Introduction
Malignant glioma is the most common malignant tumor
in the brain. It has poor prognosis and is highly heat-resistant to
conventional therapy, such as surgery, radiotherapy and
chemotherapy (1,2). Therefore, the development of new
treatments to improve the prognosis of patients with glioma is the
focus and a challenge of the current research. Hyperthermia is a
physical therapy, which has fewer side effects compared to
chemotherapy. It can be reused many times without taking into
account the accumulation of toxicity in the body (3). At present, local hyperthermia is mainly
implemented through ultrasound, microwave and radiofrequency, which
however, all have some shortcomings (4). Ultrasound cannot pass through the body
cavity that holes gas (5). There are
also issues such as the reflection of bone, bone resorption and
others. Therefore, the treatment is challenging for intracranial
tumors; microwave heating cannot be used for deep tumors, and often
with side effects like scalding; radiofrequency heating has been
widely used in local thermotherapy (6). Radiofrequency does not have a precise
positioning, it heats the normal tissue and tumor tissue together
and its capacity is affected by various factors (7). In addition, it causes complications like
severe fat superheat and skin pain (8). Thermal ablation therapy is able to kill
numerous necrosis of tumor tissue instantly due to high
temperature. However, various substances released from these
necrotic tumor tissues flowing into the blood will cause shock
syndrome and lead to high risk complications (9).
Magnetic fluid hyperthermia (MFH) is a new
hyperthermia method. Magnetic fluid is injected into the tumor
tissues, and the nanometer magnetic fluid will deposit between
cells or be engulfed by tumor cells (10). Heating magnetic medium under
alternating magnetic field will contribute to shaping local
high-heat region and avoid heating normal tissue, which can
preserve normal tissues effectively and achieve the best effect
(11). The present study explores the
effect of Fe3O4 magnetic fluid in different
concentration under alternating magnetic field on malignant glioma
cells in vitro and in vivo, which provides reference
for MFH.
Materials and methods
Main materials
Main reagents
Magnetic fluid was purchased from the magnetic fluid
research institute of Beijing Jiaotong University, China. After
intermittent sterilization, it was stored at 4°C. Before using,
RPMI-1640 medium (Gibco, Grand Island, NY, USA) and 0.9% saline
were used to dilute it to the required concentration.
Cell lines
U251 human glioma cell lines were purchased from the
Department of Neurobiology in China Medical University. Culture
conditions were: RPMI-1640 medium of l0% fetal bovine serum
(Gibco), a 37°C, saturated humidity incubator with 5%
CO2. When the cells covered the bottom of the culture
bottle, culture medium was discarded and cleaned two times with
D-Hank's liquid (Gibco), added with 0.25% trypsin digestive juice
(Sigma-Aldrich, St. Louis, MO, USA). After 1 min, trypsin was also
discarded before adding fresh medium. Then it was gently pipetted
to single cell suspension and its passage dispensed.
Experiment method
Heating experiment of Fe3O4
nanometer magnetic fluid in vitro
The magnetic fluid used in the experiments was
purchased from the magnetic fluid research institute of Beijing
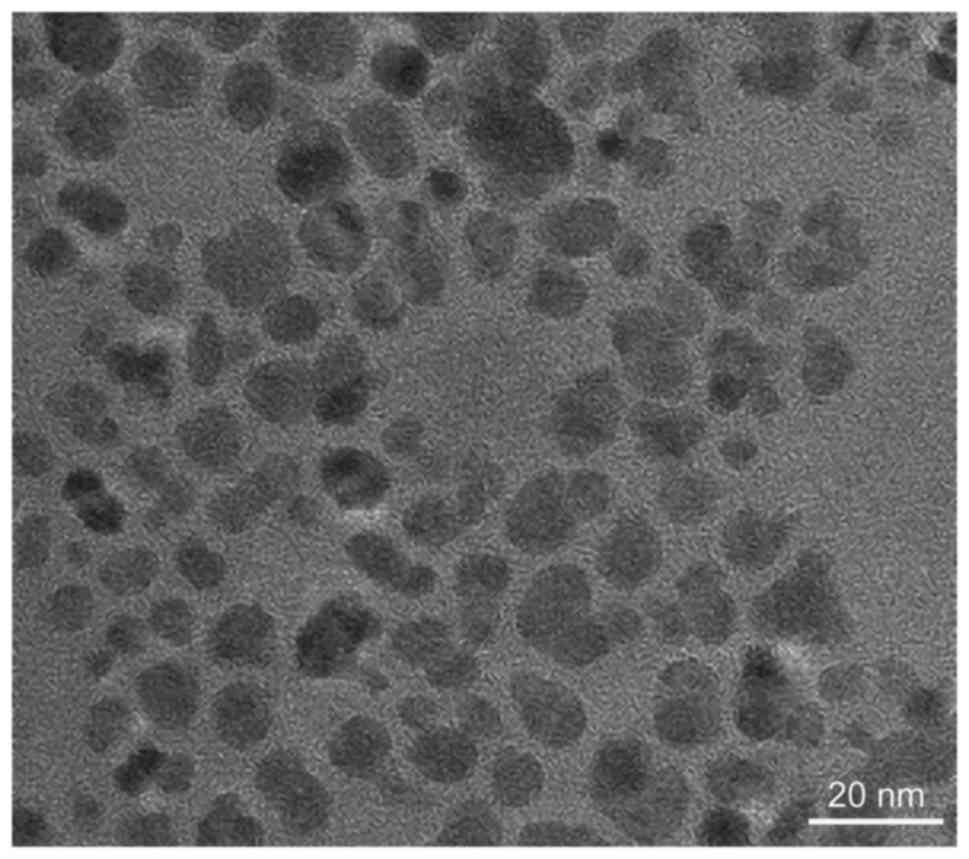
Jiaotong University. Scanning electron microscope (Quanta™ 250;
FEI, Hillsboro, OR, USA) an image of the results is shown in
Fig. 1, from which we can see that
magnetic particles dispersed clearly. Particles that have a
diameter of 5–10 nm are uniformly dispersed. The magnetic liquid
nano iron concentrations in the experiment were 2, 4, 6 and 8
g/l.
Double distilled water was used to dilute
Fe3O4 nano magnetic fluid into 2, 4, 6 and 8
g/l, then adding 5 ml into 25 mm flat tubes, respectively, placed
in a high-frequency magnetic field with a frequency of 200 KHz,
power of 4 kW, and output current of 300 A for 1 h. The starting
room temperature was 23°C. The distance between the tube bottom and
high frequency magnetic heating coil center was 0.5 cm. Temperature
was detected with TM902C digital thermometer every 5 min. The
sample's heating curve was drawn with time as the abscissa and
temperature as the ordinate.
Effect of magnetic nanoparticles on glioma in
vitro
i) U251 human glioma cell morphology observation:
Logarithmic phase cells were seeded into 6-well plates and divided
into 5 groups. The control group had only DMEM (Gibco) containing
10% fetal calf serum; then the hyperthermia group was also added,
respectively, with 5 ml magnetic fluid at concentrations of 2, 4, 6
and 8 g/l, and underwent irradiation in high frequency alternating
magnetic field (Carl Zeiss GmbH, Jena, Germany) for 1 h. The change
of cell morphology was observed at 48 h after treatment.
ii) MTT detection for U251 cell proliferation: U251
cells in logarithmic growth phase were prepared into cell
suspension (1×105/ml) as hyperthermia group (4 groups)
and control group, respectively. Hyperthermia group was added with
nano magnetic fluid of 5 ml in a concentration of 2, 4, 6 and 8 g/l
and the control group was added with RPMI-1640 medium for 5 ml and
placed into a magnetic field for 1 h. The cell suspensions were
inoculated into a 96-well plate, 100 ml per well, each group of 6
wells, for 72 h. The 96-well plate was then removed and the
original solution was discarded. A total of 100 ml fresh medium was
then added, next the 20 µl MTT (BioSharp, Hefei, China) (5 mg/ml),
continued for 4 h. Then the process was stopped, the culture medium
containing MTT was discarded, and 100 µl DMSO (Sigma-Aldrich) was
added to stop the reaction, then was pipetted gently to completely
dissolve the crystallization and to minimize bubbles. Enzyme-linked
immunosorbent assay was used to detect absorbance (OD) at a
wavelength of 570 nm. The average rate of cell proliferation was
calculated as: (1- OD of hyperthermia group/OD of control group) ×
100%.
Anti-glioma effect of nano magnetic particles in
animal experiment
After 1.25 g/l trypsin digestion of U251 human
glioma cells in logarithmic growth period, they were suspended in
the phosphate buffer saline (PBS) (BioSharp). After counting,
concentrations were adjusted. A total of 100 ml of PBS solution
containing ~1×107 cells were injected subcutaneously
into 24 male nude mice in the right lower limb. Twenty days later,
when the short diameter of subcutaneous tumors were >0.5 cm,
mice were randomly divided into the hyperthermia group, magnetic
fluid control group and blank control group with 8 rats in each
group. In magnetic fluid control group, rats underwent intratumoral
injection of 0.3 ml magnetic fluid with a concentration of 4 g/l,
and in the MFH group, rats underwent injection of 0.3 ml nano
magnetic fluid with concentrations of 2, 4, 6 and 8 g/l, while
hyperthermia group underwent anesthesia with intraperitoneal
injection of 0.5% sterile sodium phenobarbital solution (BioSharp)
in 60 mg/kg. After anesthesia, the tumors were placed on the high
frequency magnetic heater for an irradiation of 1 h with an output
current of I=300 A, twice with 24 h interval between each time
irradiation, and tumor volumes were recorded in 12 h after
hyperthermia. Two weeks later, all the animals were sacrificed to
remove the tumors and measure their lengths, diameters (a) and
short diameters (b), according to the formula of tumor volume
inhibition rate = (1- tumor volume in the experimental group/tumor
volume in control group) × 100% and tumor volume inhibition rates
were calculated. The present study was approved by the Ethics
Committee of Xuzhou Central Hospital.
Statistical analysis
Experimental results were analyzed with SPSS 18.0
(IBM Corp., Armonk, NY, USA) in a t-test or in a variance analysis,
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Heating experiment of
Fe3O4 nanometer magnetic fluid in vitro
Experimental results showed that the heating
capacity of magnetic fluid was positively correlated with the
concentration of magnetic fluid. Thus, the greater the
concentration of magnetic fluid was at a certain magnetic field
intensity, the stronger was the heating capacity. But there was a
common rule: Temperature grew rapidly in the first 20 min, but
stayed at a constant level 40 min later (Fig. 2).
Effect of magnetic nanoparticles on
glioma in vitro
i) U251 human glioma cell morphology observation:
After nano MFH, human glioma cells were observed through inverted
microscopy. Cells in the control group were the same size without
cell rupture or cell debris, and with a greater number of cells
which appeared well attached. In the hyperthermia group, the
temperature increased in response to an increase in
Fe3O4 nanometer magnetic fluid concentration.
Normal cells gradually decreased while necrotic cells and cell
debris increased gradually with less attachment to the walls, with
more magnetic material deposition. Electron microscopy showed that
in the hyperthermia group, cell chromatin was condensed with
cytoplasmic vacuoles, and apoptotic bodies were formed; nanometer
materials deposited in the nucleus, cytoplasm and lysosomes which
were confirmed as nanoparticles analyzed through energy spectrum
analysis.
ii) MTT detection of U251 cell proliferation: Nano
MFH had a significant inhibiting effect on the proliferation of
glioma cells with a dose-dependent relationship with nano magnetic
fluid concentration. The difference between the groups was
statistically significant (P<0.05) (Table I).
 | Table I.MTT results of different
concentrations of Fe3O4 nanometer magnetic
fluid hyperthermia in U251 cells (mean ± standard deviation). |
Table I.
MTT results of different
concentrations of Fe3O4 nanometer magnetic
fluid hyperthermia in U251 cells (mean ± standard deviation).
| Groups | Control group | 2 g/l | 4 g/l | 6 g/l | 8 g/l |
|---|
| OD value | 0.95±0.01 | 0.75±0.02 | 0.51±0.03 | 0.37±0.01 | 0.12±0.02 |
| Inhibition rate
(%) |
| 21.5 | 46.3 | 61.1 | 87.4 |
Anti-glioma effect of nano magnetic
particles in animal experiment
The tumors in the control group increased with the
prolongation of treatment time, with smooth surfaces and no
hemorrhage or necrosis. Tumors in the hyperthermia group decreased
gradually during the whole cycle of treatment, with hemorrhage and
necrosis by histological examination. With the rise of the
concentration, the inhibitory effect of magnetic fluid gradually
increased. The difference of tumor volumes between three groups was
statistically significant (P<0.05) (Table II). It was shown that MFH has a
certain therapeutic effect on glioma cells, and curative effect was
enhanced with higher magnetic fluid concentration.
 | Table II.Inhibiting effect of
Fe3O4 nanometer magnetic fluid hyperthermia
in different concentrations on tumor volume (mean ± standard
deviation). |
Table II.
Inhibiting effect of
Fe3O4 nanometer magnetic fluid hyperthermia
in different concentrations on tumor volume (mean ± standard
deviation).
| Groups | Blank control
group | Magnetic fluid
control group | 2 g/l | 4 g/l | 6 g/l | 8 g/l |
|---|
| Tumor volume
(cm3) | 19.58±3.53 | 17.24±2.82 | 12.17±3.21 | 9.88±2.14 | 5.69±2.03 | 2.43±1.17 |
| Inhibition rate
(%) |
| 11.9 | 37.7 | 49.5 | 69.6 | 12.4 |
Discussion
Glioma, which is originated from the neuroectoderm
in the central nervous system, is the most common primary tumor in
human intracranial tumors and a cause of death (2). Because of the infiltrating invasive
growth of glioma and the vague boundary between tumor tissue and
normal brain tissue, even surgical treatment results in 5-year
survival rate of <5% and with a high risk of recurrence
(1). These led us to identify a new
treatment option for glioma.
Jordan et al performed an antitumor study on
glioma model of rats with MFH method (12,13). The
Fe3O4 magnetic fluid covered by amino silane
was used and injected into the tumor tissue within the magnetic
field (100 kHz, 18 kA, m-1) for an irradiation of 30 min, and then
the tumor tissue temperature reached 43–47°C. Compared with the
control group, the survival rate of the model was significantly
prolonged. Pathological examination showed a large necrotic area of
tumor tissue, and nano magnetic particles were uniformly dispersed
in that tissue. Maier-Hauff et al (14) injected magnetic particles into the
glioma tumor of patients, and the temperature of the interior of
the tumor reached 44.6 Yi (42.4–49.5 Yi) in an alternating magnetic
field. Patients could tolerate the treatment without obvious side
effects. MFH can be used in applications for deep intracranial
tumors such as glioma, which indicated its safety. The present
study prepared nano magnetic material with high heating-yield, in
order to reduce the amount of the material. The heating experiment
in vitro showed that in a certain magnetic field intensity,
the magnetic fluid heating capacity was positively correlated with
magnetic fluid concentration in the magnetic field, and cell
morphology was observed by optical microscopy and electron
microscopy. MTT detection of Fe3O4 nanometer
MFH was used to detect the proliferation rate of U251 glioma cell
line. The result indicated a significant inhibitory effect on the
proliferation of glioma cells in a dose dependent manner compared
to nano magnetic fluid concentration. Liver cancer inhibition rate
of Fe3O4 nanometer MFH was detected by rat
hyperthermia experiment.
Hyperthermia has not been really practical in
clinical application, mainly due to the scarcity of detailed study
of magnetic nanoparticles in human metabolism and clearance
mechanism and toxicity. Hyperthermia could not completely inhibit
tumor and it greatly restricts the application of MFH in the
treatment of tumors (15,16). We also need many further experiments
to demonstrate the safety of application of magnetic fluid in other
aspects of the animal experiment and treatment, which may improve
the efficacy and reduce adverse reactions that can be used in the
clinical diagnosis and treatment in the future.
References
|
1
|
Hulsebos TJ, Troost D and Leenstra S:
Molecular-genetic characterisation of gliomas that recur as same
grade or higher grade tumours. J Neurol Neurosurg Psychiatry.
75:723–726. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hargrave DR and Zacharoulis S: Pediatric
CNS tumors: Current treatment and future directions. Expert Rev
Neurother. 7:1029–1042. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raut CP, Evans DB, Crane CH, Pisters PW
and Wolff RA: Neoadjuvant therapy for resectable pancreatic cancer.
Surg Oncol Clin N Am. 13639–661. (ix)2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gavrilov LR, Riabukhin VV, Elagin VA,
Ageev AA and Vykhodtseva NI: Apparatus and technical devices for
ultrasonic hyperthermia. Med Radiol (Mosk). 32:78–82. 1987.(In
Russian). PubMed/NCBI
|
|
5
|
Marmor JB, Pounds D, Postic TB and Hahn
GM: Treatment of superficial human neoplasms by local hyperthermia
induced by ultrasound. Cancer. 43:188–197. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ur Noell KT, Woodward KT, Worde BT,
Fishburn RI and Miller LS: Microwave-induced local hyperthermia in
combination with radiotherapy of human malignant tumors. Cancer.
45:638–646. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park BK, Kim CK, Park SY and Shen SH:
Percutaneous radiofrequency ablation of renal cell carcinomas in
patients with von Hippel Lindau disease: Indications, techniques,
complications, and outcomes. Acta Radiol. 54:418–427. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rhim H, Dodd GD III, Chintapalli KN, Wood
BJ, Dupuy DE, Hvizda JL, Sewell PE and Goldberg SN: Radiofrequency
thermal ablation of abdominal tumors: Lessons learned from
complications. Radiographics. 24:41–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahmed M and Goldberg SN: Thermal ablation
therapy for hepatocellular carcinoma. J Vasc Interv Radiol.
13:S231–S244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johannsen M, Thiesen B, Jordan A,
Taymoorian K, Gneveckow U, Waldöfner N, Scholz R, Koch M, Lein M,
Jung K, et al: Magnetic fluid hyperthermia (MFH)reduces prostate
cancer growth in the orthotopic Dunning R3327 rat model. Prostate.
64:283–292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kobayashi T: Cancer hyperthermia using
magnetic nanoparticles. Biotechnol J. 6:1342–1347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jordan A, Scholz R, Wust P, Fähling H,
Krause J, Wlodarczyk W, Sander B, Vogl T and Felix R: Effects of
magnetic fluid hyperthermia (MFH) on C3H mammary carcinoma in vivo.
Int J Hyperthermia. 13:587–605. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang ZY, Song J and Zhang DS: Nanosized
As2O3/Fe2O3 complexes
combined with magnetic fluid hyperthermia selectively target liver
cancer cells. World J Gastroenterol. 15:2995–3002. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maier-Hauff K, Rothe R, Scholz R,
Gneveckow U, Wust P, Thiesen B, Feussner A, von Deimling A,
Waldoefner N, Felix R, et al: Intracranial thermotherapy using
magnetic nanoparticles combined with external beam radiotherapy:
Results of a feasibility study on patients with glioblastoma
multiforme. J Neurooncol. 81:53–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Li N and Li L, Li D, Liu K, Zhao L,
Tang J and Li L: Local hyperthermia for esophageal cancer in a
rabbit tumor model: Magnetic stent hyperthermia versus magnetic
fluid hyperthermia. Oncol Lett. 6:1550–1558. 2013.PubMed/NCBI
|
|
16
|
Hu R, Ma S, Li H, Ke X, Wang G, Wei D and
Wang W: Effect of magnetic fluid hyperthermia on lung cancer
nodules in a murine model. Oncol Lett. 2:1161–1164. 2011.PubMed/NCBI
|