cancer. Bioactive compounds of natural
origin, particularly from a dietary source, are of significant
interest (2).
Plants have a long history of use in the treatment
of a number of forms of cancer, and they provide leads for the
development of potential novel agents. Corn silk is made from the
stigmas and styles of the maize plant belonging to the grass family
(3) and it is a well-known
traditional functional food and Chinese herbal medicine, which has
significant effects on human health (4). It has been reported that consumption of
corn silk has no adverse effects and that it is safe for human use.
Previous studies have confirmed the presence of multiple bioactive
compounds in corn silk, including proteins, polysaccharides
(5), flavonoids (6), vitamins, tannins, alkaloids, mineral
salts (7) and steroids. There have
been numerous studies on the bioactivity of corn silk constituents,
including its antitumor capacities (8), anti-diabetic activity in hyperglycemia
rats (5) and anti-fatigue activity
(9). In our preliminary experiments,
corn silk extract was noted to inhibit cell growth and trigger
apoptosis (8). However, the specific
active ingredients in corn silk extract that induce cancer cell
apoptosis remain unknown.
In the present study we investigated whether corn
silk extract could induce apoptosis through the mitochondrial
pathway in LoVo human colon cancer cells, and whether it could
affect cell proliferation, cell cycle progression and protein
expression.
Materials and methods
Chemicals and reagents
Collected corn silk was extracted in hot water three
times and filtered; the brown filtrate with most of the small
soluble compounds was removed. The rest of the corn silk was
air-dried and then pulverized into a homogeneous size by a
disintegrator. To obtain aqueous extracts, corn silk (500 g per
sample) was resuspended in 500 ml 96°C hot water for 1 h at 100°C.
Then, the extract was obtained by centrifugation, filtering through
a filter paper and cellulose ester membrane with 0.22-µm pores,
followed by freeze-drying. Corn silk extract, a light brown powder,
was dissolved in Dulbecco's modified Eagle's medium (DMEM),
filtered through a 0.22-µm filter and stored at −20°C.
DMEM and RPMI-1640 medium were purchased from Gibco
Chemical Co. (Invitrogen Life Technologies, Carlsbad, CA, USA).
Fetal bovine serum (FBS), ampicillin sodium and streptomycin
sulfate were purchased from Sangon Biological Engineering
Technology and Services Co., Ltd. (Shanghai, China).
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide
(MTT) was purchased from Amersco (Cleveland, OH, USA). A DNA
purification kit was purchased from Promega Corporation (Madison,
WI, USA). 3,3′-dihexyloxacarbocyanine (DiOC6) and Fluo-3/AM were
purchased from Beyotime Biological Company (Shanghai, China).
Antibodies against caspase-3, caspase-9, cytochrome c, B-cell
lymphoma 2 (Bcl-2), Bax and goat anti-rabbit IgG were obtained from
ComWin Biotechnology Company (Beijing, China).
Corn silk extract composition
analysis
The extraction of proteins was determined according
to the Kjeldahl method (10); total
sugar was determined by the phenol-sulfuric acid assay (11) and reducing sugar by the
3,5-dinitrosalicylic acid (DNS) assay (12).
Cell culture
LoVo and HT-29 human colon cancer cells and MGC-803
human gastric cancer cells were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
LoVo and MGC-803 cells were cultured in DMEM, and HT-29 cells were
cultured in RPMI-1640, containing 10% FBS, ampicillin sodium and
streptomycin sulfate. All cells were cultivated under standard
conditions at 37°C in a humidified atmosphere containing 5%
CO2.
Cell proliferation assay
The effects of corn silk extract on cell
proliferation were evaluated by MTT assay. The cells were harvested
during the logarithmic growth phase and seeded at a density of
2×104 cells/well in 96-well plates. Following overnight
growth, the culture medium was replaced with various concentrations
(1.25, 2.5, 5.0, 10.0 and 20.0 mg/ml) of corn silk extract for 24,
48 and 72 h. Approximately 100 µl MTT was added to each well for 4
h. Subsequently, the supernatant was removed and the MTT crystals
were dissolved in 200 µl dimethyl sulfoxide. Thereafter, the
absorbance was measured at 570 nm using an enzyme-linked
immunosorbent assay plate reader.
The 50% inhibitory concentration (IC50)
was determined as the anticancer drug concentration causing a 50%
reduction in cell viability, and calculated from the cytotoxicity
curves. Cell survival (percent) was calculated using the following
formula: survival (%) = [(mean experimental absorbance) / (mean
control absorbance)] × 100%.
Cell cycle analysis
LoVo cells were seeded in a six-well plate at a
density of 5×105 cells/well and treated with various
concentrations (5.0, 10.0 and 20.0 mg/ml) of corn silk extract for
48 h. Then, the cells were trypsinized, collected, washed with cold
phosphate-buffered saline (PBS) and fixed in 70% ethanol at −20°C
overnight. The cells were incubated with RNase A (0.1 mg/ml) and
propidium iodide (PI; 50 mg/l) in the dark for 30 min. The cell
cycle distribution and the percentage of apoptotic cells (sub-G1
fraction) were determined by flow cytometry (13).
DNA gel electrophoresis
LoVo cells were seeded in a six-well plate at a
density of 5×105 cells/well, incubated with various
concentrations (1.25, 2.5, 5.0, 10.0 and 20.0 mg/ml) of corn silk
extract for 24, 48 and 72 h, and then harvested. The cells were
lysed in a cell lysis buffer (10 µmol/ml Tris-HCl, 10 µmol/ml EDTA,
0.5% Triton X-100) at 4°C for 4 min. Following centrifugation, the
supernatant was treated with RNase A (20 mg/ml) and proteinase K
(20 mg/ml) for 30 min at 37°C. Then, DNA was extracted by
NaCl-isopropanol (1:6) at −20°C overnight. The extracted DNA was
resuspended and loaded in each well, and DNA gel electrophoresis
was performed using 1.5% agarose gel. Following ethidium bromide
staining, images of the DNA ladders were captured under UV as
previously described (14).
Determination of mitochondrial
transmembrane potential (ΔΨm) and Ca2+ levels
LoVo cells were seeded in six-well plates at a
density of 2×105 cells/well. Following treatment with
various concentrations (5.0, 10.0 and 20.0 mg/ml) of corn silk
extract for 48 h, the cells were pelleted by centrifugation for 5
min, washed in cold PBS twice and loaded with specific
fluorochromes. For ΔΨm analysis, the cells were resuspended in 0.5
µl DiOC6 (40 µmol/l), incubated at 37°C for 30 min in the dark and
analyzed by flow cytometry as previously described (15,16).
To detect Ca2+ release, the cells were resuspended
in 1 µl Fluo-3/AM (5 µmol/l) and incubated at 37°C for 30 min in
the dark. Following incubation, the cells were washed twice with
PBS and analyzed by flow cytometry as previously described
(16,17).
Western blot analysis
LoVo cells were seeded in six-well plates at a
density of 2×105 cells/well and incubated with various
concentrations (5.0, 10.0 and 20.0 mg/ml) of corn silk extract for
48 h. The cells were harvested and extracted using a nuclear and
cytoplasmic extraction kit. Then, the proteins were resolved by
sodium dodecyl sulphate-polyacrylamide gel electrophoresis. The
separated proteins were transferred to polyvinylidene difluoride
membranes and blocked with bovine serum albumin blocking buffer for
2 h at room temperature. The membranes were incubated with specific
antibodies overnight at 4°C and washed three times with
Tris-buffered saline containing 0.1% Tween-20. On the second day,
the membranes were incubated with the relevant secondary antibodies
(1:2,000) for 90 min at room temperature. Finally, the transferred
proteins were visualized using diaminobenzidine as previously
described (18,19).
Statistical analysis
All data are presented as the means ± standard
deviation of three experiments. Statistical differences between
corn silk extract-treated and control groups were evaluated using
Student's t-test with SPSS 18.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results and Discussion
Corn silk extract composition
analysis
As shown in Table I,
corn silk extract contained 21.80% proteins, 51.70% total sugar and
10.43% reducing sugar (Table I). In a
previous study, Ooi and Liu observed that the anticancer activity
of polysaccharides may be a consequence of the stimulation of
cell-mediated immune response (20).
In another study, Yang et al demonstrated that corn silk
polysaccharides not only inhibited the tumor growth, but also
extended the survival time of H22 tumor-bearing mice (21). Food proteins may be considered as a
source of nutraceutical peptides and amino acids that exert
biological functions to promote health and prevent disease,
including cancer (22).
 | Table I.Results of corn silk extract
composition analysis. |
Table I.
Results of corn silk extract
composition analysis.
| Composition | Content/% |
|---|
| Total sugar | 51.70 |
| Reducing sugar | 10.43 |
| Proteins | 21.80 |
| Moisture content | 11.40 |
Effects of corn silk extract on cell
proliferation of cancer cells
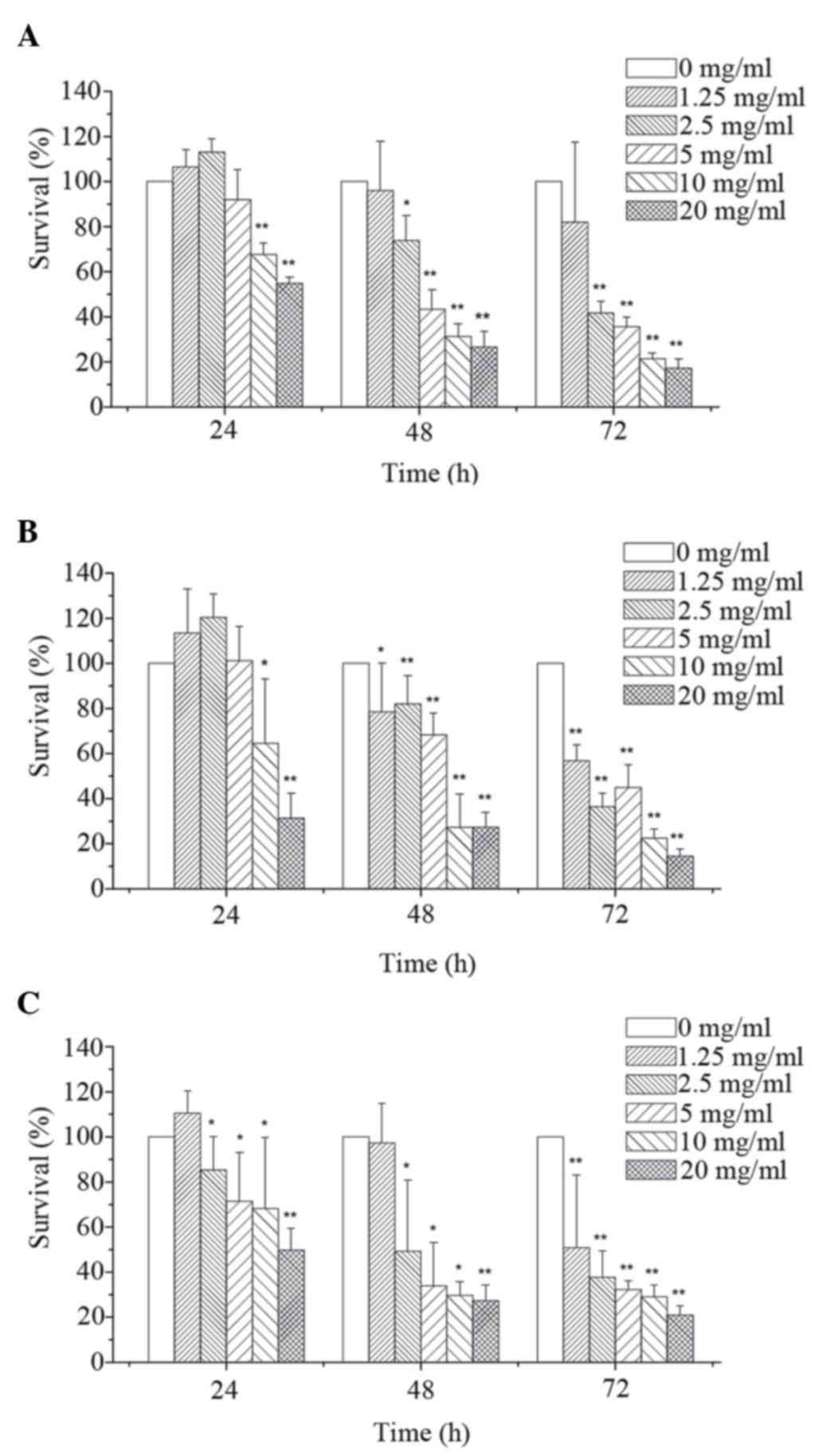
As shown in Fig. 1,
MTT assay revealed that corn silk extract inhibited cell
proliferation in a dose- and time-dependent manner. By integrating
these data, the 48 h IC50 of corn silk extract on
MGC-803 cells (Fig. 1A), HT-29 cells
(Fig. 1B) and LoVo cells (Fig. 1C) was 6.09, 5.38 and 4.52 mg/ml,
respectively. Compared with the MGC-803 cells and HT-29 cells, LoVo
cells were more sensitive to corn silk extract and thus were used
to explore the related mechanism of corn silk extract-induced
apoptosis in LoVo cells.
Effects of corn silk extract on cell
cycle arrest in LoVo cells
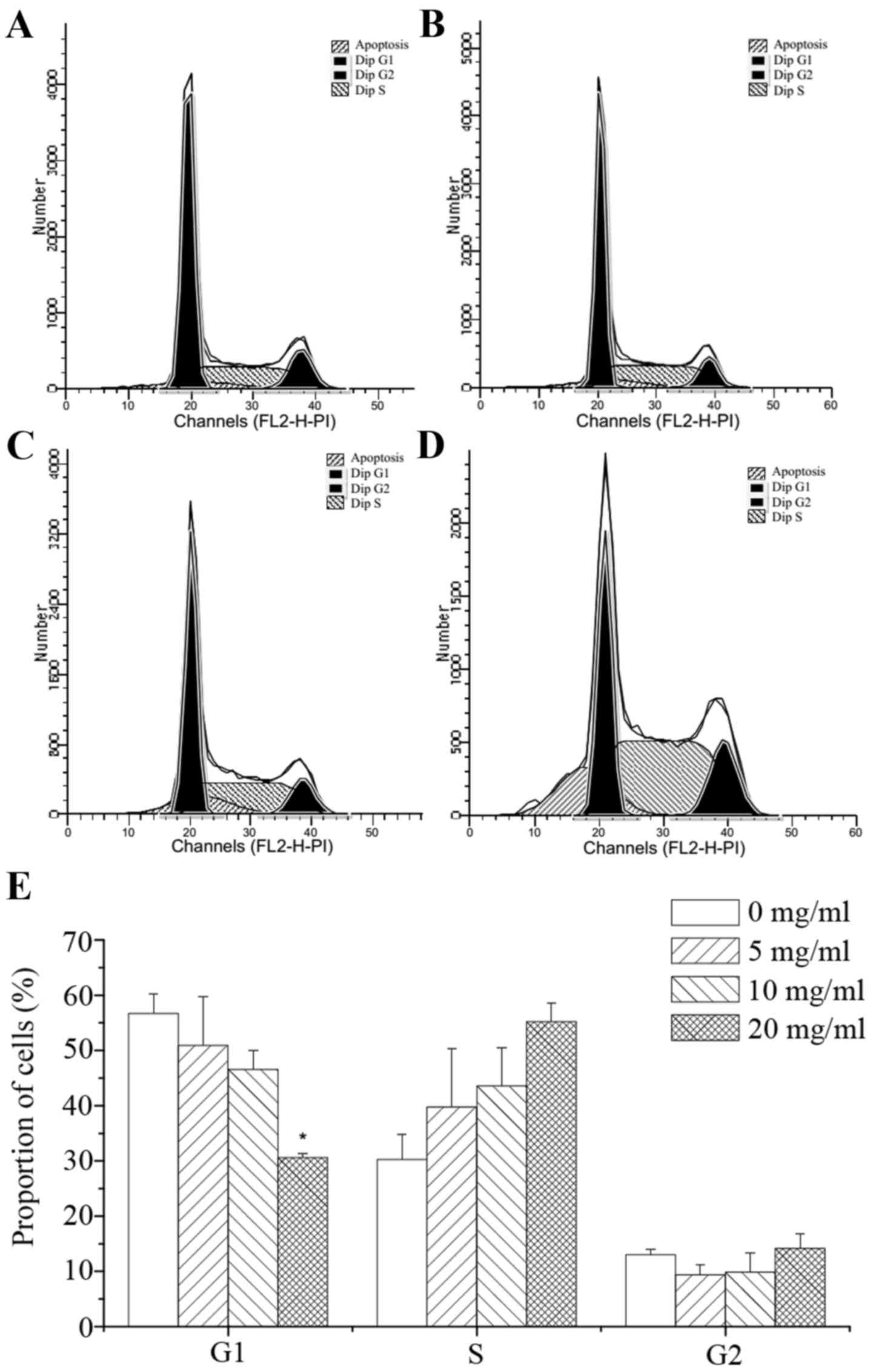
PI staining was used to detect the effects of corn
silk extract at various concentrations on colon cancer cells.
Forty-eight hours after treatment, a sub-diploid peak was present
in the left part of the graph. With the increase in corn silk
extract concentration, the sub-diploid peak became increasingly
evident, representing a gradient of mortality. As shown in Fig. 2A to D, the sub-G1 fractions in the
corn silk extract-treated groups were 7.86, 12.93 and 16.44%, which
was significantly higher than the 4.71% of the negative control
group. These results reveal that corn silk extract has a
significant inhibitory effect on cancer cell growth. Moreover, the
sub-G1 fraction was increased in a drug concentration-dependent
manner. As shown in Fig. 2E, flow
cytometry revealed that the percentage of cells in the G1 phase was
56.56, 50.92, 46.57 and 30.62% following treatment with 0, 5, 10
and 20 mg/ml corn silk extract, respectively, for 48 h. The number
of S-phase cells was 30.28, 39.72, 43.57 and 55.21% with 0, 5, 10
and 20 mg/ml corn silk extract for 48 h. The data revealed that the
relative proportion of cells in the S phase increased when the
concentration of corn silk extract was increased. Experiments
revealed that the corn silk extract inhibited the proliferation of
cells mainly through S-phase arrest, blocking cells from entering
the G2/M phase of mitosis. Correspondingly, cells in the sub-G1
phase were also increased in a drug concentration-dependent manner,
which was consistent with the flow cytometry data. Therefore, the
cell cycle inhibitory effect of the corn silk extract mainly
involved S-phase arrest.
Effects of corn silk extract on DNA
fragmentation and apoptosis in LoVo cells
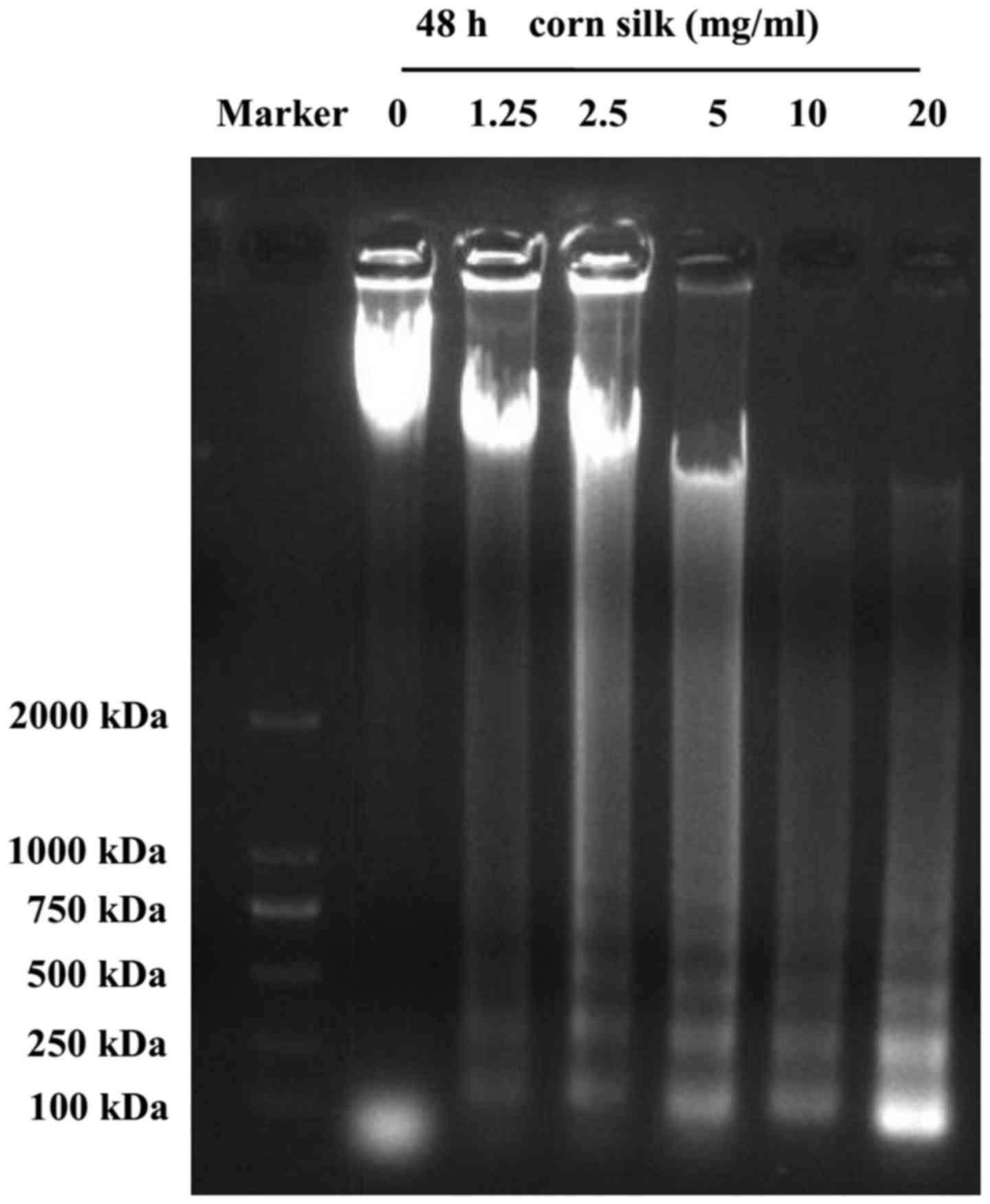
As shown in Fig. 3,
the formation of the DNA ladder occurred 48 h after treatment with
corn silk extract, and this effect was concentration-dependent from
1.25 mg/ml up to a maximum of 20 mg/ml. We observed that, in
contrast to the control group, treatment of LoVo cells with corn
silk extract for 48 h resulted in internucleosomal DNA
fragmentation consisting of 180–200 bp, a characteristic of
apoptosis, exhibiting a typical ladder-like pattern of degraded DNA
products on agarose gel electrophoresis. A previous study reported
that in the event of apoptosis, DNA gel electrophoresis exhibited a
clear ladder. DNA gel electrophoresis is considered one of the gold
standards in the determination of apoptosis (23).
Effects of corn silk extract on
mitochondrial membrane potential and Ca2+ release from LoVo
cells
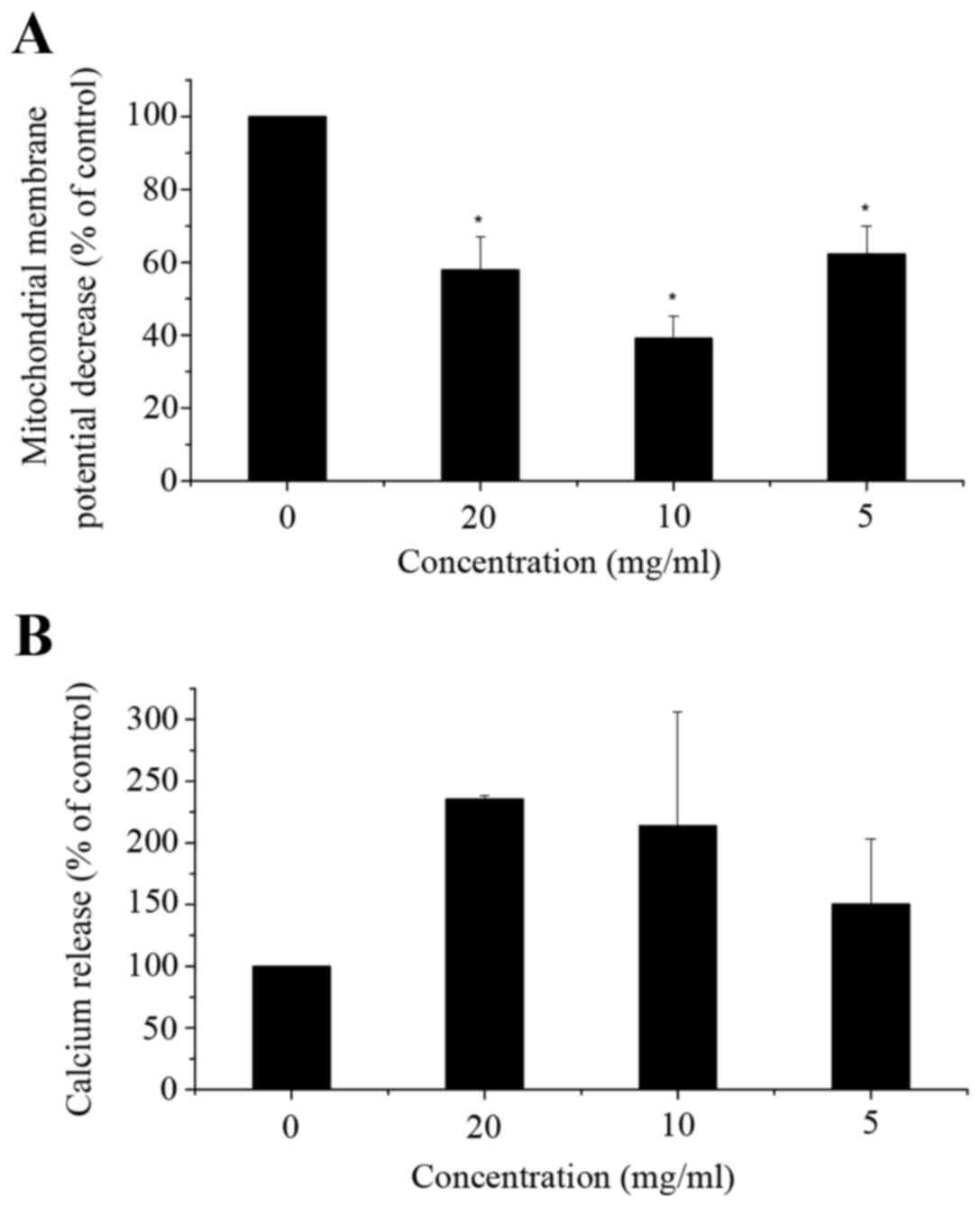
Flow cytometric analysis indicated a significant
decrease in mitochondrial membrane potential (ΔΨm) (Fig. 4A) and a significant increase in
cytosolic Ca2+ levels (Fig. 4B) in
corn silk extract-treated cells when compared with the control
cells. As illustrated in Fig. 4A, in
LoVo cells exposed to corn silk extract for 48 h, ΔΨm was
decreased, particularly in the 10 mg/ml dose group. Cytosolic Ca2+
release increased. As shown in Fig.
4B, cytosolic Ca2+ levels peaked in the 10-mg/ml dose group,
with levels being higher than those of the control cells at 48 h.
In the mitochondrial apoptosis pathway, the decrease in
mitochondrial membrane potential is an early event in apoptosis. It
was reported that the proteins extracted from corn silk could
decrease mitochondrial membrane potential (22), which is in accordance with our
results. However, in the low-dose group, the mitochondrial membrane
potential was increased compared with other corn silk
extract-treated groups, which may be related to the selection of
cells. The elevation of calcium ion levels is associated with
signal transduction in the early stages of apoptosis. Mitochondria
represent one of the intracellular calcium pools. It was reported
that stored Ca2+ was released from the mitochondria,
which significantly increased the intracellular calcium level in
the early stages of apoptosis (24).
This process was synergized by other pro-apoptotic factors leading
to apoptosis of a large number of cells. This indicates that corn
silk extract could induce apoptosis through the upregulation of
intracellular Ca2+ levels. Fan et al reported
that the changes of intracellular calcium homeostasis and ΔΨm could
contribute to apoptosis in treated cells (23).
Effects of corn silk extract on
apoptosis-associated protein expression in LoVo cells
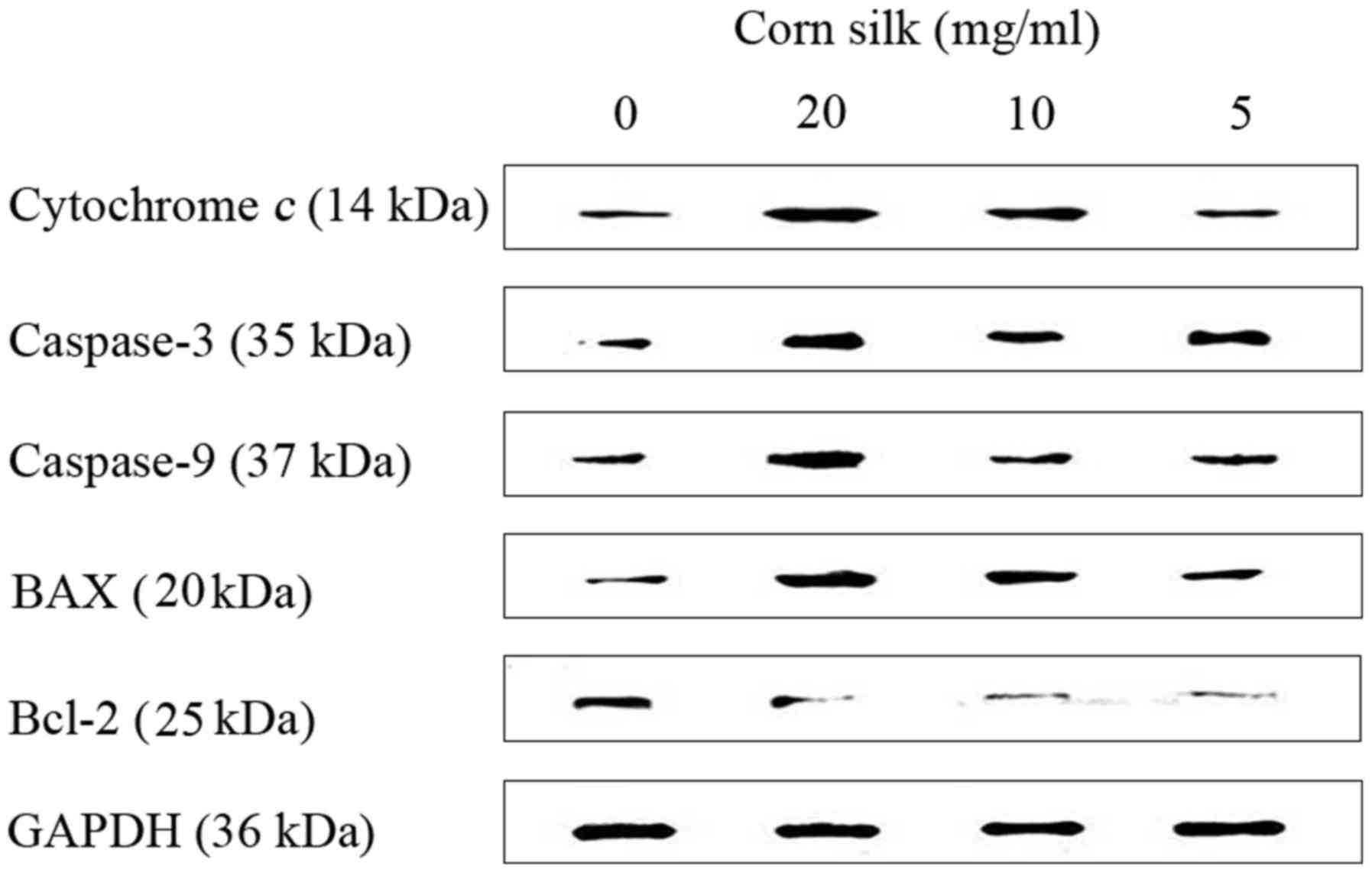
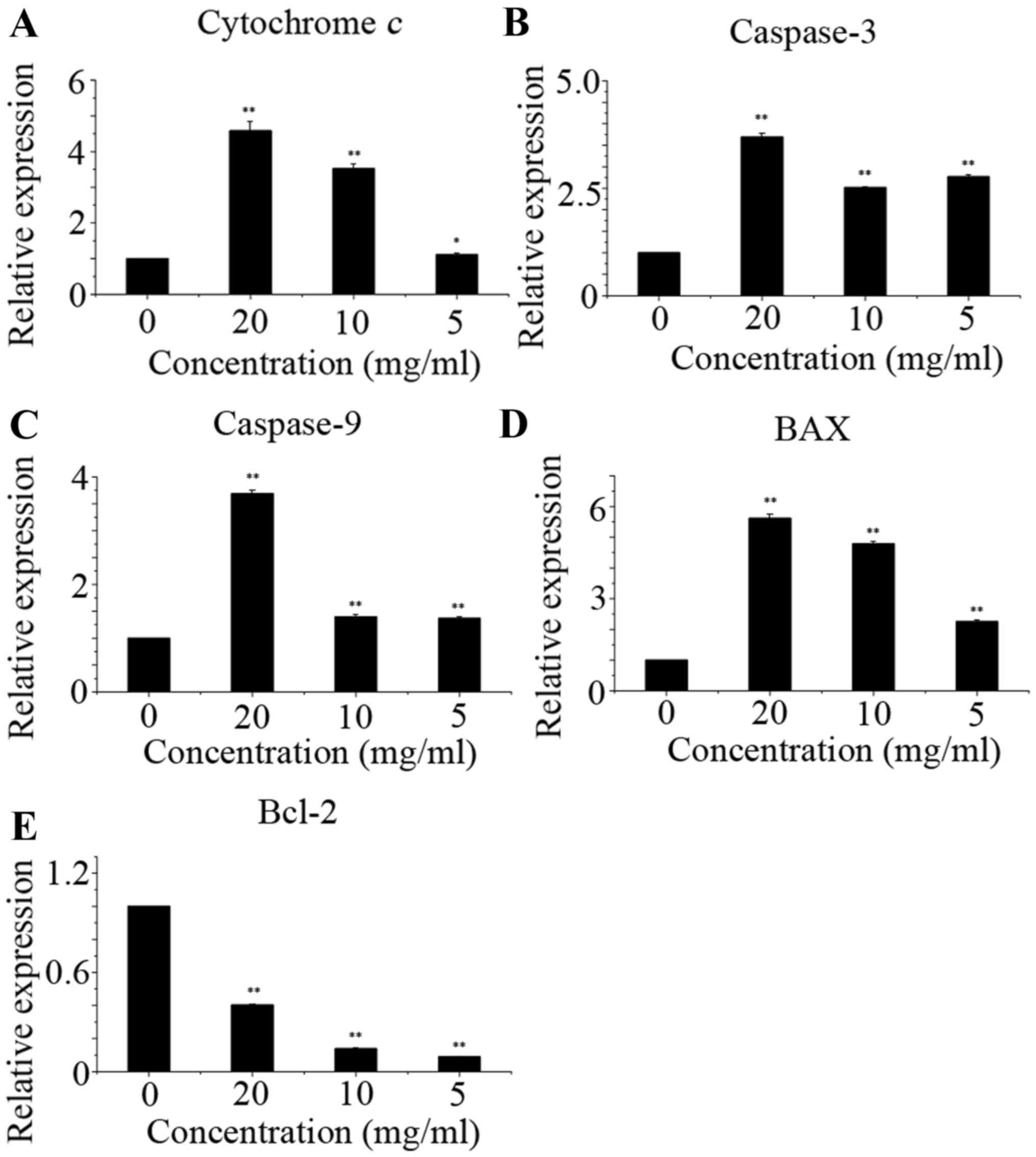
As shown in Fig. 5,
corn silk extract upregulated the levels of cytochrome c,
caspase-3, caspase-9 and Bax, but reduced the levels of Bcl-2,
which led to apoptosis. Western blot analysis data are presented as
the relative expression of selected proteins compared with GAPDH
(Fig. 6). Using western blot assay,
we have shown above that apoptotic pathway induction by corn silk
extract treatment was mediated by affecting the Bax and Bcl-2
levels, causing dysfunction of mitochondria, promoting the release
of cytochrome c and activating caspase-9 and caspase-3
(15,25).
Caspase is a family of proteases that plays a
significant role in the process of apoptosis. Caspase-9 is involved
in the upstream stages of the signal transduction cascade of
apoptosis. Following the release of cytochrome c from mitochondria,
caspase-9 is activated to form a complex with cytochrome c. The
activation of caspase-9 then activates caspase-3, thereby
facilitating subsequent apoptotic signaling. Caspase-3 is a key
enzyme in the apoptotic cascade, playing an essential role in
chromatin condensation, DNA fragmentation and other nuclear
apoptotic processes (26). This
further supports the hypothesis that corn silk extract
significantly induced apoptosis in LoVo cells. Bcl-2 family
proteins also play crucial roles in apoptosis. This family consists
of two types of proteins: anti-apoptotic and pro-apoptotic. It was
demonstrated that the change in mitochondrial outer membrane
permeability could lead to apoptosis, and this change could be
directly mediated by the Bcl-2 family proteins. In addition, the
main activity of the Bcl-2 family proteins takes place on the
mitochondrial membrane (27). In the
present study, the decrease in the Bcl-2/Bax ratio might have led
to a decrease in ΔΨm and subsequent activation of caspase-3 via the
cytochrome c and caspase-9 pathway. Corn silk extract
induced LoVo cell apoptosis features, including nuclear
condensation, DNA fragmentation, dysfunction of mitochondria, Ca2+
production and caspase activation.
Based on the results of the present study, it is
hypothesized that corn silk extract may inhibit the proliferation
of LoVo cells through S-phase arrest, and induce apoptosis through
the mitochondria-mediated pathway. At present, the purification of
the antitumor components of corn silk extract is in process, which
is likely to aid in identifying the mechanisms underlying the
antitumor activity of corn silk extract.
Acknowledgements
This study was supported by the Prophase Research
Project of Transformation of Scientific and Technological
Achievements of Heilongjiang Educational Committee of China under
grant no. 1252CGZH04 and the Scientific Research Fund Project of
Qiqihar Medical University under grant no. QY2015Q-04.
References
|
1
|
Jiang QG, Li TY, Liu DN and Zhang HT:
PI3K/Akt pathway involving into apoptosis and invasion in human
colon cancer cells LoVo. Mol Biol Rep. 41:3359–3367. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patil JR, Murthy KN Chidambara,
Jayaprakasha GK, Chetti MB and Patil BS: Bioactive compounds from
Mexican lime (Citrus aurantifolia) juice induce apoptosis in human
pancreatic cells. J Agric Food Chem. 57:10933–10942. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu QL and Deng ZH: Protective effects of
flavonoids from corn silk on oxidative stress induced by exhaustive
exercise in mice. Afr J Biotechnol. 10:3163–3167. 2011. View Article : Google Scholar
|
|
4
|
Chen S, Chen H, Tian J, Wang Y, Xing L and
Wang J: Chemical modification, antioxidant and α-amylase inhibitory
activities of corn silk polysaccharides. Carbohydrate polym.
98:428–437. 2013. View Article : Google Scholar
|
|
5
|
Zhao W, Yin Y, Yu Z, Liu J and Chen F:
Comparison of anti-diabetic effects of polysaccharides from corn
silk on normal and hyperglycemia rats. Int J Biol Macromol.
50:1133–1137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J and Mori A: Antioxidant and free
radical scavenging activities of Gastrodia elata Bl. and Uncaria
rhynchophylla (Miq.) Jacks. Neuropharmacology. 31:1287–1298. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Namba T, Xu H, Kadota S and Hattori M:
Inhibition of IgE formation in mice by glycoproteins from corn
silk. Phytother Res. 7:227–230. 1993. View Article : Google Scholar
|
|
8
|
Habtemariam S: Extract of corn silk
(stigma of Zea mays) inhibits the tumour necrosis factor-alpha- and
bacterial lipopolysaccharide-induced cell adhesion and ICAM-1
expression. Planta Med. 64:314–318. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu QL, Zhang LJ, Li YN, Ding YJ and Li FL:
Purification and anti-fatigue activity of flavonoids from corn
silk. Int J Phys Sci. 5:321–326. 2010.
|
|
10
|
Lynch JM and Barbano DM: Kjeldahl nitrogen
analysis as a reference method for protein determination in dairy
products. J AOAC Int. 82:1389–1398. 1999.PubMed/NCBI
|
|
11
|
Rover MR, Johnston PA, Lamsal BP and Brown
RC: Total water-soluble sugars quantification in bio-oil using the
phenol-sulfuric acid assay. J Anal Appl Pyrolysis. 104:194–201.
2013. View Article : Google Scholar
|
|
12
|
de Wit JN: Marschall Rhône-Poulenc Award
Lecture. Nutritional and functional characteristics of whey
proteins in food products. J Dairy Sci. 81:597–608. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tai KW, Chou MY, Hu CC, Yang JJ and Chang
YC: Induction of apoptosis in KB cells by pingyangmycin. Oral
Oncol. 36:242–247. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu HF, Lai TY, Hsia TC, Tang YJ, Yang JS,
Chiang JH, Lu CC, Liu CM, Wang HL and Chung JG: Danthron induces
DNA damage and inhibits DNA repair gene expressions in GBM 8401
human brain glioblastoma multiforms cells. Neurochem Res.
35:1105–1110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin SY, Lai WW, Ho CC, Yu FS, Chen GW,
Yang JS, Liu KC, Lin ML, Wu PP, Fan MJ and Chung JG: Emodin induces
apoptosis of human tongue squamous cancer SCC-4 cells through
reactive oxygen species and mitochondria-dependent pathways.
Anticancer Res. 29:327–335. 2009.PubMed/NCBI
|
|
16
|
Lu HF, Chen YS, Yang JS, Chen JC, Lu KW,
Chiu TH, Liu KC, Yeh CC, Chen GW, Lin HJ and Chung JG: Gypenosides
induced G0/G1 arrest via inhibition of cyclin E and induction of
apoptosis via activation of caspases-3 and −9 in human lung cancer
A-549 cells. In Vivo. 22:215–221. 2008.PubMed/NCBI
|
|
17
|
Wu CC, Lin JP, Yang JS, Chou ST, Chen SC,
Lin YT, Lin HL and Chung JG: Capsaicin induced cell cycle arrest
and apoptosis in human esophagus epidermoid carcinoma CE 81T/VGH
cells through the elevation of intracellular reactive oxygen
species and Ca2+ productions and caspase-3 activation. Mutat Res.
601:71–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohan KV, Gunasekaran P, Varalakshmi E,
Hara Y and Nagini S: In vitro evaluation of the anticancer effect
of lactoferrin and tea polyphenol combination on oral carcinoma
cells. Cell Biol Int. 31:599–608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng L, Wang X, Luo W, Zhan Y and Zhang
Y: Brucine, an effective natural compound derived from nux-vomica,
induces G1 phase arrest and apoptosis in LoVo cells. Food Chem
Toxicol. 58:332–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ooi VE and Liu F: Immunomodulation and
anti-cancer activity of polysaccharide-protein complexes. Curr Med
Chem. 7:715–729. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang J, Li X, Xue Y, Wang N and Liu W:
Anti-hepatoma activity and mechanism of corn silk polysaccharides
in H22 tumor-bearing mice. Int J Biol Macromol. 64:276–280. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee J, Lee S, Kim SL, Choi JW, Seo JY,
Choi DJ and Park YI: Corn silk maysin induces apoptotic cell death
in PC-3 prostate cancer cells via mitochondria-dependent pathway.
Life Sci. 119:47–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan MJ, Lin YC, Shih HD, Yang JS, Liu KC,
Yang ST, Lin CY, Wu RS, Yu CS, Ko YC and Chung JG: Crude extracts
of Agaricus brasiliensis induce apoptosis in human oral cancer CAL
27 cells through a mitochondria-dependent pathway. In Vivo.
25:355–366. 2011.PubMed/NCBI
|
|
24
|
Wang L, Hu T, Shen J, Zhang L, Li LF, Chan
RL, Li MX, Wu WK and Cho CH: Miltirone induced mitochondrial
dysfunction and ROS-dependent apoptosis in colon cancer cells. Life
Sci. 151:224–234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abdel-Latif AM, Abuel-Ela HA and
El-Shourbagy SH: Increased caspase-3 and altered expression of
apoptosis-associated proteins, Bcl-2 and Bax in lichen planus. Clin
Exp Dermatol. 34:390–395. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su TR, Tsai FJ, Lin JJ, Huang HH, Chiu CC,
Su JH, Yang YT, Chen JY, Wong BS and Wu YJ: Induction of apoptosis
by 11-dehydrosinulariolide via mitochondrial dysregulation and ER
stress pathways in human melanoma cells. Mar Drugs. 10:1883–1898.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Z, Liu B, Cailing E, Liu J, Zhang Q,
Liu J, Chen N, Chen R and Zhu R: Resveratrol inhibits the
proliferation of human melanoma cells by inducing G1/S cell cycle
arrest and apoptosis. Mol Med Rep. 11:400–404. 2015.PubMed/NCBI
|