Introduction
Lung cancer is one of the most prevalent types of
malignant tumor, with 1.83 million new diagnoses per year
worldwide, and is a leading cause of cancer-associated mortality
globally (1,2). The morbidity associated with lung cancer
also is constantly increasing and threatens human health globally
(3). The number of lung
cancer-associated mortalities worldwide is expected to grow to up
to 3 million by 2035 (4). Due to the
difficulty in determining an early diagnosis and the high
metastatic potential of this form of cancer, metastasis develops
prior to the diagnosis of lung cancer in the majority of cases.
Approximately 90% of patients with lung cancer succumb to the
disease due to tumor metastasis (5).
Conventional chemotherapy is often ineffective for patients with
metastatic lung cancer and frequently causes toxic side effects.
Therefore, the development of novel therapeutic agents for patients
with lung cancer is required (6).
Cancer cell migration is necessary for tumor
development. Previous studies have revealed that tumor migration
and invasion depends on the degradation of the extracellular matrix
(ECM), which forms a barrier to tumor invasion (7,8). Matrix
metalloproteinases (MMPs) are a large family of zinc-dependent
endopeptidases capable of degrading the majority of ECM components
(9,10). Overexpression of MMPs is frequently
detected in various types of cancer and has been observed to
facilitate tumor cell metastasis (11). Therefore, the inhibition of the
activities of MMPs in the ECM may prevent the invasion of tumor
cells and form the basis for an efficient anticancer therapy.
Pinus massoniana is a tree species native to
Southern China (12) and P.
massoniana bark extract (PMBE) is an established traditional
Chinese medicine used for the treatment of rheumatism, arthralgia,
inflammation and cancer (13,14). The primary active component of PMBE is
anthocyanin series B (15). Certain
studies have reported that PMBE induces the apoptosis of hepatoma
and cervical cancer cells (16,17).
However, the effects of PMBE on the migration and invasion of lung
cancer have yet to be elucidated. In the present study, the effect
of PMBE on the migration and invasion of human lung cancer A549
cells was investigated in order to examine the molecular mechanisms
underlying this process.
Materials and methods
Reagents and chemicals
RPMI-1640 medium and fetal bovine serum (FBS) were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Transwells (polyethylene terephthalate; 8.0-µm pore size)
were purchased from BD Biosciences (San Jose, CA, USA). Antibodies
against MMP-9 (catalog no. 10375-2-AP; 1:500 dilution) and β-actin
(catalog no. 60008-1-Ig; 1:2,000 dilution) were obtained from
ProteinTech Group, Inc. (Chicago, IL, USA). An enhanced
chemiluminescence (ECL) kit was purchased from GE Healthcare Life
Sciences (Chalfont, UK). MTT was obtained from Ameresco, Inc.
(Framingham, MA, USA) and PMBE powder was purchased from Shaanxi
Tianrun Pytochemical Co. Ltd. (Shaanxi, China).
Cell culture
The human lung cancer A549 cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA) and
maintained in RPMI-1640 medium supplemented with 10% FBS at 37°C in
an incubator containing 5% CO2. The cells were harvested
upon reaching 80% confluency.
MTT assay
The cells were plated in 96-well plates at a density
of 1×104/well and incubated for 24 h at 37°C.
Subsequently, 200 µl RPMI-1640 medium containing 0, 50, 100, 150 or
200 µg/ml PMBE was added to the wells. The cells treated without
PMBE served as the control for the MTT assay. The cells were then
cultured for 24 h and incubated with 20 µl MTT solution (5 mg/ml)
for 4 h in 5% CO2 at 37°C. Subsequently, 150 µl dimethyl
sulfoxide was added into each well and the absorbance was evaluated
at a wavelength of 589 nm in a Multiskan Ascent plate reader
(Thermo Fisher Scientific, Inc.).
Wound healing assay
A549 cells were seeded into 6-well plates at a
density of 5×104/ml and then the cell monolayer was
scratched with the end of a 200-µl pipette tip. The plates were
washed with phosphate-buffered saline to remove detached cells.
Subsequently, the cells were treated with 0, 25 or 50 µg/ml PMBE
solution for 48 h. The cells treated without PMBE served as the
control for the wound healing assay. The migration of the lung
cancer cells was observed under a phase-contrast microscope
(magnification, ×40) at 0 and 48 h post-wounding. The cells that
had migrated into the denuded area in each of six random fields
were evaluated and quantified using a computer-assisted microscope
(Novel, Inc. Ningbo, Zhejiang, China).
Transwell chamber assay
The migration ability of the A549 cells was
quantified using a Transwell assay. The A549 cells were treated
with 0, 25 or 50 µg/ml PMBE solution for 24 h at 37°C. The cells
treated without PMBE served as the control for the Transwell
chamber assay. A total of 2×105 cells in RPMI-1640
medium without serum were added to each upper chamber, and
RPMI-1640 supplemented with 10% FBS was added to the lower chamber
as a chemoattractant. After 48 h of treatment at 37°C, the cells
remaining on the upper surface of the membrane were removed with
cotton swabs, and the cells that had migrated to the underside of
the membrane were fixed using 4% paraformaldehyde and stained with
0.1% crystal violet for 10 min at room temperature. The migrated
cells on the underside of membrane were washed with PBS and counted
under a microscope (magnification, ×100).
Western blot analysis
The A549 cells were harvested and lysed in cold
radioimmunoprecipitation assay buffer. The cells treated without
PMBE served as the control group in western blot assay. Total
proteins (20 µg) were separated by 10% SDS-PAGE, transferred to
nitrocellulose membranes using a semi-dry apparatus for 40 min and
then blocked with blocking buffer (5% skimmed milk in Tris-buffered
saline) for 1.5 h at room temperature. Specific primary antibodies
against MMP-9 (catalog no. 10375-2-AP) and β-actin (catalog no.
60008-1-Ig) (ProteinTech Group, Inc.) at 1:500 and 1:2,000
dilution, respectively, were added and incubated overnight at 4°C.
Following incubation with corresponding anti-mouse (catalog no.
SA00001-1)/rabbit (catalog no. SA00001-2) horseradish
peroxidase-conjugated secondary antibodies at 1:2,000 dilution for
2 h at room temperature, the protein bands were visualized using an
enhanced chemiluminescence (ECL) kit (GE Healthcare Life Sciences)
by Image Lab 4.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The bands were quantified by Image J software (National Institutes
of Health, Bethesda, MD, USA). β-actin was used as the loading
control.
Statistical analysis
Statistical analyses were performed using SPSS
version 13.0 (SPSS Inc., Chicago, IL, USA). The data were presented
as the mean ± standard deviation. One-way analysis of variance with
Bonferroni's multiple comparison test was used to compare between
the groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of PMBE on human lung cancer
cell proliferation
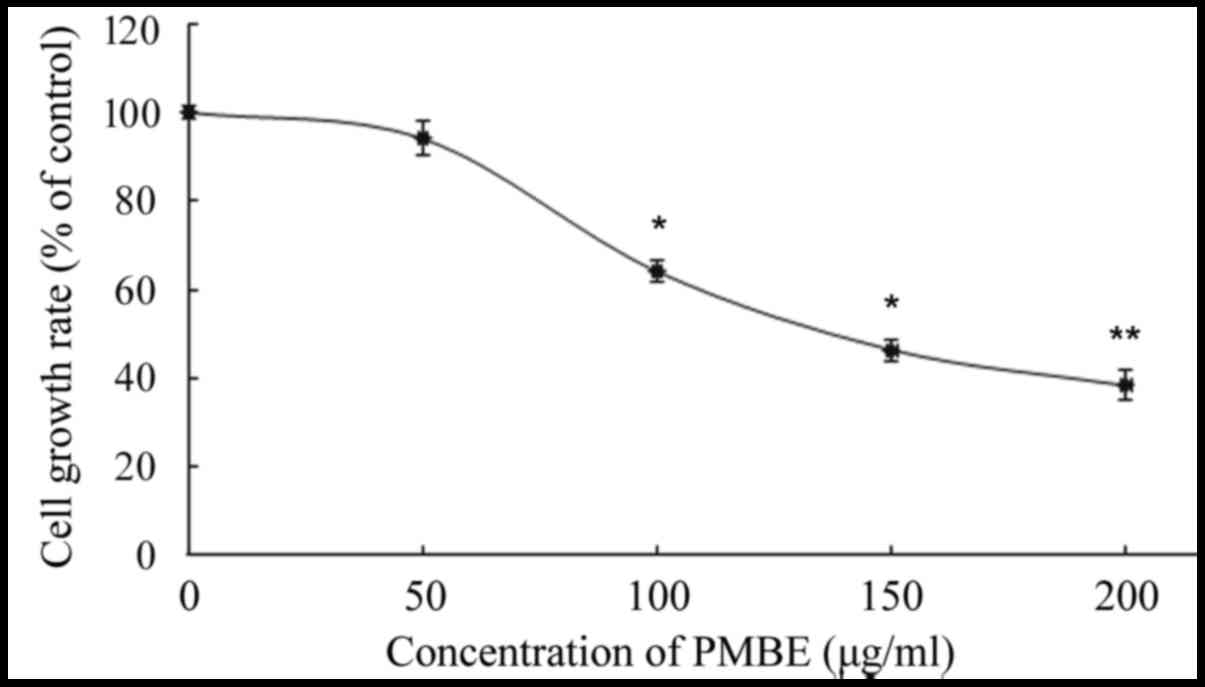
The A549 cells were treated with various
concentrations of PMBE (0, 50, 100, 150 and 200 µg/ml) for 24 h.
The inhibitory effect of PMBE on the lung cancer cells was
evaluated using an MTT assay. The results revealed that PMBE
exhibited antiproliferative effects on the lung cancer A549 cells.
There was a significant dose-dependent decrease in the
proliferation of lung cancer cells that were treated with PMBE
(P=0.0338, P=0.0173 and P=0.0024 for the cells treated with 100,
150 and 200 µg/ml PMBE, respectively, compared with the control
group). The IC50 value of PMBE was 148 µg/ml for 24 h
(Fig. 1).
Effects of PMBE on human lung cancer
cell migration
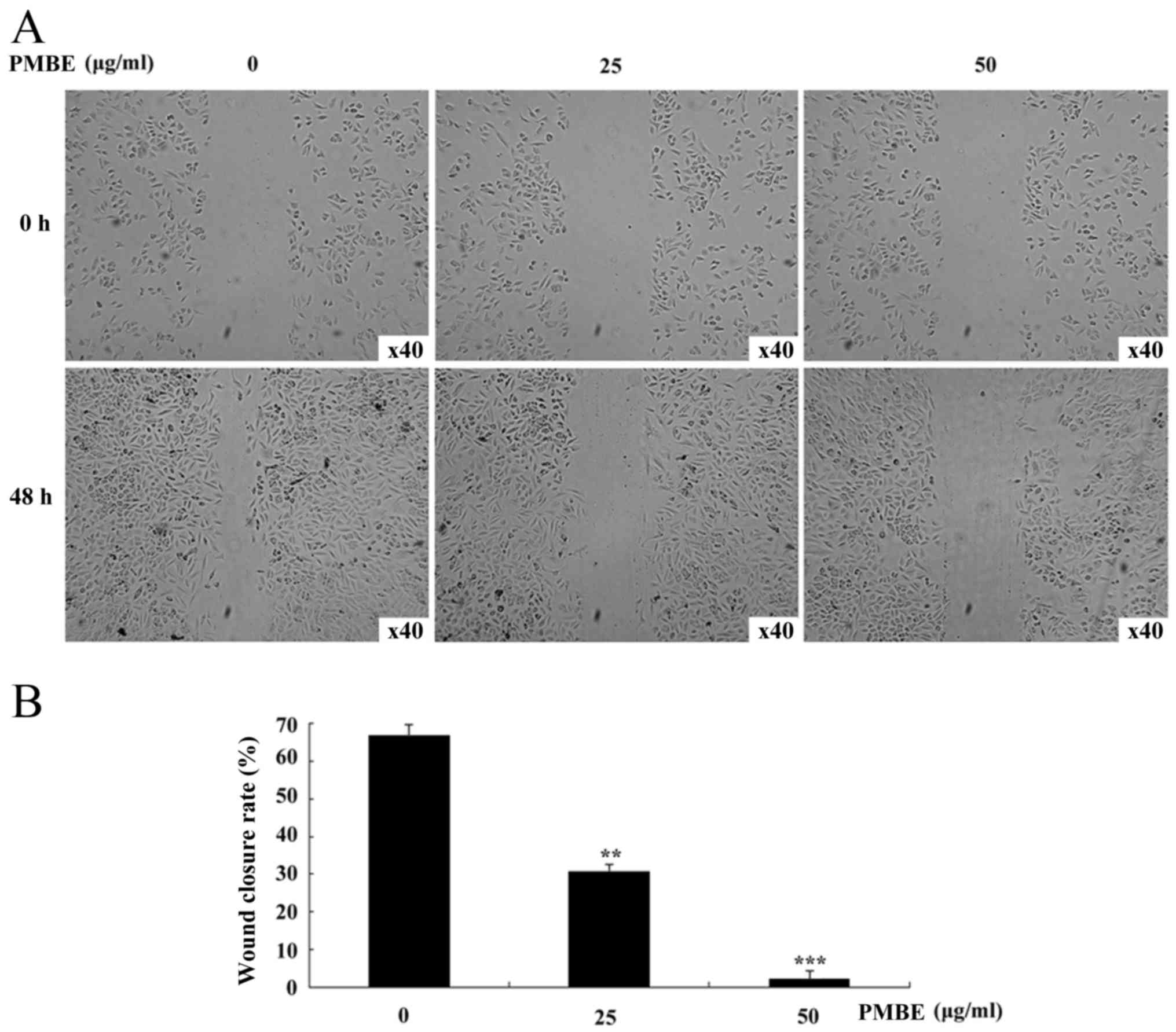
To investigate whether PMBE is able to affect the
migration of lung cancer cells, a wound healing assay was
performed. PMBE solution dose-dependently reduced the movement of
A549 cells in the wound-healing assay (Fig. 2A). With the increase in PMBE
concentration, the movement of the lung cancer cells was
significantly decreased (P=0.0081 and P=0.0006 for the cells
treated with 25 and 50 µg/ml PMBE, respectively, compared with the
control group). The wound closure/migration rates of the lung
cancer A549 cells treated with 0, 25 and 50 µg/ml PMBE were 66.67,
30.61 and 2.08%, respectively (Fig.
2B). Therefore, the results of the wound-healing assay
suggested that PMBE maybe suppress the migration of lung cancer
cells.
Effects of PMBE on human lung cancer
cell migration
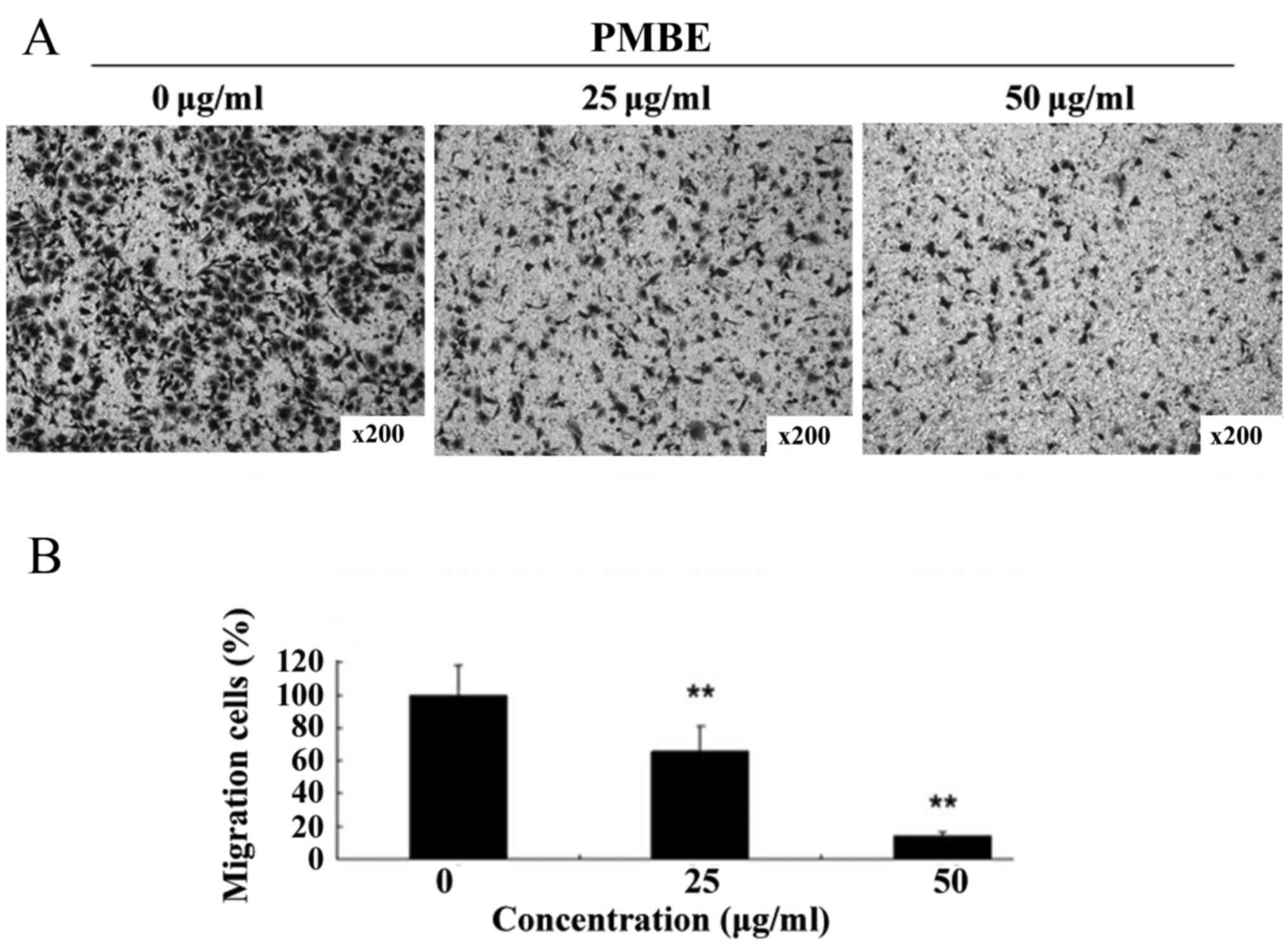
A Transwell assay was performed to investigate the
anti-migration effects of PMBE on lung cancer A549 cells. PMBE
inhibited the migration of the lung cancer cells in a
dose-dependent manner for 48 h (Fig.
3A). The number of cells that had migrated to the lower surface
of the membrane was significantly reduced compared with the control
group (Fig. 3B; P=0.0064 and P=0.0028
for the cells treated with 25 and 50 µg/ml PMBE, respectively,
compared with the control group). This result suggested that PMBE
is able to suppress the migration of lung cancer cells.
Effects of PMBE on the expression of
MMP-9 in lung cancer cells
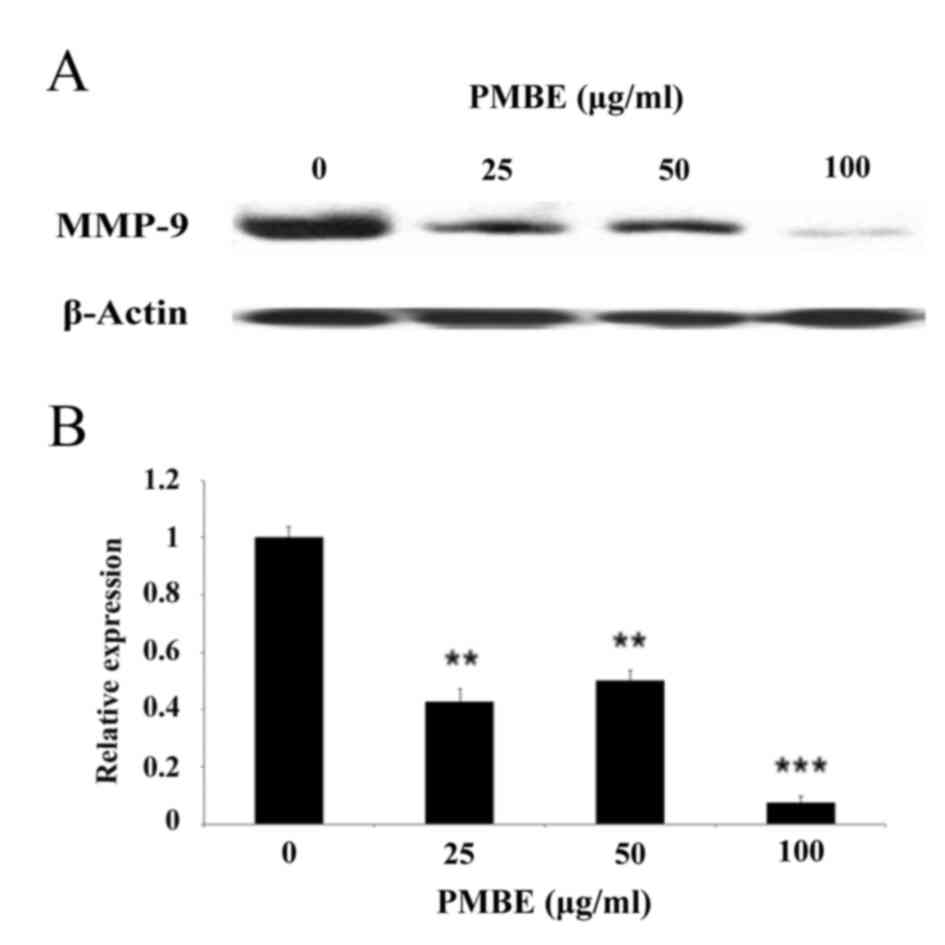
To further investigate the underlying molecular
mechanisms by which PMBE may suppress the migration of lung cancer
cells, the expression levels of MMP-9 were examined following PMBE
treatment using western blotting. The results revealed that PMBE
dose-dependently reduced the expression levels of MMP-9 protein
(Fig. 4; P=0.0049, P=0.0032 and
P=0.0007 for the cells treated with 25, 50 and 100 µg/ml PMBE,
respectively, compared with the control group. These data suggested
that the inhibitory effect of PMBE on the migration of lung cancer
cells may, in part, be due to the downregulation of MMP-9
expression levels.
Discussion
Metastasis in patients with malignant neoplasms is
the leading cause of cancer-associated mortality (18–20).
Despite recent advancements, there are limited effective therapies
available for patients with metastatic lung cancer due to drug
resistance or serious side effects (21,22)
Therefore, novel alternative therapies to prevent tumor migration
and invasion are required for patients with lung cancer.
Plant-derived therapies have been the subject of a
number of studies with regard to their pharmacological effects for
the treatment and prevention of cancer, due to their high potency
and low toxicity (22–24). PMBE has previously been used in
traditional Chinese medicine for patients with cancer (15,25,26). The
present study investigated the effect of PMBE on lung cancer cells
and the potential mechanisms underlying this process.
Firstly, the results of the current study indicated
that PMBE was able to significantly prevent the growth of lung
cancer cells, and that the cell survival rate was decreased in a
dose-dependent manner. A previous study revealed that PMBE exhibits
anticancer effects in murine sarcoma S180 cells (25). In addition, PMBE is able to inhibit
cell proliferation and induce apoptosis in human hepatoma cells
(17). These results suggest that
PMBE possesses potential antitumor activity.
Furthermore, the present study demonstrated that
PMBE suppressed the migration of lung cancer cells. Further
investigation of the molecular mechanisms underlying this process
demonstrated that PMBE was able to reduce the expression levels of
MMP-9 in the lung cancer cells. MMP-9 is an important member of the
MMP family that is used for the degradation of the ECM (27). A previous study revealed that the
increased expression of MMP-9 is associated with the development of
lung cancer and the promotion of cell metastasis (28). The results of the present study
suggested that the effect of PMBE was mediated, at least in part,
by the downregulation of MMP-9 expression in the lung cancer
cells.
In conclusion, to the best of our knowledge, the
current study is the first to indicate that PMBE can inhibit the
growth of lung cancer cells. Furthermore, PMBE was able suppress
the migration and invasion of the lung cancer cells, which was
associated with reduced levels of MMP-9 protein expression. These
results suggest that PMBE may be a novel candidate for the
treatment of lung cancer.
Acknowledgements
This study was supported by The Chinese National
Natural Science Fund (grant no. 81302282).
References
|
1
|
Wagland R, Brindle L, Ewings S, James E,
Moore M, Rivas C, Esqueda AI and Corner J: Promoting help-seeking
in response to symptoms amongst primary care patients at high risk
of lung cancer: A mixed method study. PLoS One. 11:e01656772016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biaoxue R, Hua L, Wenlong G and Shuanying
Y: Increased serum amyloid A as potential diagnostic marker for
lung cancer: A meta-analysis based on nine studies. BMC Cancer.
16:8362016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amoori N, Mirzaei M and Cheraghi M:
Incidence of cancers in Kuzestan province of Iran: Trend from 2004
to 2008. Asian Pac J Cancer Prev. 15:8345–8349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Didkowska J, Wojciechowska U, Mańczuk M
and Łobaszewski J: Lung cancer epidemiology: Contemporary and
future challenges worldwide. Ann Transl Med. 4:1502016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prabhu VV and Guruvayoorappan C:
Inhibition of metastatic lung cancer in C57BL/6 mice by marine
mangrove Rhizophora apiculata. Asian Pac J Cancer Prev. 14:1833–40.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khozin S, Blumenthal GM, Jiang X, He K,
Boyd K, Murgo A, Justice R, Keegan P and Pazdur R: U.S. Food and
Drug Administration approval summary: Erlotinib for the first-line
treatment of metastatic non-small cell lung cancer with epidermal
growth factor receptor exon 19 deletions or exon 21 (L858R)
substitution mutations. Oncologist. 19:774–779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kurniawan NA, Chaudhuri PK and Lim CT:
Concentric Gel System to Study the Biophysical Role of Matrix
Microenvironment on 3D Cell Migration. J Vis Exp.
e527352015.PubMed/NCBI
|
|
8
|
Alfano M, Nebuloni M, Allevi R, Zerbi P,
Longhi E, Lucianò R, Locatelli I, Pecoraro A, Indrieri M, Speziali
C, et al: Linearized texture of three-dimensional extracellular
matrix is mandatory for bladder cancer cell invasion. Sci Rep.
6:361282016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu D, Guo H, Li Y, Xu X, Yang K and Bai
Y: Association between polymorphisms in the promoter regions of
matrix metalloproteinases (MMPs) and risk of cancer metastasis: A
meta-analysis. PLoS One. 7:e312512012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang TH, Chiu YH, Chan YL, Chiu YH, Wang
H, Huang KC, Li TL, Hsu KH and Wu CJ: Prophylactic administration
of fucoidan represses cancer metastasis by inhibiting vascular
endothelial growth factor (VEGF) and matrix metalloproteinases
(MMPs) in lewis tumor-bearing mice. Mar Drugs. 13:1882–1900. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hassan ZK, Elamin MH, Daghestani MH, Omer
SA, Al-Olayan EM, Elobeid MA, Virk P and Mohammed OB: Oleuropein
induces anti-metastatic effects in breast cancer. Asian Pac J
Cancer Prev. 13:4555–4559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma H, Liu B, Feng D, Xie H, Li R, Yuchi Y,
Wang H and Wang J: Pinus massoniana bark extract selectively
induces apoptosis in human hepatoma cells, possibly through
caspase-dependent pathways. Int J Mol Med. 25:751–759.
2010.PubMed/NCBI
|
|
13
|
Cui Y, Xie H and Wang J: Potential
biomedical properties of Pinus massoniana bark extract. Phytother
Res. 19:34–38. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Bai J, Jiang G, Li X, Wang J, Wu D,
Owusu L, Zhang E and Li W: Anti-tumor effect of Pinus massoniana
bark proanthocyanidins on ovarian cancer through induction of cell
apoptosis and inhibition of cell migration. PLoS One.
10:e01421572015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang C, Zhang L, Cheng P and Zhang Q:
Inhibitory effects of Pinus massoniana bark extract on hepatitis C
virus in vitro. Pharm Biol. 53:451–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma H, Lai F, Xie H, Wang J and Wang H:
Involvement of the Bcl-2 family members in Pinus massoniana bark
extract induced apoptosis in HeLa cells. Phytother Res.
22:1472–1476. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui YY, Xie H, Qi KB, He YM and Wang JF:
Effects of Pinus massoniana bark extract on cell proliferation and
apoptosis of human hepatoma BEL-7402 cells. World J Gastroenterol.
11:5277–5282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan QL, Hou XS and Zhao G: Utility of
serum peptidome patterns of esophageal squamous cell carcinoma
patients for comprehensive treatment. Asian Pac J Cancer Prev.
14:2919–2923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu YC, Zhao J, Hu CE, Gan J, Zhang WH and
Huang GJ: Comprehensive analysis of vascular endothelial growth
factor-C related factors in stomach cancer. Asian Pac J Cancer
Prev. 15:1925–1929. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi Y, Li X, Zhao S and Han Y: Value of
porous titanium alloy plates for chest wall reconstruction after
resection of chest wall tumors. Asian Pac J Cancer Prev.
15:4535–4538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pradhan S, Mahajan D, Kaur P, Pandey N,
Sharma C and Srivastava T: Scriptaid overcomes hypoxia-induced
cisplatin resistance in both wild-type and mutant p53 lung cancer
cells. Oncotarget. 10.18632/oncotarget: 18632, 2016 (Epub ahead of
print).
|
|
22
|
Park KI, Park HS, Kang SR, Nagappan A, Lee
DH, Kim JA, Han DY and Kim GS: Korean Scutellaria baicalensis water
extract inhibits cell cycle G1/S transition by suppressing cyclin
D1 expression and matrix-metalloproteinase-2 activity in human lung
cancer cells. J Ethnopharmacol. 133:634–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin J, Li Q, Chen H, Lin H, Lai Z and Peng
J: Hedyotis diffusa willd. extract suppresses proliferation and
induces apoptosis via IL-6-inducible STAT3 pathway inactivation in
human colorectal cancer cells. Oncol Lett. 9:1962–1970.
2015.PubMed/NCBI
|
|
24
|
Cai Q, Lin J, Wei L, Zhang L, Wang L, Zhan
Y, Zeng J, Xu W, Shen A, Hong Z and Peng J: Hedyotis diffusa Willd
inhibits colorectal cancer growth in vivo via inhibition of STAT3
signaling pathway. Int J Mol Sci. 13:6117–6128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang JH, Feng DR, Ma HL, Liu B, Wang HB,
Xie H, Li RD and Wang JF: Antitumor effects of Pinus massoniana
bark extract in murine sarcoma S180 both in vitro and in vivo. Am J
Chin Med. 40:861–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu DC, Li S, Yang DQ and Cui YY: Effects
of Pinus massoniana bark extract on the adhesion and migration
capabilities of HeLa cells. Fitoterapia. 82:1202–1205. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pintha K, Yodkeeree S and Limtrakul P:
Proanthocyanidin in red rice inhibits MDA-MB-231 breast cancer cell
invasion via the expression control of invasive proteins. Biol
Pharm Bull. 38:571–581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Balla MM, Desai S, Purwar P, Kumar A,
Bhandarkar P, Shejul YK, Pramesh CS, Laskar S and Pandey BN:
Differential diagnosis of lung cancer, its metastasis and chronic
obstructive pulmonary disease based on serum Vegf, Il-8 and MMP-9.
Sci Rep. 6:360652016. View Article : Google Scholar : PubMed/NCBI
|