Introduction
Colorectal cancer is one of the most common types of
cancer in the world (1). In 2008,
colorectal cancer was the leading cause of cancer-associated
mortality in Japanese women and the third leading cause in Japanese
men (2). The biology of colorectal
carcinoma may differ by location and it remains unclear whether
carcinoma of the colon (caecum to sigmoid) and rectum should be
considered as a single entity or as two distinct entities (3).
There are two major arguments for dividing
colorectal carcinoma according to location. Firstly, rectal
carcinoma may have a more malignant nature than colon carcinoma; it
is more likely to metastasize to the lymph nodes or to recur
(4). Furthermore, the risk of local
recurrence following endoscopic resection has been reported to be
significantly higher for rectal T1 carcinoma than for colon T1
carcinoma (5). Secondly, rectal
surgery is more invasive than colon surgery, with higher rates of
post-operative complications, including anastomotic leakage
(6). In addition, a number of
patients with rectal cancer are left with permanent stomas
following standard abdominoperineal resection, and low anterior
resection may lead to disorders and complications of anal
functions, thus reducing patient quality of life (7).
In general, lymph node metastasis occurs in
approximately 10% of patients with colorectal T1 carcinoma
(8,9),
therefore, it is essential to thoroughly assess the need for
surgical resection in such patients. The present study was designed
to compare the clinicopathological characteristics of patients with
colon and rectal T1 carcinoma, and to determine whether these
carcinomas should be considered as a single entity or two distinct
entities when deciding on surgical treatment.
Patients and methods
Patients
The study cohort included 557 patients with T1
colorectal carcinoma who were treated at Showa University Northern
Yokohama Hospital (Yokohama, Japan) between April 2001 and March
2013. Patients were eligible for inclusion if they underwent
endoscopic resection followed by additional surgery or surgical
resection as a first-line treatment. Patients were excluded if they
underwent endoscopic resection alone; exhibited evidence of
inflammatory bowel disease, familial adenomatous polyposis or Lynch
syndrome; had synchronous or metachronous advanced colorectal
carcinoma or exhibited evidence of malignant disease in any other
organ. None of the patients included in the study had received
preoperative radiotherapy or neoadjuvant chemotherapy.
The rectum was defined as the area between the upper
border of the anal canal and the lower border of the second sacral
vertebra. Patient characteristics that were analysed included age,
gender, tumor size, morphology, histological type, tumor budding,
invasion depth, intramural lymphatic invasion, intramural vascular
invasion and lymph node metastasis.
In a subanalysis, the sizes of colon and rectal T1
carcinomas were evaluated according to their morphologies. Tumor
morphology was classified into three types according to the Paris
endoscopic categorization of superficial neoplastic lesions
(10): Depressed type; slightly
elevated and flat type, including laterally spreading tumors
(LSTs); and protruded type. LSTs were further divided into granular
type (LST-G) and nongranular type (LST-NG) (11).
The protocol of the current study was approved by
the ethics committee of Showa University Northern Yokohama Hospital
(no. 1201-05) and registered in the UMIN Clinical Trials Registry
(UMIN000010979). Written informed consent was obtained from all
patients prior to treatment.
Endoscopic procedure
Examinations were performed using magnifying
colonoscopies (CF-H260AZI or PCF-Q240ZI; Olympus Optical
Corporation, Tokyo, Japan), which enhance images ~75- to 100-fold.
All colorectal lesions were evaluated in real-time under magnifying
chromoendoscopy in the presence of 0.40% indigo carmine (Nagase
Medicals Co., Ltd., Hyogo, Japan) to determine pit pattern
classification (12,13), and tumors with a type V pit pattern
were evaluated using 0.05% crystal violet (Koso Chemical Co., Ltd.,
Gyoda, Japan) (13). Lesions observed
to have III, IV, or VI low-grade pit patterns (i.e.,
adenomas, intramucosal colorectal carcinomas and slightly invasive
submucosal colorectal carcinomas) were resected endoscopically.
Patients with lesions exhibiting a VI high-grade or
VN pit pattern (i.e., massively invasive submucosal
colorectal carcinomas) were referred for surgery. Patients who
refused surgery underwent endoscopic resection as a first-line
treatment.
Surgical procedure
Patients who were endoscopically diagnosed with
massively invasive submucosal carcinoma and agreed to undergo
surgical resection underwent curative resection with lymph node
dissection, either laparoscopically or using an open procedure.
Patients were considered to be at high risk for lymph node
metastasis if they exhibited vertically positive margins, an
unfavourable histological type, evidence of vascular or lymphatic
invasion, high-grade tumor budding or massively invasive carcinoma
following endoscopic resection. Additional curative surgery with
lymph node dissection was recommended for these patients (2).
Histological examination
Resected specimens were immediately fixed in 10%
buffered formalin solution for at least 24 h at room temperature,
and stained with hematoxylin and eosin. Histological specimens were
subsequently cut into parallel sections 2–3 mm thick. Pathological
specimens were assessed for resection margin status and
histological characteristics by a single experienced pathologist.
Tumor size was defined as the maximum tumor diameter on the
original pathology report.
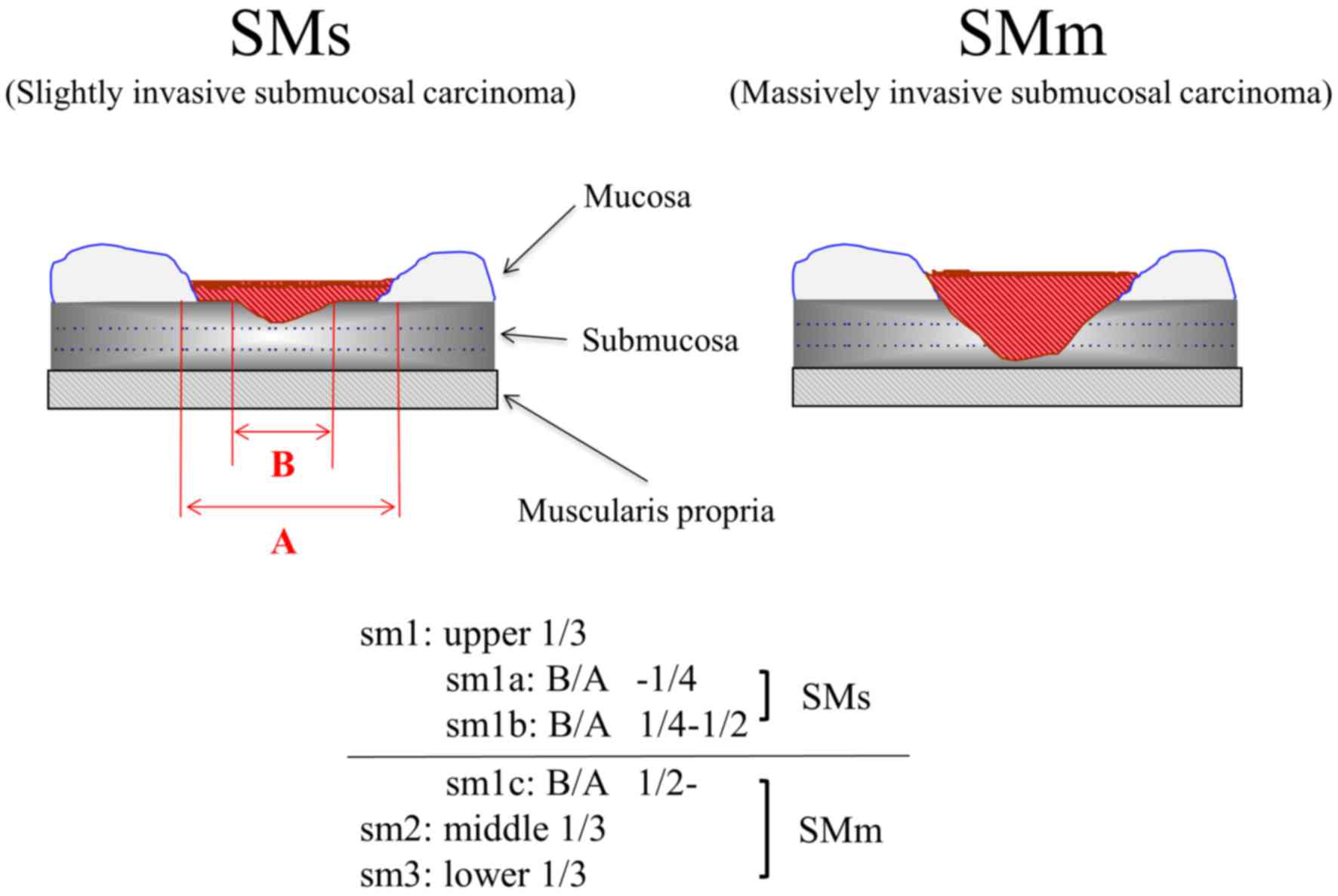
The degree of submucosal invasion was determined
according to the Kudo classification (Fig. 1) (14).
Specifically, the degree of submucosal invasion was classified into
three categories based on invasion depth in surgically resected
specimens: Infiltration into the upper third (sm1), middle third
(sm2) and lower third (sm3) of the submucosal layer. In
endoscopically resected specimens, the resected submucosal layer
was vertically divided into two components. Submucosal invasion
within the upper layer was regarded as sm1, invasion of the deeper
layer was regarded as sm2 and a vertically positive margin was
regarded as sm3 (15). Furthermore,
sm1 cases were subclassified into sm1a, sm1b, and sm1c based on the
horizontal extension of the submucosally invaded area. Sm1a and
sm1b carcinomas were defined as slightly invasive, while sm1c, sm2,
and sm3 carcinomas were considered massively invasive submucosal
carcinomas.
Histological type was based on the World Health
Organization Classification of Tumors (16). Tumors were examined histochemically
using Victoria blue (Muto Pure Chemicals Co., Ltd., Tokyo, Japan)
staining for vascular invasion and D2-40 (Dako North America, Inc.,
Carpinteria, CA, USA) expression for lymphatic invasion.
Haematoxylin and eosin staining was also performed. Tumor budding
was defined as an isolated single carcinoma cell or a cluster
composed of <5 cells. After choosing one field in which budding
was the most intense, a budding count was conducted in a field
measuring 0.785 mm2 using an objective lens
(magnification, ×20). A field with ≥5 buds was regarded as grade 2
or 3 (17).
Statistical analysis
Nominal and ordinal variables are expressed as
frequencies and percentages. Continuous variables are reported as
mean ± standard deviation (SD) and were compared using Student's
t-test, whereas dichotomous variables were compared using
χ2 or Fisher exact tests, as appropriate. All
statistical analyses were performed using R ver. 2.10.0 (R
Foundation for Statistical Computing, Vienna, Austria). All
P-values were two sided and P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient population
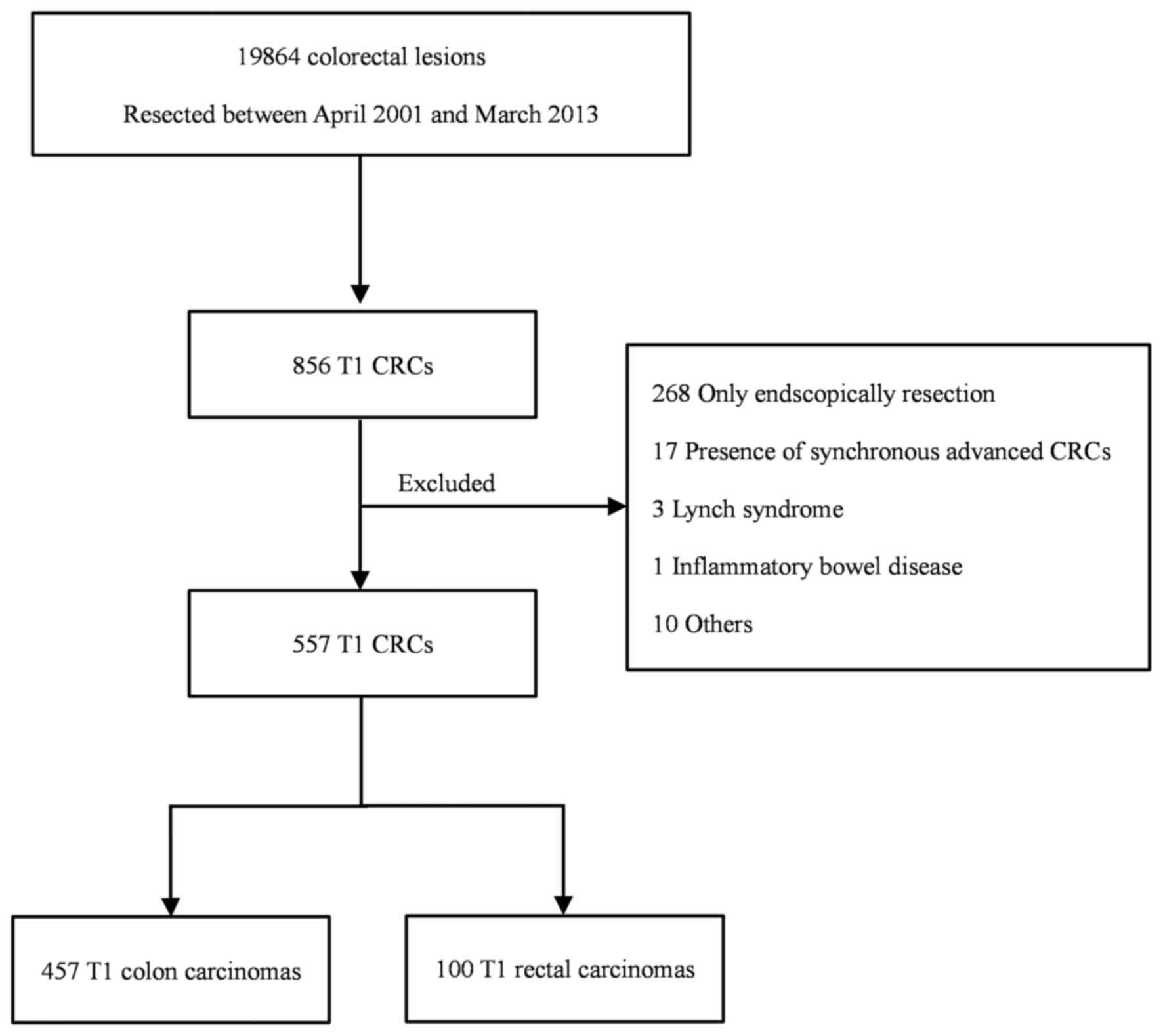
A total of 19,864 patients with colorectal neoplasms
underwent endoscopic or surgical tumor resection at the Showa
University Northern Yokohama Hospital (Yokohama, Japan) between
April 2001 and March 2013 (Fig. 2).
Of these, 856 (4.3%) patients exhibited colorectal T1 carcinoma.
However, 299 patients with colorectal T1 carcinoma were excluded:
268 underwent endoscopic resection alone, 17 had synchronous
advanced colorectal carcinoma, 3 had Lynch syndrome, 1 had
inflammatory bowel disease and 10 were excluded for other reasons.
Thus, the final study cohort consisted of 557 patients with
colorectal T1 carcinoma, including 457 with colon carcinoma and 100
with rectal carcinoma. Of these, 293 patients (233 with colon and
60 with rectal carcinoma) underwent first-line surgery and lymph
node dissection and 264 (224 with colon and 40 with rectal tumors)
underwent first-line endoscopic resection followed by additional
surgery. The total cohort of 557 patients included 210 women
(37.7%) and 347 men (62.3%), among whom the mean (± SD) age was
64.9±11.4 years.
The median number of lymph nodes dissected per
patient was 13 (range, 1–53). The median number of lymph nodes in
cases of colon carcinoma was 13 (range, 1–53), while the median
number of lymph nodes in cases of rectal carcinoma was 13 (range,
2–41). Of the 557 patients, 54 (9.7%) exhibited evidence of lymph
node metastasis.
Clinicopathological features of colon
and rectal T1 carcinoma
Table I presents the
clinicopathological characteristics of the patient groups. Rectal
T1 carcinomas were significantly larger (23.7±13.1 mm vs. 19.9±11.0
mm, P<0.01) and exhibited significantly higher rates of vascular
invasion (48.0% vs. 30.2%, P<0.01) than colon T1 carcinomas. The
rates of lymphatic invasion (46.0% vs. 41.4%, P=0.43) and lymph
node metastasis (9.0% vs. 9.8%, P=0.80) were similar in patients
with rectal and colon T1 carcinomas. None of the other
clinicopathological factors assessed differed significantly between
patients with colon and rectal T1 carcinomas.
 | Table I.Clinicopathological characteristics of
patients with colon and rectal T1 carcinoma. |
Table I.
Clinicopathological characteristics of
patients with colon and rectal T1 carcinoma.
| Characteristic | Colon (n=457) | Rectum (n=100) | P-value |
|---|
| Age, years ± SD | 65.3±11.4 | 62.9±10.8 | 0.06 |
| Males, n (%) | 285 (62.5) | 62 (62.0) | 0.96 |
| Tumor size, mm ±
SD | 19.9±11.0 | 23.7±13.1 | <0.01a |
| Invasion depth, SMm
(%) | 422 (92.3) | 93 (93.0) | 0.99 |
| Histological type,
por or muc (%) | 89
(19.5) | 15 (15.0) | 0.33 |
| Vascular invasion, +
(%) | 138 (30.2) | 48 (48.0) |
<0.001a |
| Lymphatic invasion, +
(%) | 189 (41.4) | 46 (46.0) | 0.43 |
| Tumor budding, grade
2 or 3 (%) | 123 (26.9) | 32 (32.0) | 0.33 |
| Lymph node
metastasis, + (%) |
45 (9.8) |
9 (9.0) | 0.94 |
Morphology and size of colon and
rectal T1 carcinomas
Table II presents
comparisons between the morphology and size of colon and rectal T1
carcinomas. Morphologically, 28.2% (129/457) and 36.0% (36/100), of
colon and rectal T1 carcinomas were depressed, respectively, 26.3%
(120/457) and 30.0% (30/100) were flat and 45.5% (208/457) and
34.0% (34/100) were protruded. There were no significant
differences in tumor morphology between the groups (P=0.10). In
addition, 24.5% (112/457) of colon and 26.0% (26/100) of rectal T1
carcinomas were LSTs. LST-Gs accounted for 7.0% (32/457) of colon
and 22.0% (22/100) of rectal T1 carcinomas, with mean tumor sizes
of 41.1±18.1 mm and 38.1±16.2 mm, respectively (P=0.54). LST-NGs
accounted for 17.5% (80/457) of colon and 4.0% (4/100) of rectal T1
carcinomas, with mean tumor sizes of 24.4±9.5 mm and 26.3±4.9 mm,
respectively (P=0.70). However, colon T1 carcinomas classified as
LST-Gs were significantly larger than those classified as LST-NGs
(41.1±18.1 mm vs. 24.4±9.5 mm, P<0.01). In addition, rectal T1
carcinomas classified as LST-Gs were significantly larger than
rectal tumors classified as LST-NGs (38.1±16.4 mm vs. 26.3±4.9 mm,
P<0.01).
 | Table II.Morphology and tumor size according to
LST subtypes of colon and rectal carcinoma. |
Table II.
Morphology and tumor size according to
LST subtypes of colon and rectal carcinoma.
| Characteristic | Colon (n=457) | Rectum (n=100) | P-value |
|---|
| Morphology |
|
| 0.10 |
|
Depressed, n (%) | 129 (28.2) | 36 (36.0) |
|
| Flat, n
(%) | 120 (26.3) | 30 (30.0) |
|
|
Protruded, n (%) | 208 (45.5) | 34 (34.0) |
|
| LST, n (%) | 112 (24.5) | 26 (26.0) | 0.85 |
| LST-G, n (%) | 32
(7.0) | 22 (22.0) |
<0.01a |
| Tumor size ± SD,
mm | 41.1±18.1 | 38.1±16.4 | 0.54 |
| LST-NG, n (%) | 80
(17.5) |
4 (4.0) |
<0.01a |
| Tumor
size ± SD, mm | 24.4±9.5 | 26.3±4.9 | 0.70 |
Discussion
The present study investigated the
clinicopathological differences between T1 rectum and colon
carcinomas to assess whether they should be classified as a single
entity or two distinct entities when considering the indications
for surgical resection. Colon and rectal carcinoma share many
features, however, they exhibit important clinicopathological and
genetic differences (3). The results
of the current study include three important clinical observations:
i) The rates of lymph node metastasis were similar in rectal and
colon T1 carcinomas; ii) rectal T1 carcinomas were generally larger
than colon T1 carcinomas; iii) rectal T1 carcinomas were
accompanied by significantly higher rates of vascular invasion than
colon T1 carcinomas.
Lymph node metastasis is an important prognostic
factor in patients with rectal and colon and T1 carcinoma and
affects disease management. Nodal involvement is associated with an
increased risk of local recurrence and shorter overall and
disease-free survival time (18–20). Lymph
node metastasis generally occurs in approximately 10% of patients
with T1 colorectal carcinoma (8,9). In the
clinical guidelines of the Japanese Society for Cancer of the Colon
and Rectum (JSCCR), patients who undergo endoscopic resection for
T1 colorectal carcinoma are considered to be at extremely low risk
of developing lymph node metastasis if they have negative vertical
margins, well or moderately differentiated adenocarcinoma, absence
of vascular or lymphatic invasion, grade 0 or 1 tumor budding and
submucosal invasion <1,000 µm (2).
These patients should be monitored periodically, however, they
should not require additional surgery. Patients with ≤1 of these
factors are considered to be high risk and surgery, including lymph
node dissection, is recommended.
Surgery for rectal carcinoma is more invasive than
surgery for colon carcinoma. Standard abdominoperineal resection
for low rectal carcinoma leaves many patients with permanent
stomas. Advanced anus-preserving low anterior resection and
intersphincteric resection have become more common as treatments
for lower rectal carcinoma that avoid colostomies (21). However, some anal function disorders
and complications have been reported following such resections,
reducing patient quality of life (8).
Due to similar rates of lymph node metastasis in patients with
colon and rectal T1 carcinoma, surgical indications following
endoscopic resection should be similar. However, it has been
reported that high-risk patients with rectal T1 carcinoma have a
significantly higher risk of local recurrence than high-risk
patients with colon T1 carcinoma treated with endoscopic resection
alone (5). Although rectal surgery is
more invasive than colon surgery, additional surgery involving
dissection of the lymph nodes is recommended for high-risk patients
with rectal T1 carcinoma.
The present study indicated that rectal T1
carcinomas were larger than colon T1 carcinomas. This may be
explained by differences in their morphologies, particularly in
terms of the percentage of cases classified as LST-Gs. LSTs
typically extend laterally and circumferentially rather than
vertically along the colonic wall (22) and are associated with a lower
frequency of invasion than polypoid lesions of similar size.
Although a similar proportion of colon and rectal T1 carcinomas
were classified as LSTs (24.5% vs. 26.0%, P=0.80), LST-Gs accounted
for a significantly higher proportion of rectal LSTs than colon
LSTs [84.6% (22/26) vs. 28.6% (32/112), P<0.01]. In accordance
with previous results (23), LST-Gs
were observed to be significantly larger than LST-NGs (39.9±17.4 mm
vs. 24.5±9.3 mm, P<0.01), which may have affected the
differences between the size and growth patterns of colon and
rectal T1 carcinomas. Among patients who underwent endoscopic
resection alone during the same period, rectal T1 carcinomas were
significantly larger than colon T1 carcinomas (25.1±19.7 mm vs.
19.7±12.1 mm, P=0.016).
In agreement with a previous study (5), the rate of vascular invasion was
observed to be significantly higher in rectal than colon T1
carcinomas. Differences in vascular invasion may have resulted from
differences between the anatomical features of the rectum and
colon. The rectum is supplied by both the inferior mesenteric
artery and the internal iliac artery, with veins from the rectum
returning to the vena cava and portal vein. Although blood vessel
density differs in the colon and rectum, its association with
vascular invasion remains unclear. It has been reported that tumor
location in the rectum is a significant independent risk factor for
delayed bleeding following endoscopic submucosal dissection for
colorectal neoplasms, potentially as a consequence of differences
in blood vessel density (24).
Guidelines from the National Comprehensive Cancer Network, the
European Society for Medical Oncology and JSCCR identify vascular
invasion as a risk factor for lymph node metastasis and recommend
that such patients undergo surgery with lymph node dissection
(2,25,26).
Additionally, vascular invasion is an independent predictor of
distant recurrence and survival in all patients with colorectal
carcinoma (27,28), suggesting the need for surgery in
patients with colon and rectal T1 carcinomas who exhibit vascular
invasion. However, as reported in a meta-analysis performed by
Bosch et al (9), lymphatic
invasion is the most powerful predictor of lymph node metastasis,
whereas vascular invasion is a weaker predictor. This may explain
the absence of a significant difference between rates of lymph node
metastasis for colon and rectal T1 carcinoma in the current
study.
The present study had three primary limitations: i)
It was a retrospective analysis of patients treated at a single
center, although it did include a larger cohort of patients than in
previous, similar studies (29–32). ii)
The retrospective design of the current study may have introduced
some selection bias. When evaluating the incidence of lymph node
metastasis, only patients who had undergone surgery were included.
Patients treated by endoscopic resection alone were excluded as the
incidence of lymph node metastasis this group was not precisely
assessed. iii) The pathological diagnosis or features of these
patients were not re-evaluated. Therefore, a large-scale
prospective trial is necessary to verify the current management
strategy for colorectal T1 carcinoma.
In conclusion, the results of the present study
clearly demonstrate that patterns of lymph node metastasis did not
differ between rectal and colon T1 carcinomas, even though rectal
T1 carcinomas were larger and accompanied by a significantly higher
rate of vascular invasion. Surgery should be the first-line
treatment for high-risk patients with colon or rectal T1 carcinoma,
even though rectal surgery is comparatively more invasive. Although
investigated lymph node metastasis and other clinicopathological
characteristics were investigated in the current study, survival
and recurrence should be considered in future work.
Acknowledgements
The authors wish to express their gratitude to all
members of the Digestive Disease Center of Showa University
Northern Yokohama Hospital.
References
|
1
|
Matsuda T, Marugame T, Kamo K, Katanoda K,
Ajiki W and Sobue T: Japan Cancer Surveillance Research Group:
Cancer incidence and incidence rates in Japan in 2002: Based on
data from 11 population-based cancer registries. Jpn J Clin Oncol.
38:641–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese Society for Cancer of the Colon and Rectum (JSCCR)
Guidelines 2014 for treatment of colorectal cancer. Int J Clin
Oncol. 20:207–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frattini M, Balestra D, Suardi S, Oggionni
M, Alberici P, Radice P, Costa A, Daidone MG, Leo E, Pilotti S, et
al: Different genetic features associated with colon and rectal
carcinogenesis. Clin Cancer Res. 10:4015–4021. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Wei XZ, Fu CG, Zhao RH and Cao FA:
Patterns of lymph node metastasis are different in colon and rectal
carcinomas. World J Gastroenterol. 16:5375–5379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ikematsu H, Yoda Y, Matsuda T, Yamaguchi
Y, Hotta K, Kobayashi N, Fujii T, Oono Y, Sakamoto T, Nakajima T,
et al: Long-term outcomes after resection for submucosal invasive
colorectal cancers. Gastroenterology. 144:551–559; quiz e14. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pommergaard HC, Gessler B, Burcharth J,
Angenete E, Haglind E and Rosenberg J: Preoperative risk factors
for anastomotic leakage after resection for colorectal cancer: A
systematic review and meta-analysis. Colorectal Dis. 16:662–671.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kakodkar R, Gupta S and Nundy S: Low
anterior resection with total mesorectal excision for rectal
cancer: Functional assessment and factors affecting outcome.
Colorectal Dis. 8:650–656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kobayashi H, Mochizuki H, Morita T, Kotake
K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hase K, Oya M, et
al: Characteristics of recurrence after curative resection for T1
colorectal cancer: Japanese multicenter study. J Gastroenterol.
46:203–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bosch SL, Teerenstra S, de Wilt JH,
Cunningham C and Nagtegaal ID: Predicting lymph node metastasis in
pT1 colorectal cancer: A systematic review of risk factors
providing rationale for therapy decisions. Endoscopy. 45:827–834.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
The Paris endoscopic classification of
superficial neoplastic lesions, . Esophagus, stomach and colon:
November 30 to December 1, 2002. Gastrointest Endosc. 58:(Suppl 6).
S3–S43. 2003.PubMed/NCBI
|
|
11
|
Kudo S, Lambert R, Allen JI, Fujii H,
Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T, et al:
Nonpolypoid neoplastic lesions of the colorectal mucosa.
Gastrointest Endosc. 68:(Suppl 4). S3–S47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kudo S, Tamura S, Nakajima T, Yamano H,
Kusaka H and Watanabe H: Diagnosis of colorectal tumorous lesions
by magnifying endoscopy. Gastrointest Endosc. 44:8–14. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuda T, Fujii T, Saito Y, Nakajima T,
Uraoka T, Kobayashi N, Ikehara H, Ikematsu H, Fu KI, Emura F, et
al: Efficacy of the invasive/non-invasive pattern by magnifying
chromoendoscopy to estimate the depth of invasion of early
colorectal neoplasms. Am J Gastroenterol. 103:2700–2706. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kudo S, Tamegai Y, Yamano H, Imai Y,
Kogure E and Kashida H: Endoscopic mucosal resection of the colon:
The Japanese technique. Gastrointest Endosc Clin N Am. 11:519–535.
2001.PubMed/NCBI
|
|
15
|
Tsuruta O, Toyonaga A, Ikeda H, Tanikawa K
and Morimatsu M: Clinicopathological study of superficial-type
invasive carcinoma of the colorectum. Int J Oncol. 10:1003–1008.
1997.PubMed/NCBI
|
|
16
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO Classification of Tumours of the Digestive System
Lyon. France: 2010
|
|
17
|
Ueno H, Mochizuki H, Hashiguchi Y,
Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H,
Ozawa K, et al: Risk factors for an adverse outcome in early
invasive colorectal carcinoma. Gastroenterology. 127:385–394. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zenni GC, Abraham K, Harford FJ, Potocki
DM, Herman C and Dobrin PB: Characteristics of rectal carcinomas
that predict the presence of lymph node metastases: Implications
for patient selection for local therapy. J Surg Oncol. 67:99–103.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saclarides TJ, Bhattacharyya AK,
BrittonKuzel C, Szeluga D and Economou SG: Predicting lymph node
metastases in rectal cancer. Dis Colon Rectum. 37:52–57. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chok KS and Law WL: Prognostic factors
affecting survival and recurrence of patients with pT1 and pT2
colorectal cancer. World J Surg. 31:1485–1490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paty PB, Enker WE, Cohen AM and Lauwers
GY: Treatment of rectal cancer by low anterior resection with
coloanal anastomosis. Ann Surg. 219:365–373. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kudo S: Endoscopic mucosal resection of
flat and depressed types of early colorectal cancer. Endoscopy.
25:455–461. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Uraoka T, Saito Y, Matsuda T, Ikehara H,
Gotoda T, Saito D and Fujii T: Endoscopic indications for
endoscopic mucosal resection of laterally spreading tumours in the
colorectum. Gut. 55:1592–1597. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Terasaki M, Tanaka S, Shigita K, Asayama
N, Nishiyama S, Hayashi N, Nakadoi K, Oka S and Chayama K: Risk
factors for delayed bleeding after endoscopic submucosal dissection
for colorectal neoplasms. Int J Colorectal Dis. 29:877–882. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Labianca R, Nordlinger B, Beretta GD,
Mosconi S, Mandalà M, Cervantes A and Arnold D: ESMO Guidelines
Working Group: Early colon cancer: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
24:(Suppl 6). vi64–vi72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Benson AB III, BekaiiSaab T, Chan E, Chen
YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fenton
MJ, et al: Localized colon cancer, version 3.2013: Featured updates
to the NCCN Guidelines. J Natl Compr Canc Netw. 11:519–528.
2013.PubMed/NCBI
|
|
27
|
Brown CE and Warren S: Visceral metastasis
from rectal carcinoma. Surg Gynecol Obstet. 66:611–621. 1938.
|
|
28
|
Messenger DE, Driman DK and Kirsch R:
Developments in the assessment of venous invasion in colorectal
cancer: Implications for future practice and patient outcome. Hum
Pathol. 43:965–973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kikuchi R, Takano M, Takagi K, Fujimoto N,
Nozaki R, Fujiyoshi T and Uchida Y: Management of early invasive
colorectal cancer. Risk of recurrence and clinical guidelines. Dis
Colon Rectum. 38:1286–1295. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okuyama T, Oya M and Ishikawa H: Budding
as a risk factor for lymph node metastasis in pT1 or pT2
well-differentiated colorectal adenocarcinoma. Dis Colon Rectum.
45:628–634. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nascimbeni R, Burgart LJ, Nivatvongs S and
Larson DR: Risk of lymph node metastasis in T1 carcinoma of the
colon and rectum. Dis Colon Rectum. 45:200–206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sakuragi M, Togashi K, Konishi F, Koinuma
K, Kawamura Y, Okada M and Nagai H: Predictive factors for lymph
node metastasis in T1 stage colorectal carcinomas. Dis Colon
Rectum. 46:1626–1632. 2003. View Article : Google Scholar : PubMed/NCBI
|