Introduction
Cholangiocarcinoma (CCA) is one of the most common
causes of cancer-associated mortality in Thailand, and is one of
the most aggressive types of cancer due to its resistance to
chemotherapy and propensity for local and distant invasion
(1). Currently, no effective therapy
has been identified for the disease, and alternative therapeutic
options are urgently required. Certain dietary phytochemicals have
been previously observed to exert chemopreventive and anticancer
effects, and have been demonstrated to modulate apoptotic signaling
pathways, which may be targeted for the prevention and treatment of
CCA (2). To the best of our
knowledge, the present study is the first to extensively examine
the use of phytochemicals in CCA.
Ten different medicinal plants were selected for the
current study and evaluated with regard to their cytotoxicity and
cytotoxic mechanisms in a CCA cell line. The selected medicinal
plants included Phyllanthus emblica fruit pulp (PEf) and
seed (PEs), which contains polyphenols and hydrolysable
tannin-derived compounds (3);
Phyllanthus extracts have been demonstrated to trigger
apoptosis and regulate multiple survival signal pathways in some
cancer cell types (4,5). The plants also included Curcuma
longa (CL), Terminalia chebula fruit pulp (TCf) and seed
(TCs), Moringa oleifera seed (MOs), Momordica
charantia, Areca catechu seed (ACs), Brassica
oleracea var. rubra and Tinospora crispa (TiC).
These extracts have previously demonstrated anti-proliferative
effects via modulation of the apoptotic pathways (6–14).
Curcumin, derived from CL, has previously been shown
to exhibit apoptotic effects in various cancer cell lines through
the activation of caspase-3 and the downregulation of B-cell
lymphoma-2 (BCL-2). Curcumin also activates the expression of tumor
protein p53, peroxisome proliferator-activated receptor (PPAR), and
other tumor suppressor genes, and downregulates specific oncogenes,
including c-Myc, human epidermal growth factor receptor 2 and
epidermal growth factor receptor (6).
Chebulagic acid from the TCf has previously been demonstrated to
induce G1 arrest, inhibit nuclear factor κ-light-chain-enhancer of
activated B cells (NF-κB) and induce apoptosis in retinoblastoma
cells (7). MOs and M. oleifera
leaf extracts have been observed to induce CCA cell apoptosis by
inducing reactive oxygen species accumulation and mitochondrial
dysfunction (8). M. oleifera
leaf extract inhibits pancreatic cancer cells by targeting the
NF-κB signaling pathway, increasing the efficacy of
chemotherapeutic agents in human pancreatic cancer cells (9). Cucurbitae-type triterpene glycosides
from M. charantia fruit (MCf) inhibit NF-κB and activate
PPAR in HepG2 cells (10). In
addition, M. charantia leaf extract induces apoptosis
through caspase- and mitochondria-dependent pathways in human
cancer cells (11). Arecoline from
ACs has been demonstrated to induce HaCaT cell apoptosis by
upregulating the expression and activation of cleaved-BH3
interacting-domain death agonist, cleaved-PARP and
cleaved-caspase-3 (12). Sulforaphane
from red cabbage [B. oleracea var. capitata L. f.
rubra (BO)] has been demonstrated to reduce BCL-2 expression
levels (antiapoptotic) and reduce the induction of p53,
BCL-2-associated X protein (proapoptotic) and caspase-3 in HEp-2
cells (13). Methanol extract of the
T. crispa stem has been observed to promote apoptosis by
upregulating caspase-3 expression levels and insulin sensitization,
through the inhibition of the insulin-like growth factor-1 receptor
in HepG2 cells (14). Additionally,
Solanum torvum (ST) and Allium ascalonicum (AA) have
demonstrated cytotoxic effects in multiple cancer cell lines
(15,16); however, the mechanisms underlying
these effects require further study for elucidation.
The aim of the present study was to investigate the
roles of specific medicinal plants that inhibit CCA cell
proliferation and identify the molecular mechanisms underlying
this.
Materials and methods
Plant materials and extraction
procedures
All plant samples including PEf, PEs, TCf, TCs, ACs,
BO, CL, ST, AA, MCf pulp, M. charantia seed (MCs), TiC,
M. oleifera fruit (MOf) pulp and MOs were purchased from
Rangsit market in Amphoe Mueang, Pathum Thani province, Thailand.
The fruits were separated into pulp and seed and all of the samples
were dried at 55°C and ground into powder. Powder samples (15 g)
were macerated with 300 ml 95% ethanol for 24 h. Extract solutions
were subsequently dried by rotary evaporation and freeze-drying,
and stored at −20°C until required. The sample extracts were
freshly prepared in dimethyl sulfoxide (DMSO) to the concentration
of 50 mg/ml then diluted with HAM's F12 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) to the concentration of
1–500 µg/ml.
Cell proliferation assay
The human CCA cell line RMCCA1 (16) was grown in HAM's F12 medium
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a 5% CO2 humidified
atmosphere. For the proliferation assays, cells were seeded into
96-well culture plates at a density of 10,000 cells per well,
followed by the addition of sample extracts in various
concentrations (1–500 µg/ml) or control medium (Ham's F12 medium
containing 0.01% DMSO). The cells were subsequently incubated for
48 h prior to the application of Cell Proliferation Reagent WST-1
(Roche Diagnostics GmbH, Mannheim, Germany) according to the
manufacturer's instructions. Cell viability was calculated as a
percentage, relative to the control cells. The extracts that
exhibited anti-proliferative effects were selected for further
assays investigating the effects of stress and apoptosis on the
RMCCA1 cell line.
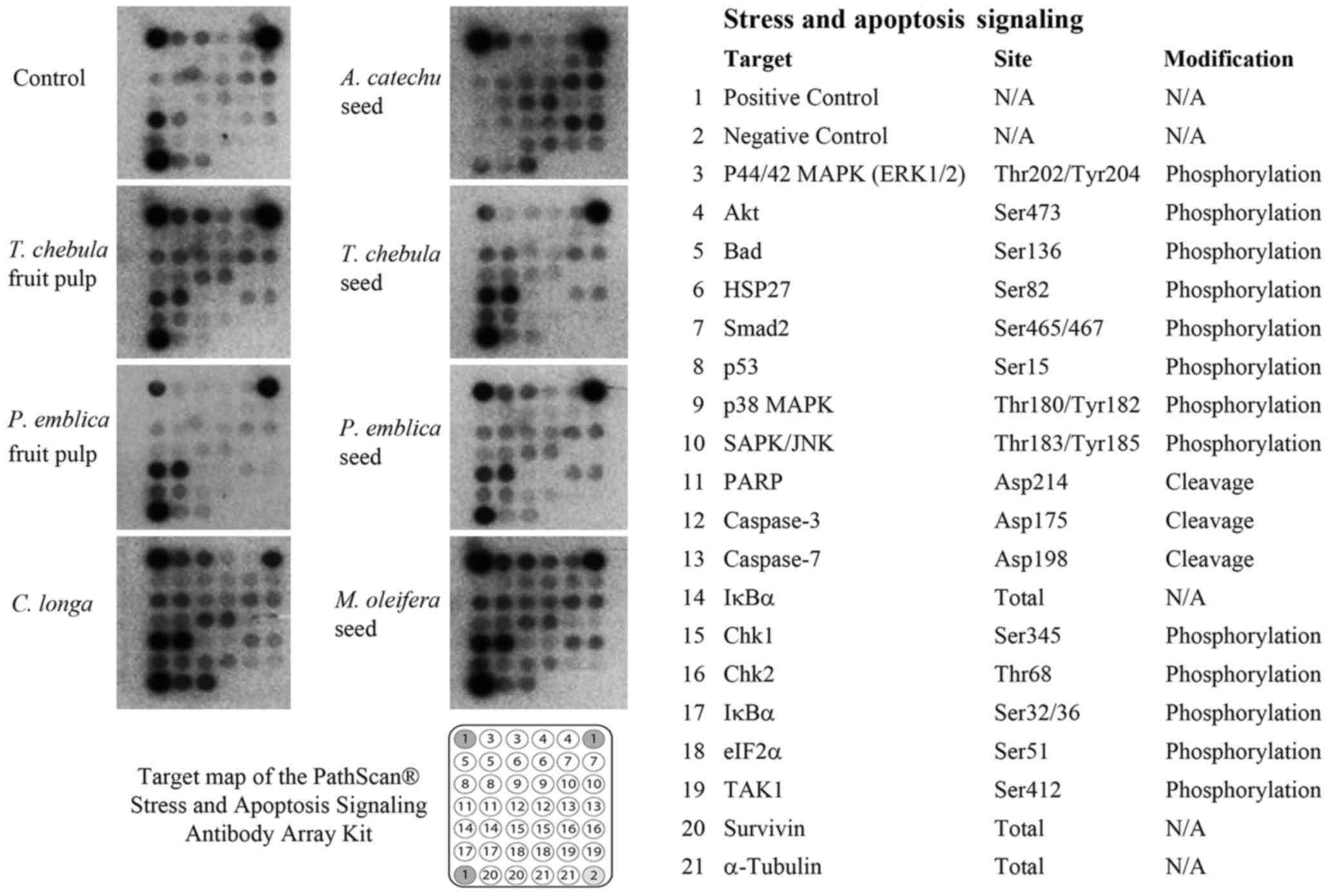
Cellular stress and apoptosis
signaling assay
RMCCA1 cells were treated with seven selected
extracts (PEf, PEs, TCf, TCs, ACs, CL and MOs) prior to using the
PathScan® Stress and Apoptosis Signaling Antibody Array
kit with a chemiluminescent readout (cat. 12856; Cell Signaling
Technology, Inc., Danvers, MA, USA) according to the manufacturer's
instructions. The kit facilitated the simultaneous detection of the
phosphorylation of 13 signaling molecules, including extracellular
signal-regulated kinase (ERK)-1/2 (Thr202/tyr204), protein kinase B
(Akt; Ser473), BCL-2-associated death promoter (Bad; Ser136), heat
shock protein 27 (HSP27; Ser82), Smad2 (Ser465/467), p53 (Ser15),
p38 mitogen activated protein kinase (MAPK; Thr180/Tyr182),
stress-activated protein kinases (SAPK)/Jun amino-terminal kinases
(JNK; Thr183/Tyr185), checkpoint kinase (Chk) 1 (Ser345), Chk2
(Thr68), NF-κB inhibitor α (IκBα Ser32/36) eukaryotic translation
initiation factor 2 subunit α (elF2; Ser51) and transforming growth
factor-β-activated kinase 1 (TAK1) (Ser412). In addition, the kit
also allowed for simultaneous detection of 3 cleaved molecules,
including poly (ADP-ribose) polymerase (PARP) (Asp214), caspase-3
(Asp175) and caspase-7 (Asp198), and detection of 3 total proteins,
including IκBα, survivin and α-tubulin. Cell lysates were incubated
on the slide followed by a biotinylated detection antibody
cocktail. Streptavidin-conjugated HRP and LumiGLO®
Reagent were then used to visualize the bound detection antibody by
chemiluminescence. Images were acquired by briefly exposing the
slide to standard chemiluminescent film. The spot intensity was
quantified using ImageJ software (National Institutes of Health,
Bethesda, MD, USA). The intensity of each signal was calculated
relative to the positive control signal of the array kit.
Statistical analysis
The experiments were performed in triplicate and the
data are presented as the mean ± standard deviation. Data between
three or more groups were compared using one-way analysis of
variance, followed by Dunnett's post hoc test. P<0.05 was
considered to indicate a statistically significant result.
Results
Effects of plant extracts on cell
proliferation
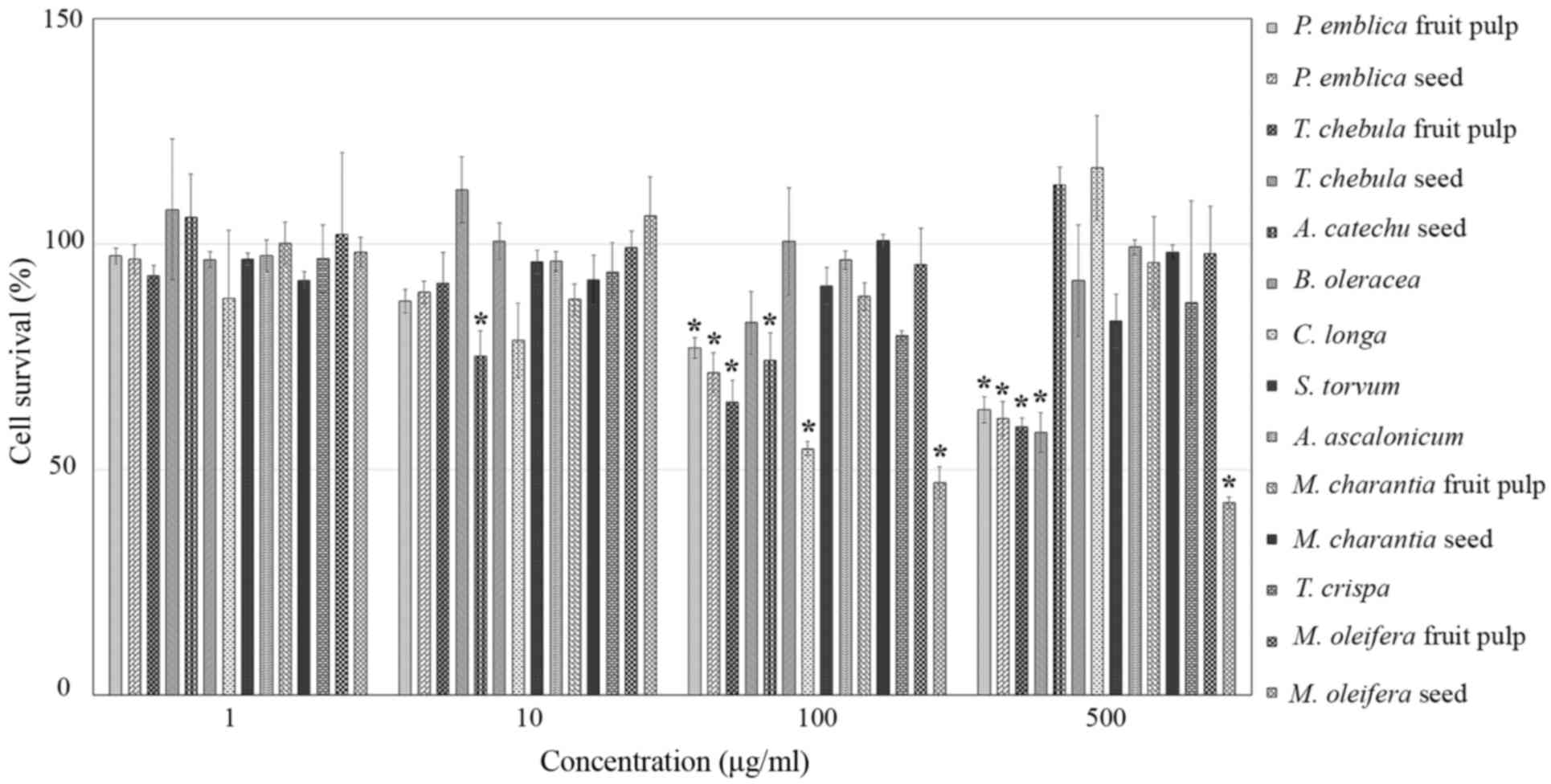
Cell proliferation assays were performed in RMCCA1
cells treated with 14 extract samples at concentrations of 1, 10,
100 and 500 µM or control medium. After 48 h of incubation, the
results demonstrated that TCs (500 µg/ml), PEf, PEs, TCf, CL and
MOs (100 µg/ml), and ACs (10 µg/ml) significantly inhibited RMCCA1
cell proliferation (P=0.002, <0.001, <0.001, <0.001,
0.002, <0.001 and 0.008, respectively). However, BO, ST, AA,
MCf, MCs, TiC and MOf had no significant inhibitory effect on
RMCCA1 cell proliferation, as compared with control cells
(P>0.05; Fig. 1). Therefore, PEf,
PEs, TCf, TCs, ACs, CL and MOs were selected to further study the
mechanisms underlying CCA cell proliferation inhibition.
Effects of plant extracts on cellular
stress and apoptosis signaling
A PathScan® Stress and Apoptosis
Signaling Antibody Array kit with a chemiluminescent readout was
used for the simultaneous detection of 19 signaling molecules that
are involved in the regulation of the cellular stress response and
apoptosis. The expression levels of α-tubulin were used to
normalize the signals between various samples, and the α-tubulin
levels were observed to be relatively constant between the samples.
The RMCCA1 cells treated with PEs, TCf, TCs, ACs, CL or MOs
exhibited high intensity of the apoptotic signals p53, PARP and
caspase-3 whereas PEf did not appear to induce apoptotic signals
(Fig. 2). The intensity of each
signal was measured by ImageJ software. The expression level was
calculated relative to the positive control signal as intensity
ratio.
Caspase-3 and −7 proteases exert pro-apoptotic
functions through the cleavage of a number of cellular targets
(18). Increased activation of
caspase-3 was demonstrated in RMCCA1 cells treated with the
extracts of PEs, TCf, TCs, ACs, CL and MOs (P=0.018, <0.001,
0.018, 0.032, 0.029 and 0.006, respectively); however, this was not
observed in RMCCA1 cells treated with the extract of PEf (P=0.183).
No significant differences in caspase-7 expression levels were
identified.
Other pro-apoptotic molecules, including
cleaved-PARP, phosphorylated-Chk2 and phosphorylated-p53, were
demonstrated to be upregulated in RMCCA1 cells treated with certain
plant extracts. The expression level of cleaved-PARP was
significantly increased in RMCCA1 cells treated with the extracts
of PEs, TCf, TCs, CL and MOs (P=0.018, 0.014, 0.003, 0.039 and
0.005, respectively). Phosphorylated-Chk2 was significantly
upregulated in RMCCA1 cells treated with the extracts of PEs, TCf,
TCs, ACs and MOs (P=0.048, 0.005, 0.012, 0.029 and 0.007,
respectively). The activation of phosphorylated-p53 was
demonstrated in RMCCA1 cells treated with the extracts of PEs, TCf,
TCs, ACs, CL and MOs (P=0.014, 0.005, 0.001, 0.028, 0.041 and
0.002, respectively; Fig. 3).
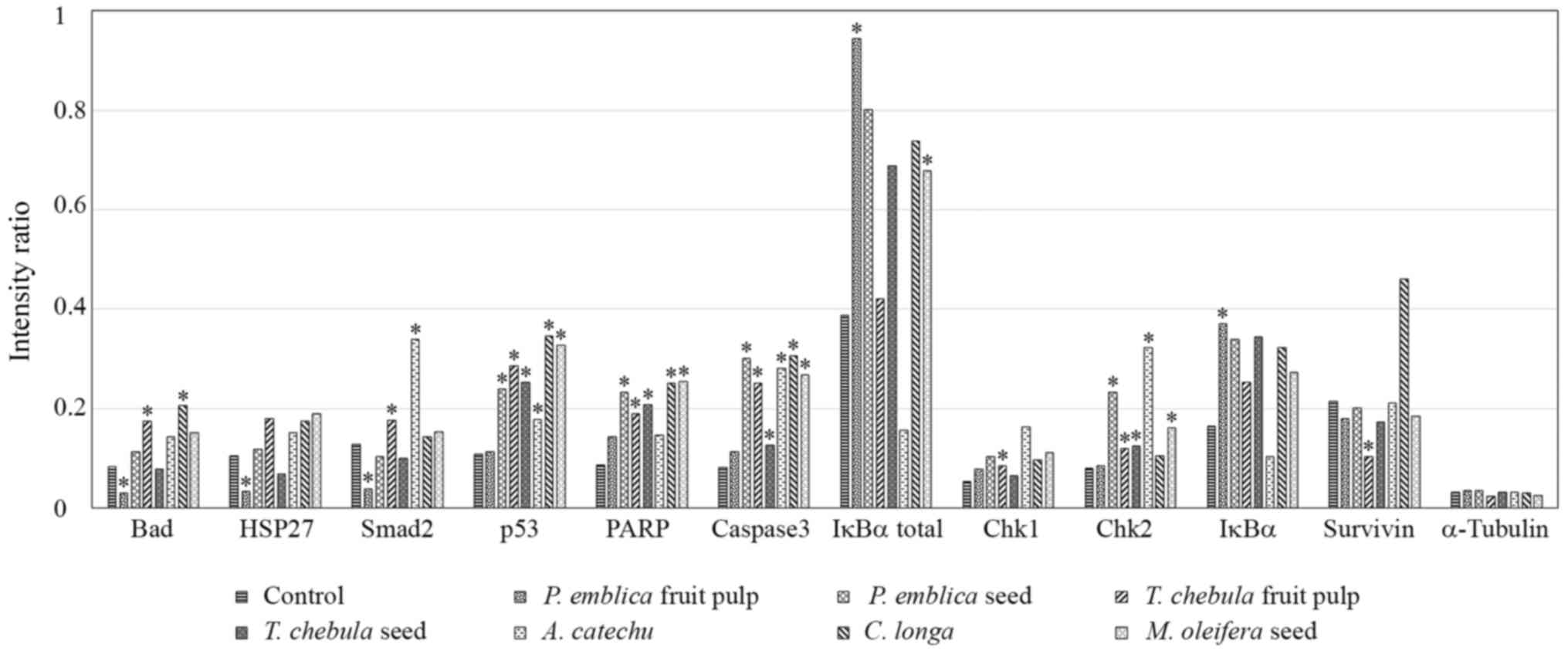 | Figure 3.Array image pixel intensity ratio of
phosphorylated/cleaved pro-apoptotic signaling molecules in RMCCA1
cells treated with medicinal plants. RMCCA1 cells were treated with
seven selected plants that had exhibited apoptotic signals. The
phosphorylation levels of Bad at Ser136, HSP27 at Ser82, Smad2 at
Ser465/467, p53 at Ser15, IκBα at Ser32/36, Chk1 at Ser345 and Chk2
at Thr68, and the cleavage of PARP at Asp214 and caspase-3 at
Asp175, were significantly altered. The expression of survivin
decreased only in RMCCA1 cells treated with the extract of TCf. The
expression levels of α-tubulin were used to normalize the signals
between various samples, and these were observed to be consistent.
*P<0.05 vs. control. Bad, BCL-2-associated death promoter;
HSP27, heat shock protein 27; PARP, poly (ADP-ribose) polymerase;
IκBα, NF-κB inhibitor α; Chk, checkpoint kinase. |
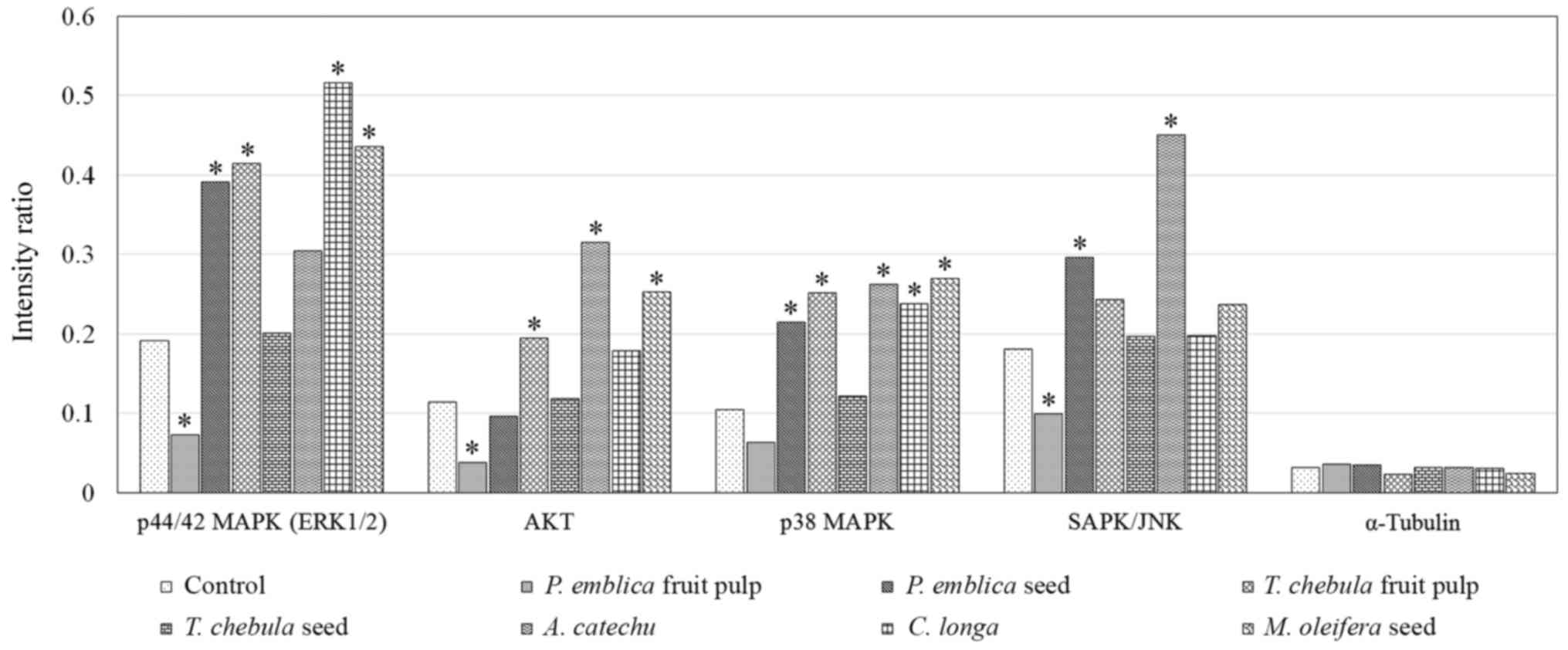
Activation of the Akt and MAPK pathways, including
ERK1/2 and p38 MAPK, were demonstrated in RMCCA1 cells. The
expression level of phosphorylated-ERK1/2 was demonstrated to be
upregulated in RMCCA1 cells treated with the extract of PEs, TCf,
CL and MOs (P=0.010, 0.003, <0.001 and 0.001, respectively)
while RMCCA1 cells treated with the extracts of PEs, TCf, ACs, CL
and MOs exhibited significantly increased expression levels of
phosphorylated-p38 MAPK (P=0.004, 0.002, 0.042, 0.037 and 0.002,
respectively). The expression level of phosphorylated-AKT was
significantly upregulated in RMCCA1 cells treated with the extract
of TCf, ACs and MOs (P=0.001, 0.034 and <0.001, respectively).
However, the expression level of phosphorylated-ERK1/2 was
significantly downregulated in the RMCCA1 cells treated with PEf
extract (P=0.005), while the expression level of phosphorylated-p38
MAPK appeared downregulated but was not significant (P=0.054). In
addition, the expression of phosphorylated-SAPK/JNK in the RMCCA1
cells treated with PEf extract was significantly downregulated
(P=0.004). The activation of SAPK/JNK was demonstrated in RMCCA1
cells treated with the extracts of PEs and ACs (P=0.043 and 0.023,
respectively; Fig. 4).
Discussion
The present study investigated the inhibition of CCA
cell proliferation by various types of medicinal plants. Each plant
extract was composed of mixture of bioactive compounds (19); however, the current study evaluated
the activity of the entire extract rather than the activities of
the individual compounds. Inhibition of CCA cell proliferation was
associated with the upregulation of numerous intracellular
signaling molecules and pro-apoptotic molecules. A total of 19
signaling molecules involved in the regulation of the stress
response and apoptosis were simultaneously detected in the present
study.
Caspase-3 protease exerts a pro-apoptotic function
through the cleavage of multiple cellular targets, and is activated
in apoptotic cells by extrinsic (death ligand) and intrinsic
(mitochondrial) pathways (20). The
present study demonstrated that caspase-3 is activated (via
cleavage at Asp175) in CCA cells treated with the extracts of PEs,
TCf, TCs, ACs, CL and MOs. The results revealed that these same
extracts also inhibit PARP function via cleavage at Asp214. PARP is
a DNA repair enzyme that is activated in response to DNA strand
breaks (21). During apoptosis,
cleavage of PARP-1 at Asp214 by caspase-3 is a useful marker of
this type of cell death (21). The
results of the current study suggested that the extracts of PEs,
TCf, TCs, ACs, CL and MOs induced apoptosis in RMCCA1 cells.
The extracts of PEs, TCf, TCs, ACs and MOs were
identified to increase Chk2 phosphorylation in CCA cells. Chk2
functions as a cell cycle checkpoint regulator and is a putative
tumor suppressor protein; it is phosphorylated in response to DNA
damage and prevents the cell from entering mitosis, thus arresting
the cell cycle in the G1 phase (22).
Previous studies have demonstrated that gallic acid (a hydrolysable
tannin present in TCf) and quercetin induce cell cycle arrest in
the G1 phase and induce apoptosis in certain cancer cells by
inactivating the phosphorylation of Cdc25A/Cdc25C-Cdc2 via Chk2
activation (23,24). Therefore, the induction of Chk2 by the
aforementioned plant extracts may have an important role in CCA
apoptosis.
Accumulation of p53 has a crucial role in cell
apoptosis. The phosphorylation of p53 underlies p53 accumulation
and its subsequent prolonged protein half-life due to
ubiquitination and degradation (25).
The phosphorylation of p53 at Ser15 promotes the accumulation and
functional activation of p53 in response to apoptotic stimuli or
DNA damage (26). The current study
demonstrated that p53 phosphorylation at Ser15 is increased in CCA
cells treated with extracts of PEs, TCf, TCs, ACs, CL and MOs.
Furthermore, it has previously been demonstrated that the
phosphorylation of p53 at Ser15 may induce protein translocation to
the mitochondria (intrinsic pathway), thereby inducing apoptosis by
the mitochondrial pathway (27).
Consequently, the present study found that the induction of p53
phosphorylation of at Ser15 in CCA cells treated with the extracts
of PEs, TCf, TCs, ACs, CL and MOs, may also induce apoptosis by
activating caspase-3 which serve a critical role in the induction
of apoptosis in mitochondrial pathway and inhibiting PARP function.
Wu et al (28) identified that
the silencing of p53 by specific small interfering RNA reduced the
effect of CL on the induction of nasopharyngeal carcinoma cell
apoptosis. Therefore, p53 function may be important to the ability
of the specified plant extracts to induce CCA cell apoptosis.
ERK1/2 and p38 kinase are activated in CCA cells
that have been treated with the extracts of PEs, TCf, ACs, CL and
MOs. Our previous study demonstrated that ERK1/2 functions to
promote survival in CCA cells, whereas p38 kinase activation is a
primary signal for apoptosis (29).
ERK1/2 signaling is required for the proliferation of CCA cells and
the inhibition of ERK1/2 contributes to cell apoptosis (30). A previous study suggested that the
opposing effects of ERK1/2 and p38 kinase in apoptosis are
regulated by the phosphorylation and accumulation of p53 (31). In addition, p38 kinase may physically
interact with and phosphorylate p53 at Ser15 in response to
ultraviolet B radiation, and induce apoptosis in vitro and
in vivo (32). The present
study demonstrated that the simultaneous activation of p38 kinase
and p53 could be observed in CCA cells treated with extracts of
PEs, TCf, ACs, CL and MOs, which suggests that the interaction of
p53 and p38 MAPK induces apoptosis in CCA cells.
The current study also demonstrated that JNK is
activated in CCA cells that have been treated with extracts of PEs
and ACs. Consistently, a previous study revealed that curcumin
induced osteoclastoma cell apoptosis by activating the JNK
signaling pathway (33). JNK belongs
to the superfamily of MAPKs and has a critical role in death
receptor-initiated (extrinsic) and mitochondrial (intrinsic)
apoptotic pathways (34). The
induction of apoptosis by PEs and ACs extracts may be regulated by
the JNK pathway.
In the present study, PEf extract was demonstrated
to inhibit CCA cell proliferation; however, apoptotic signals in
the cells treated with PEf were not observed. We hypothesized that
the anti-proliferative effect of PEf occurs via the inhibition of
Akt activation. Akt is a protein kinase that promotes cell growth
and survival (35), and our previous
study demonstrated that Akt is involved in the regulation of CCA
cell proliferation and invasion (36). The results of the present study are
concordant with another previous study, which demonstrated that
Phyllanthus extracts inhibit prostate cancer cell
proliferation by inhibiting Akt activation (37).
The present study investigated the interaction of
certain signaling molecules in CCA cells that have important roles
in the regulation of apoptosis by specific plant extracts. Certain
plants exhibited anti-proliferative effects in CCA cells by
inducing pro-apoptotic signals; however, they also exhibited
anti-apoptotic signals, including the phosphorylation of Bad at
Ser136 and ERK1/2. Furthermore, the concentrations of the extracts
required to inhibit CCA cells are relatively high, as the selected
samples were from dietary medicinal plants. Further studies must be
performed in in vivo to investigate the potential
applications of these plant extracts in the clinical treatment of
CCA.
Acknowledgements
This study was funded by the Research Institute of
Rangsit University (grant no., 37/2555). The manuscript underwent
English language editing by editors at American Journal
Experts.
References
|
1
|
Sripa B and Pairojkul C:
Cholangiocarcinoma: Lessons from Thailand. Curr Opin Gastroenterol.
24:349–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shanmugam MK, Kannaiyan R and Sethi G:
Targeting cell signaling and apoptotic pathways by dietary agents:
Role in the prevention and treatment of cancer. Nutr Cancer.
63:161–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao T, Sun Q, Marques M and Witcher M:
Anticancer properties of Phyllanthus emblica (Indian gooseberry).
Oxid Med Cell Longev. 2015:9508902015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mahata S, Pandey A, Shukla S, Tyagi A,
Husain SA, Das BC and Bharti AC: Anticancer activity of Phyllanthus
emblica Linn. (Indian gooseberry): Inhibition of transcription
factor AP-1 and HPV gene expression in cervical cancer cells. Nutr
Cancer. 65:(Suppl). S88–S97. 2013. View Article : Google Scholar
|
|
5
|
Zhu X, Wang J, Ou Y, Han W and Li H:
Polyphenol extract of Phyllanthus emblica (PEEP) induces inhibition
of cell proliferation and triggers apoptosis in cervical cancer
cells. Eur J Med Res. 18:462013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahmani AH, Al Zohairy MA, Aly SM and Khan
MA: Curcumin: A potential candidate in prevention of cancer via
modulation of molecular pathways. BioMed Res Int. 2014:7616082014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumar N, Gangappa D, Gupta G and Karnati
R: Chebulagic acid from Terminalia chebula causes G1 arrest,
inhibits NFκB and induces apoptosis in retinoblastoma cells. BMC
Complement Altern Med. 14:3192014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leelawat S and Leelawat K: Moringa
oleifera extracts induce cholangiocarcinoma cell apoptosis by
induction of reactive oxygen species production. Int J Pharmacog
Phytochem Res. 6:183–189. 2014.
|
|
9
|
Berkovich L, Earon G, Ron I, Rimmon A,
Vexler A and Lev-Ari S: Moringa Oleifera aqueous leaf extract
down-regulates nuclear factor-kappaB and increases cytotoxic effect
of chemotherapy in pancreatic cancer cells. BMC Complement Altern
Med. 13:2122013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nhiem NX, Yen PH, Ngan NT, Quang TH, Kiem
PV, Minh CV, Tai BH, Cuong NX, Song SB and Kim YH: Inhibition of
nuclear transcription factor-κB and activation of peroxisome
proliferator-activated receptors in HepG2 cells by cucurbitane-type
triterpene glycosides from Momordica charantia. J Med Food.
15:369–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li CJ, Tsang SF, Tsai CH, Tsai HY, Chyuan
JH and Hsu HY: Momordica charantia extract induces apoptosis in
human cancer cells through caspase- and mitochondria-dependent
pathways. Evid-Based Complement Altern Med. 2012:2619712012.
View Article : Google Scholar
|
|
12
|
Li M, Gao F, Zhou ZS, Zhang HM, Zhang R,
Wu YF, Bai MH, Li JJ, Lin SR and Peng JY: Arecoline inhibits
epithelial cell viability by upregulating the apoptosis pathway:
Implication for oral submucous fibrosis. Oncol Rep. 31:2422–2428.
2014.PubMed/NCBI
|
|
13
|
Devi JR and Thangam EB: Mechanisms of
anticancer activity of sulforaphane from Brassica oleracea in HEp-2
human epithelial carcinoma cell line. Asian Pac J Cancer Prev.
13:2095–2100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abu MN, Salleh MAM, Radzman NHM, Ismail
WIW, Yusoff RM and Hasan HF: Insulin sensitivity enhancement of the
mixture of Tinospora Crispa and Gelam (Melaleuca Cajuputi) honey
and its antiproliferative activity on hepatocellular carcinoma,
HepG2: A preliminary study. J Med Res Devel. 2:48–54. 2013.
|
|
15
|
Yousafa Z, Wanga Y and Baydounc E:
Phytochemistry and pharmacological studies on Solanum torvum
Swartz. J Appl Pharm Sci. 3:152–160. 2013.
|
|
16
|
Mohammadi-Motlagh HR, Mostafaie A and
Mansouri K: Anticancer and anti-inflammatory activities of shallot
(Allium ascalonicum) extract. Arch Med Sci. 7:38–44. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rattanasinganchan P, Leelawat K,
Treepongkaruna SA, Tocharoentanaphol C, Subwongcharoen S,
Suthiphongchai T and Tohtong R: Establishment and characterization
of a cholangiocarcinoma cell line (RMCCA-1) from a Thai patient.
World J Gastroenterol. 12:6500–6506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nowsheen S and Yang ES: The intersection
between DNA damage response and cell death pathways. Exp Oncol.
34:234–254. 2012.
|
|
19
|
Sasidharan S, Chen Y, Saravanan D, Sundram
KM and Yoga Latha L: Extraction, isolation and characterization of
bioactive compounds form plants' extracts. Afr J Tradit Complement
Altern Med. 8:1–10. 2011.PubMed/NCBI
|
|
20
|
Shalini S, Dorstyn L, Dawar S and Kumar S:
Old, new and emerging functions of caspases. Cell Death Differ.
22:526–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu SW, Wang H, Dawson TM and Dawson VL:
Poly(ADP-ribose) polymerase-1 and apoptosis inducing factor in
neurotoxicity. Neurobiol Dis. 14:303–317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith J, Tho LM, Xu N and Gillespie DA:
The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and
cancer. Adv Cancer Res. 108:73–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suh DK, Lee EJ, Kim HC and Kim JH:
Induction of G(1)/S phase arrest and apoptosis by quercetin in
human osteosarcoma cells. Arch Pharm Res. 33:781–785. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agarwal C, Tyagi A and Agarwal R: Gallic
acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via
ATM-Chk2 activation, leading to cell cycle arrest, and induces
apoptosis in human prostate carcinoma DU145 cells. Mol Cancer Ther.
5:3294–3302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meek DW: Regulation of the p53 response
and its relationship to cancer. Biochem J. 469:325–346. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dmitrieva NI, Michea LF, Rocha GM and Burg
MB: Cell cycle delay and apoptosis in response to osmotic stress.
Comp Biochem Physiol A Mol Integr Physiol. 130:411–420. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chi SW: Structural insights into the
transcription-independent apoptotic pathway of p53. BMB Rep.
47:167–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu J, Tang Q, Zhao S, Zheng F, Wu Y, Tang
G and Hahn SS: Extracellular signal-regulated kinase
signaling-mediated induction and interaction of FOXO3a and p53
contribute to the inhibition of nasopharyngeal carcinoma cell
growth by curcumin. Int J Oncol. 45:95–103. 2014.PubMed/NCBI
|
|
29
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leelawat K, Keeratichamroen S, Leelawat S
and Tohtong R: CD24 induces the invasion of cholangiocarcinoma
cells by upregulating CXCR4 and increasing the phosphorylation of
ERK1/2. Oncol Lett. 6:1439–1446. 2013.PubMed/NCBI
|
|
31
|
Kim SJ, Ju JW, Oh CD, Yoon YM, Song WK,
Kim JH, Yoo YJ, Bang OS, Kang SS and Chun JS: ERK-1/2 and p38
kinase oppositely regulate nitric oxide-induced apoptosis of
chondrocytes in association with p53, caspase-3, and
differentiation status. J Biol Chem. 277:1332–1339. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gong X, Liu A, Ming X, Deng P and Jiang Y:
UV-induced interaction between p38 MAPK and p53 serves as a
molecular switch in determining cell fate. FEBS Lett.
584:4711–4716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao F, Liu T, Xu Y, Xu D and Feng S:
Curcumin inhibits cell proliferation and promotes apoptosis in
human osteoclastoma cell through MMP-9, NF-κB and JNK signaling
pathways. Int J Clin Exp Pathol. 8:6037–6045. 2015.PubMed/NCBI
|
|
34
|
Nishina H, Wada T and Katada T:
Physiological roles of SAPK/JNK signaling pathway. J Biochem.
136:123–126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Davis WJ, Lehmann PZ and Li W: Nuclear
PI3K signaling in cell growth and tumorigenesis. Front Cell Dev
Biol. 3:242015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leelawat K, Udomchaiprasertkul W, Narong S
and Leelawat S: Induction of MKP-1 prevents the cytotoxic effects
of PI3K inhibition in hilar cholangiocarcinoma cells. J Cancer Res
Clin Oncol. 136:1537–1544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang YQ, Jaganath I, Manikam R and Sekaran
SD: Phyllanthus suppresses prostate cancer cell, PC-3,
proliferation and induces apoptosis through multiple signaling
pathways (MAPKs, PI3K/Akt, NFκB, and hypoxia). Evid-Based
Complement Altern Med. 2013:1–13. 2013. View Article : Google Scholar
|