Introduction
Gastric cancer is the fourth most common cancer
globally and the second leading cause of cancer-associated
mortality (1). Gastrectomy with D2
dissection and progress in chemotherapy have significantly improved
survival times in patients with gastric cancer (2,3). The
standard treatment for stage II/III gastric cancer is curative
resection and adjuvant chemotherapy. On the basis of the results of
the Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer, patients
with stage II/III gastric cancer in Japan usually receive S-1 for 1
year after gastrectomy. However, despite treatment with S-1, the
5-year overall survival rate is 71.7% and thus remains
unsatisfactory (4). Therefore, great
hope has been placed on the development of personalized medicine
using biomarkers to improve outcomes in stage II/III gastric
cancer.
Platelet-derived growth factor receptor-β
(PDGFR-β) is a cell surface tyrosine kinase receptor for
members of the PDGF family. PDGFR-β is expressed in
numerous types of human neoplasms, including gastric cancer
(5–7).
PDGFR-β signaling has been reported to increase tumor cell
proliferation in an autocrine manner (8), and to stimulate angiogenesis (9), recruit pericytes (8,10) and
regulate interstitial fluid pressure (IFP) in the stroma;
PDGFR-β thereby affects the transvascular transport of
chemotherapeutic agents in a paracrine manner (11).
The present study was designed to evaluate the
clinical significance of PDGFR-β gene expression in patients
with stage II/III gastric cancer who received curative resection
followed by adjuvant chemotherapy with S-1.
Materials and methods
Patients and samples
The present study focused on surgically resected
specimens of cancer tissue and adjacent normal mucosa obtained from
134 patients with stage II/III gastric cancer who underwent
curative surgery without receiving pre-operative chemotherapy or
radiotherapy. Tumor stage was evaluated according to the 7th
edition of the International Union Against Cancer (UICC)-TNM
classification of malignant tumors (12). The patients post-operatively received
adjuvant chemotherapy with S-1. All patients were treated in the
Department of Surgery, Yokohama City University (Yokohama,
Kanagawa, Japan), the Gastroenterological Center, Yokohama City
Medical Center (Yokohama, Kanagawa, Japan) or the Department of
Gastrointestinal Surgery, Kanagawa Cancer Center (Yokohama,
Kanagawa, Japan) between March 2002 and July 2010. Informed consent
was obtained from all patients, and the study protocol was approved
by the Ethics Committees of Yokohama City University Medical
Center, Yokohama City University and Kanagawa Cancer Center. All
tissue samples were embedded in optimal cutting temperature
compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan) and
immediately stored at −80°C until use. No patient had any other
malignancies. The tissue specimens were stained with hematoxylin
and eosin, and examined histopathologically. Sections that
consisted of >80% carcinoma cells were used to prepare total
RNA.
RNA extraction and complementary DNA
(cDNA) synthesis
Total RNA isolated from gastric cancer tissues and
adjacent normal mucosa was prepared using TRIzol (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). cDNA was synthesized
from 0.4 µg total RNA using an iScript cDNA synthesis kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer's instructions. Following synthesis, the cDNA was
diluted to 0.2 µg/µl with water and stored at −20°C until use.
Oligonucleotide primers for PDGFR-β
cDNA amplification by reverse-transcription polymerase chain
reaction (RT-PCR)
The oligonucleotide primers for PDGFR-β were
as follows: Sense, 5′-CGTGGCTTTTCTGGTATCTTTGAG-3′ and antisense,
5′-CGTTGATGGATGACACCTGGAG-3′. β-actin was used as the internal
control. The oligonucleotide primers for β-actin were as follows:
Sense, 5′-AGTTGCGTTACACCCTTTCTTGAC-3′ and antisense,
5′-GCTCGCTCCAACCGACTGC-3′.
Amplification of PDGFR-β was performed for 40
cycles of 10 sec at 95°C, 10 sec at 58°C and 20 sec at 72°C.
Amplification of β-actin was performed for 40 cycles of 15 sec at
95°C, 15 sec at 60°C and 30 sec at 72°C. A 10-µl aliquot of each
amplified PCR product underwent electrophoresis in a 3% agarose gel
containing ethidium bromide.
Immunohistochemical staining
Immunohistochemical studies of PDGFR-β were
performed on formalin-fixed, paraffin-embedded surgical specimens
obtained from the gastric cancer patients. The section width was
specified as 4 µm. The tissue sections were deparaffinized and
soaked in 10 mM sodium citrate buffer (pH 6.0) at 121°C for 15 min
to retrieve cell antigens. Blocking was performed twice. First,
endogenous peroxidase activity was blocked with 3% hydrogen
peroxide solution for 5 min. Next, protein activity was blocked
with 5% skimmed milk for 5 min. Subsequent to blocking, the
sections were incubated overnight at 4°C to allow antigen-antibody
reactions to occur. Peroxidase-labeled polymer (EnVision+, rabbit;
Dako, Glostrup, Denmark) was used to detect signals of the
antigen-antibody reactions. All sections were counterstained with
hematoxylin. The primary rabbit polyclonal antibodies against
PDGFR-β (catalog no., HPA028499; Atlas Antibodies, Stockholm,
Sweden) were used at a dilution of 1:100.
RT-quantitative (q)PCR
RT-qPCR was performed using iQ SYBR Green Supermix
(Bio-Rad Laboratories, Inc.). PCR was performed in a total volume
of 15 µl, which included 0.2 µg cDNA, 0.4 µM of each primer, 7.5 µl
iQ SYBR-Green Supermix containing dATP, dCTP, dGTP and dTTP at
concentrations of 400 µM each, and 50 U/ml of iTag DNA polymerase.
The PCR cycling conditions consisted of 10 min at 95°C, followed by
40 cycles of denaturation of the cDNA for 10 sec at 95°C, annealing
for 10 sec at 56°C (60°C for β-actin) and a primer extension for 20
sec at 72°C, followed by 10 min at 72°C. To distinguish specific
from non-specific products and primer dimers, melting curve
analyses were performed. To evaluate specific mRNA expression in
the samples, a standard curve was created for each run, based on
three points from human control cDNA (Clontech Laboratories, Inc.,
CA, USA). The concentrations of each sample were calculated by
relating their crossing point to the standard curve. The number of
experimental repeats was three times, and the method used for
quantitation was relative quantities (iQ5 software version 2.0;
Bio-Rad Laboratories, Inc.).
Statistical analysis
Differences between gene expression levels in
the gastric cancer and adjacent normal mucosa samples were compared
using the Wilcoxon test. The associations between gene expression
and potential explanatory variables, including age, gender, tumor
size, histological type, serosal invasion, lymph node metastasis,
tumor-node-metastasis (TNM) stage (12), lymphatic invasion and venous invasion,
were evaluated using the χ2 test. Associations between
PDGFR-β gene expression and survival were assessed using the
Kaplan-Meier method and were compared by the log-rank test. A Cox
proportional-hazards model was used to perform univariate analyses
and stepwise multivariate analyses to determine risk factors. All
statistical analyses were performed with the Dr. SPSS II program,
version 11.0.1 J for Windows (SPSS, Inc., Chicago, IL, USA).
Two-sided P-values were calculated, and a difference was considered
statistically significant at P<0.05. Data are expressed as
median (range).
Results
PDGFR-β mRNA expression in specimens
of cancer tissue and adjacent normal mucosa
PDGFR-β gene expression was examined by
RT-PCR, using gastric cancer tissue and normal gastric mucosal
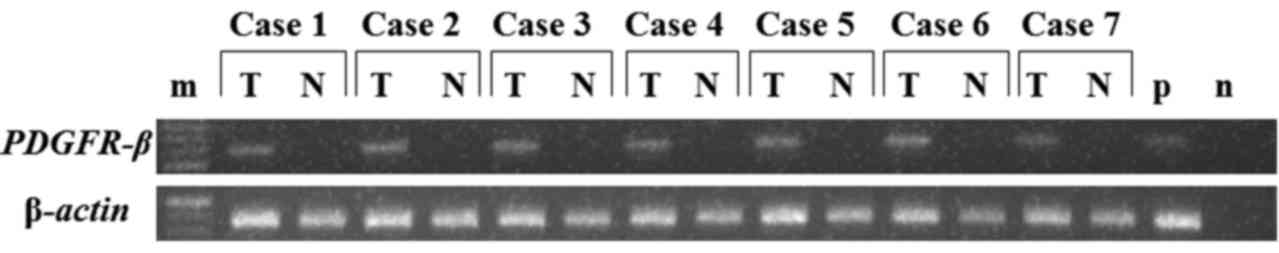
tissue obtained from 7 patients (Fig.
1). The results revealed that PDGFR-β expression was
significantly higher in tumor tissue than in normal gastric mucosal
tissue. Therefore, PDGFR-β gene expression was examined by
RT-qPCR in gastric cancer tissue and normal gastric mucosa in 75
patients for whom samples of both gastric cancer tissue and normal
gastric mucosal tissue were available. The mRNA expression levels
of PDGFR-β in specimens of cancer tissue and adjacent normal
mucosa obtained from 7 patients with gastric cancer were analyzed
by RT-PCR (Fig. 1). PDGFR-β
mRNA expression was significantly higher in the cancer tissues than
in the paired normal adjacent mucosa. PDGFR-β mRNA
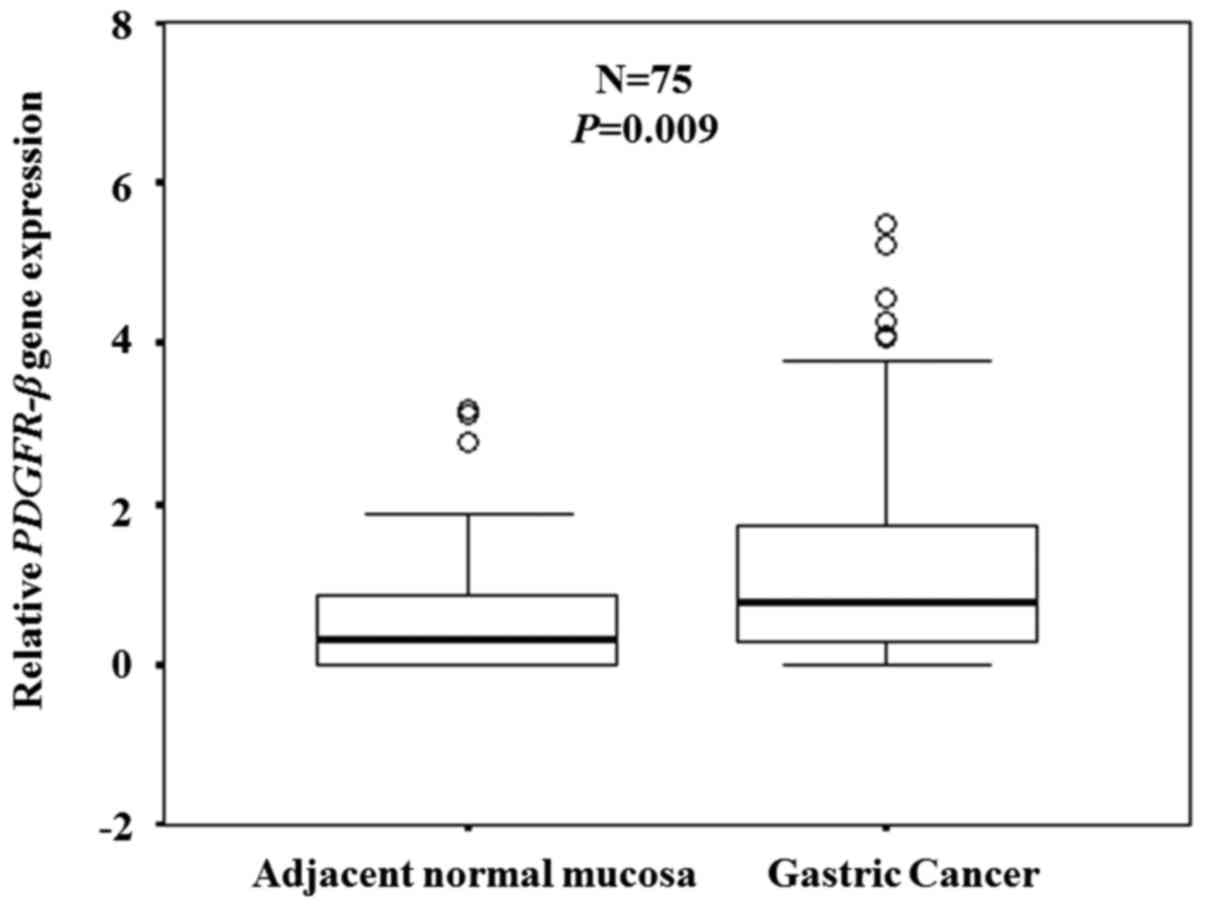
expression was confirmed in clinical samples (n=75) by RT-qPCR.
Expression levels of the PDGFR-β gene were significantly
higher in the cancer tissues compared with the adjacent normal
mucosa (P=0.009; Fig. 2).
Immunohistochemistry of PDGFR-β
expression
Expression of PDGFR-β protein was evaluated
by immunohistochemical analysis of resected specimens of gastric
cancer. Positive staining for PDGFR-β was observed in the
tumor stromal cells of the gastric cancer tissue in differentiated
and undifferentiated types of gastric cancer (Fig. 3).
Associations between PDGFR-β mRNA
expression levels and clinicopathological features
Expression levels of the PDGFR-β gene were
categorized as low or high according to the median value. The
associations between gene expression and clinicopathological
features were then examined. Expression levels of the
PDGFR-β gene were not found to be associated with any
clinicopathological variable (Table
I).
 | Table I.Association between PDGFR-β
mRNA expression and clinicopathological features. |
Table I.
Association between PDGFR-β
mRNA expression and clinicopathological features.
| Variable | Low PDGFR-β
expression (n=67) | High PDGFR-β
expression (n=67) | P-value |
|---|
| Age, years |
|
|
|
|
<65 | 26 | 30 | 0.484 |
|
≥65 | 41 | 37 |
|
| Gender |
|
|
|
|
Male | 45 | 47 | 0.710 |
|
Female | 22 | 20 |
|
| Tumor size, cm |
|
|
|
|
<6 | 27 | 33 | 0.297 |
| ≥6 | 40 | 34 |
|
| Histological
type |
|
|
|
|
Differentiated | 24 | 29 | 0.377 |
|
Undifferentiated | 43 | 38 |
|
| Serosal
invasion |
|
|
|
|
Absent | 27 | 26 | 0.860 |
|
Present | 40 | 41 |
|
| Lymph node
metastasis |
|
|
|
|
Absent | 8 | 8 | 1.000 |
|
Present | 59 | 59 |
|
| TNM stage |
|
|
|
| II | 19 | 21 | 0.706 |
|
III | 48 | 46 |
|
| Lymphatic
invasion |
|
|
|
|
Absent | 14 | 16 | 0.679 |
|
Present | 53 | 51 |
|
| Venous
invasion |
|
|
|
|
Absent | 21 | 12 | 0.071 |
|
Present | 46 | 55 |
|
Associations between PDGFR-β mRNA
expression levels and outcome
The expression levels of the PDGFR-β gene
were categorized as low or high according to the median expression
value. Overall survival was significantly poorer in the patients
with high expression levels (5-year overall survival rate, 58.8%)
of the PDGFR-β gene than in those with low expression levels
(5-year overall survival rate, 79.4%) (P=0.045; Fig. 4).
Univariate and multivariate analyses
of the associations between clinicopathological factors and PDGFR-β
mRNA expression levels with regard to outcome
The following variables were included in univariate
analysis: Age, gender, tumor size, histological grade, serosal
invasion, lymph-node metastasis, TNM stage, lymphatic invasion,
venous invasion and PDGFR-β gene expression. Only TNM stage
and PDGFR-β gene expression were significantly associated
with the outcome. Upon multivariate Cox proportional-hazards
regression analysis, TNM stage (P=0.035) and high PDGFR-β
gene expression (P=0.040) was independently and inversely
associated with outcome (Table
II).
 | Table II.Univariate and multivariate Cox
proportional hazards analysis of clinicopathological factors. |
Table II.
Univariate and multivariate Cox
proportional hazards analysis of clinicopathological factors.
|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|
|---|
| Variable | n | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
<65 | 56 | 1 | 0.356–1.319 | 0.258 |
|
|
|
|
≥65 | 78 | 0.685 |
|
|
|
|
|
| Gender |
|
|
|
|
|
|
|
|
Male | 92 | 1 | 0.318–1.438 | 0.310 |
|
|
|
|
Female | 42 | 0.676 |
|
|
|
|
|
| Tumor size, cm |
|
|
|
|
|
|
|
|
<6 | 60 | 1 | 0.553–2.060 | 0.847 |
|
|
|
| ≥6 | 74 | 1.067 |
|
|
|
|
|
| Histological
type |
|
|
|
|
|
|
|
|
Differentiated | 53 | 1 | 0.497–1.907 | 0.937 |
|
|
|
|
Undifferentiated | 81 | 0.973 |
|
|
|
|
|
| Serosal
invasion |
|
|
|
|
|
|
|
|
Absent | 53 | 1 | 0.753–3.118 | 0.239 |
|
|
|
|
Present | 81 | 1.532 |
|
|
|
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
Absent | 16 | 1 | 0.655–11.356 | 0.168 |
|
|
|
|
Present | 118 | 2.726 |
|
|
|
|
|
| TNM stage |
|
|
|
|
|
|
|
| II | 40 | 1 | 1.039–6.019 | 0.041a | 1 | 1.071–6.220 | 0.035a |
|
III | 94 | 2.501 |
|
| 2.581 |
|
|
| Lymphatic
invasion |
|
|
|
|
|
|
|
|
Absent | 30 | 1 | 0.456–2.201 | 0.997 |
|
|
|
|
Present | 104 | 1.002 |
|
|
|
|
|
| Venous
invasion |
|
|
|
|
|
|
|
|
Absent | 33 | 1 | 0.685–3.580 | 0.288 |
|
|
|
|
Present | 101 | 1.566 |
|
|
|
|
|
| PDGFR-β
expression |
|
|
|
|
|
|
|
|
Low | 67 | 1 | 1.002–3.887 | 0.049a | 1 | 1.032–4.029 | 0.040a |
|
High | 67 | 1.973 |
|
| 2.039 |
|
|
Discussion
The standard treatment for stage II/III gastric
cancer is a curative resection and adjuvant chemotherapy. The
overall survival rate of patients with stage II/III gastric cancer
remains unsatisfactory, even after curative surgery and adjuvant
chemotherapy with S-1 (2–4). Improved risk stratification and
personalized medicine based on biomarker analysis will hopefully
improve outcomes in stage II/III gastric cancer. The present study
therefore focused on PDGFR-β and examined the clinical
significance of PDGFR-β expression in patients with stage
II/III gastric cancer who received curative surgery and
post-operative adjuvant chemotherapy with S-1.
The present study examined the expression levels of
PDGFR-β mRNA in gastric cancer and adjacent normal mucosa.
Studies have previously compared the relative mRNA expression
levels of the PDGFR-β gene between various types of cancer
tissue and adjacent normal mucosa. Erben et al reported that
PDGFR-β mRNA expression is higher in rectal cancer tissue
than in normal rectal mucosa (13).
Antoniades et al found that PDGF and PDGFR-β
mRNA are strongly expressed in lung carcinoma tissue, but not in
normal lung tissue (14). Vrekoussis
et al showed that immunohistochemical expression levels of
endothelial PDGFR-β are significantly higher in breast cancer
tissue than in normal breast tissue (15), and Guo et al reported that the
positive rate of immunohistochemical PDGFR-β expression is
significantly higher in gastric carcinoma tissue than in normal
gastric mucosa tissue (16). The
present results are consistent with these findings. PDGFR-β
mRNA expression levels were significantly higher in 75 specimens of
gastric cancer tissue than in the paired adjacent normal
mucosa.
The associations between PDGFR-β mRNA
expression levels and clinicopathological features in gastric
cancer were then evaluated. Kodama et al reported that
PDGFR-β expression correlates with lymph node metastasis in
gastric cancer (5). Suzuki et
al showed that activation of PDGFR-β correlates with the
depth of tumor invasion in gastric cancer (17), and Guo et al reported that
PDGFR-β expression positively correlates with the depth of
tumor invasion, lymph node metastasis and TNM stage in gastric
cancer (16). By contrast, the
expression levels of PDGFR-β mRNA were not associated with
any clinicopathological features in the present study.
Finally, the present study assessed the associations
between PDGFR-β gene expression levels and outcome in stage
II/III gastric cancer after curative resection and adjuvant
chemotherapy with S-1. Paulsson et al reported that high
stromal PDGFR-β expression significantly correlates with
shorter recurrence-free and cancer-specific survival times in
various types of breast cancer (18),
while Hägglöf et al showed that stromal PDGFR-β
expression predicts cancer-specific survival in various types of
prostate cancer (19). Sato et
al (20) reported that
PDGFR-β expression is a significant prognostic factor in
grade II/III astrocytoma and grade IV glioblastoma. Chen et
al (21) found that
PDGFR-β overexpression alone is not a significant predictor
of either disease-free survival or overall survival, whereas
PDGFR-α/PDGFR-β/vascular endothelial growth factor
coexpression significantly correlates with poorer disease-free
survival and overall survival in patients with stage I–IV
hepatocellular carcinoma. By contrast, Shinohara et al
reported that the levels of PDGFR -β expression are not a
statistically significant predictor of 5-year overall survival in
either limited or extensive small-cell lung cancer (22). The present results showed that high
PDGFR-β expression levels were a significant independent
predictor of a poorer 5-year overall survival rate in patients with
stage II/III gastric cancer after curative resection and
post-operative adjuvant chemotherapy with S-1. Upon multivariate
Cox proportional hazards regression analysis, high levels of
PDGFR-β gene expression were independently and inversely
associated with outcome.
The molecular mechanisms and functional impact of
PDGFR-β expression in cancer remain to be fully elucidated.
PDGFR-β promotes the proliferation of tumor-associated
fibroblasts and neovascular endothelial cells participating in
tumor angiogenesis; it also increases IFP, and a higher IFP is
associated with greater PDGFR-β expression. Tailor et
al reported that the IFP was higher in the high PDGFR-β
group in nude mice bearing human non-small-cell lung cancer A549
xenografts (23). Increased IFP acts
as a barrier to drug distribution and the migration of cytotoxic T
lymphocytes that target tumor cells (24). High IFP is thus a prognostic factor of
tumors. Yeo et al reported that IFP is a prognostic factor
in patients with cervical cancer (25). Milosevic et al (26) also showed that IFP is a predictor of
survival in patients with cervical cancer. These findings suggest
that reducing the IFP may contribute to improved survival. Pietras
et al (27) demonstrated that
the inhibition of PDGFR reduces the IFP and increases the
capillary-to-interstitium transport of 51Cr-EDTA in PROb
rat colonic carcinomas. Pietras et al (28) also reported that the inhibition of
PDGFR signaling enhances the antitumor effect of
chemotherapy on subcutaneous KAT-4 tumors in FOX Chase severe
combined immune deficiency mice. Emerich et al (29) showed that infusion of the bradykinin
agonist Cereport (labradimil or RMP-7) in rats bearing experimental
tumors reduces the IFP and increases deposition of
[14C]-carboplatin in tumor tissue. These findings
suggest that high PDGFR-β expression is associated with high
IFP and causes resistance to chemotherapy by reducing blood flow to
tumors and decreasing drug delivery in patients with gastric cancer
after curative surgery and post-operative adjuvant chemotherapy
with S-1. Imatinib is a PDGFR inhibitor. Wiig et al
(30) reported that rats with colonic
carcinomas treated with imatinib exhibited reduced IFP. Kim et
al (24) demonstrated that
treatment with imatinib markedly enhances the chemotherapeutic
effects of antitumor drugs such as 5-fluorouracil and paclitaxel
in vivo in gastric carcinoma MKN-45 cells transplanted into
nude mice. These findings indirectly suggest that PDGFR-β
expression may antagonize the antitumor effects of chemotherapy.
However, further investigations are necessary to elucidate the
underlying mechanisms.
In conclusion, PDGFR-β gene expression levels
were higher in gastric cancer tissue than in adjacent normal mucosa
in the present study, and the high expression of this gene was
significantly associated with a poor outcome in patients with stage
II/III gastric cancer who underwent curative resection and received
adjuvant chemotherapy with S-1. These findings suggest that
PDGFR-β overexpression is a useful, independent predictor of
outcome in patients with stage II/III gastric cancer who receive
curative surgery and post-operative adjuvant chemotherapy with
S-1.
Acknowledgements
The authors would like to thank Medical Network K.K
for preparing the original manuscript. The study was supported by
the Yokohama Foundation for Advanced Medical Science (grant no.
18-7A-4).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sano T, Sasako M, Yamamoto S, Nashimoto A,
Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y
and Okajima K: Gastric cancer surgery: Morbidity and mortality
results from a prospective randomized controlled trial comparing D2
and extended para-aortic lymphadenectomy-Japan Clinical Oncology
Group study 9501. J Clin Oncol. 22:2767–2773. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sasako M, Sakuramoto S, Katai H, Kinoshita
T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T and
Ohashi Y: Five-year outcomes of a randomized phase III trial
comparing adjuvant chemotherapy with S-1 versus surgery alone in
stage II or III gastric cancer. J Clin Oncol. 29:4387–4393. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kodama M, Kitadai Y, Sumida T, Ohnishi M,
Ohara E, Tanaka M, Shinagawa K, Tanaka S, Yasui W and Chayama K:
Expression of platelet-derived growth factor (PDGF)-B and
PDGF-receptor β is associated with lymphatic metastasis in human
gastric carcinoma. Cancer Sci. 101:1984–1989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sumida T, Kitadai Y, Shinagawa K, Tanaka
M, Kodama M, Ohnishi M, Ohara E, Tanaka S, Yasui W and Chayama K:
Anti-stromal therapy with imatinib inhibits growth and metastasis
of gastric carcinoma in an orthotopic nude mouse model. Int J
Cancer. 128:2050–2062. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsuda T, Yoshida K, Tsujino T, Nakayama H,
Kajiyama G and Tahara E: Coexpression of platelet-derived growth
factor (PDGF) A-chain and PDGF receptor genes in human gastric
carcinomas. Jpn J Cancer Res. 80:813–817. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ostman A: PDGF receptors-mediators of
autocrine tumor growth and regulators of tumor vasculature and
stroma. Cytokine Growth Factor Rev. 15:275–286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Risau W, Drexler H, Mironov V, Smits A,
Siegbahn A, Funa K and Heldin CH: Platelet-derived growth factor is
angiogenic in vivo. Growth Factors. 7:261–266. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bergers G, Song S, MeyerMorse N, Bergsland
E and Hanahan D: Benefits of targeting both pericytes and
endothelial cells in the tumor vasculature with kinase inhibitors.
J Clin Invest. 111:1287–1295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pietras K: Increasing tumor uptake of
anticancer drugs with imatinib. Semin Oncol. 31(2): Suppl 6.
S18–S23. 2004. View Article : Google Scholar
|
|
12
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer (UICC) TNM Classification of
Malignant Tumors. 7th. Wiley-Blackwell; Oxford: pp. 73–77. 2009
|
|
13
|
Erben P, Horisberger K, Muessle B, Müller
MC, Treschl A, Ernst T, Kähler G, Ströbel P, Wenz F, Kienle P, et
al: mRNA expression of platelet-derived growth factor receptor-beta
and C-KIT: Correlation with pathologic response to cetuximab-based
chemoradiotherapy in patients with rectal cancer. Int J Radiat
Oncol Biol Phys. 72:1544–1550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Antoniades HN, Galanopoulos T,
NevilleGolden J and O'Hara CJ: Malignant epithelial cells in
primary human lung carcinomas coexpress in vivo platelet-derived
growth factor (PDGF) and PDGF receptor mRNAs and their protein
products. Proc Natl Acad Sci USA. 89:3942–3946. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vrekoussis T, Stathopoulos EN, Kafousi M,
Navrozoglou I and Zoras O: Expression of endothelial PDGF receptors
alpha and beta in breast cancer: Up-regulation of endothelial PDGF
receptor beta. Oncol Rep. 17:1115–1119. 2007.PubMed/NCBI
|
|
16
|
Guo Y, Yin J, Zha L and Wang Z:
Clinicopathological significance of platelet-derived growth factor
B, platelet-derived growth factor receptor-β, and E-cadherin
expression in gastric carcinoma. Contemp Oncol (Pozn). 17:150–155.
2013.PubMed/NCBI
|
|
17
|
Suzuki S, Dobashi Y, Hatakeyama Y, Tajiri
R, Fujimura T, Heldin CH and Ooi A: Clinicopathological
significance of platelet-derived growth factor (PDGF)-B and
vascular endothelial growth factor-A expression, PDGF receptor-β
phosphorylation, and microvessel density in gastric cancer. BMC
cancer. 10:6592010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paulsson J, Sjöblom T, Micke P, Pontén F,
Landberg G, Heldin CH, Bergh J, Brennan DJ, Jirström K and Ostman
A: Prognostic significance of stromal platelet-derived growth
factor beta-receptor expression in human breast cancer. Am J
Pathol. 175:334–341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hägglöf C, Hammarsten P, Josefsson A,
Stattin P, Paulsson J, Bergh A and Ostman A: Stromal PDGFRbeta
expression in prostate tumors and non-malignant prostate tissue
predicts prostate cancer survival. PloS One. 5:e107472010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato S, Sato Y, Hatakeyama K, Marutsuka K,
Yamashita A, Takeshima H and Asada Y: Quantitative analysis of
vessels with smooth muscle layer in astrocytic tumors: correlation
with histological grade and prognostic significance. Histol
Histopathol. 26:497–504. 2011.PubMed/NCBI
|
|
21
|
Chen L, Shi Y, Jiang CY, Wei LX, Lv YL,
Wang YL and Dai GH: Coexpression of PDGFR-alpha, PDGFR-beta and
VEGF as a prognostic factor in patients with hepatocellular
carcinoma. Int J Biol Markers. 26:108–116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shinohara ET, Gonzalez A, Massion PP,
Olson SJ, Albert JM, Shyr Y, Carbone DP, Johnson DH, Hallahan DE
and Lu B: PDGFR-beta expression in small cell lung cancer patients.
Int J Radiat Oncol Biol Phys. 67:431–437. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tailor TD, Hanna G, Yarmolenko PS, Dreher
MR, Betof AS, Nixon AB, Spasojevic I and Dewhirst MW: Effect of
pazopanib on tumor microenvironment and liposome delivery. Mol
Cancer Ther. 9:1798–1808. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim R, Emi M, Arihiro K, Tanabe K, Uchida
Y and Toge T: Chemosensitization by STI571 targeting the
platelet-derived growth factor/platelet-derived growth factor
receptor-signaling pathway in the tumor progression and
angiogenesis of gastric carcinoma. Cancer. 103:1800–1809. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yeo SG and Kim JS, Cho MJ, Kim KH and Kim
JS: Interstitial fluid pressure as a prognostic factor in cervical
cancer following radiation therapy. Clin Cancer Res. 15:6201–6207.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Milosevic M, Fyles A, Hedley D, Pintilie
M, Levin W, Manchul L and Hill R: Interstitial fluid pressure
predicts survival in patients with cervix cancer independent of
clinical prognostic factors and tumor oxygen measurements. Cancer
Res. 61:6400–6405. 2001.PubMed/NCBI
|
|
27
|
Pietras K, Ostman A, Sjöquist M,
Buchdunger E, Reed RK, Heldin CH and Rubin K: Inhibition of
platelet-derived growth factor receptors reduces interstitial
hypertension and increases transcapillary transport in tumors.
Cancer Res. 61:2929–2934. 2001.PubMed/NCBI
|
|
28
|
Pietras K, Rubin K, Sjöblom T, Buchdunger
E, Sjöquist M, Heldin CH and Ostman A: Inhibition of PDGF receptor
signaling in tumor stroma enhances antitumor effect of
chemotherapy. Cancer Res. 62:5476–5484. 2002.PubMed/NCBI
|
|
29
|
Emerich DF, Dean RL, Snodgrass P,
Lafreniere D, Agostino M, Wiens T, Xiong H, Hasler B, Marsh J, Pink
M, et al: Bradykinin modulation of tumor vasculature: II.
activation of nitric oxide and phospholipase A2/prostaglandin
signaling pathways synergistically modifies vascular physiology and
morphology to enhance delivery of chemotherapeutic agents to
tumors. J Pharmacol Exp Ther. 296:632–641. 2001.PubMed/NCBI
|
|
30
|
Wiig H, Tveit E, Hultborn R, Reed RK and
Weiss L: Interstitial fluid pressure in DMBA-induced rat mammary
tumours. Scand J Clin Lab Invest. 42:159–164. 1982. View Article : Google Scholar : PubMed/NCBI
|