Introduction
Atomic bombs were dropped on Nagasaki and Hiroshima
in August 1945. The Life Span Study (LSS) cohort from the Radiation
Effects Research Foundation (RERF) was established and included
120,000 subjects who were exposed to radiation from the atomic
bombs (1). During the initial 6–8
years following the bombings, the frequency of leukemia reached a
peak and then reduced dramatically to an excess relative risk (ERR)
of 0 (2). Conversely, frequencies of
various types of solid cancer, including bladder, breast, lung,
stomach and brain, were relatively high with an ERR of 0.47
(3,4).
Gastric cancer (GC) is the fifth most common
malignancy in the world and the second highest cause of mortality
for men and women (5). Over 60% of GC
cases worldwide occur in Asian countries, including Japan, China,
Korea and Vietnam (6,7). While radiation is currently widely used
in medicine, industry and nuclear power, the effect of radiation on
GC development has been estimated on the basis of LSS with ERRs per
Gy of 1.20 for mortality (8) and 1.32
for incidence (1). However, Preston
et al (9) estimated that,
following exposure at 30 years of age, the risk of cancer at age 70
increased by ~35% per Gy for males and 58% per Gy for females.
Various studies have identified mutations in TP53 and
BRAF in atomic bomb survivors (10–12), but
no study has examined the specific alterations in
radiation-associated cancer. A previous study demonstrated that
alteration of gene expression occurs in radiation-associated GC
(13). It was observed that versican
and osteonectin genes are expressed at much lower levels in
tumor-associated stroma in a group of subjects exposed to a high
dose of radiation, compared with a group exposed to a low dose
(13). In addition, more frequent GC
cases with high microRNA (miR)-24 expression levels were noted in
the high dose-exposed group than in the low dose-exposed group
(14). However, these findings cannot
fully explain the pathogenesis of radiation-associated GC.
An Escherichia coli ampicillin secretion trap
was performed in previous studies using GC cell lines and tissue
samples (15–17), which demonstrated that the FKTN
gene is overexpressed in GC (18).
FKTN, which encodes fukutin protein, is responsible for
Fukuyama-type congenital muscular dystrophy (19). Fukutin is presumed to be involved in
the glycosylation of α-dystroglycan, which functions in basement
membrane formation (19). Although
overexpression of fukutin has been reported in GC (18), its association with radiation exposure
has not yet been investigated.
In the present study, immunohistochemical analysis
of fukutin was performed in order to elucidate the association
between fukutin expression and radiation-associated GC. In
addition, a focus was placed upon the gastric and intestinal mucin
phenotype of GC, as fukutin expression is observed more commonly in
the intestinal phenotype of GC than in other phenotypes (18).
Materials and methods
Tissue samples
The present study included formalin-fixed and
paraffin-embedded archival tissues from 278 patients with GC who
underwent surgery. The patients were treated at Hiroshima Red Cross
Hospital and Atomic-Bomb Survivors Hospital (HRCHABSH; Hiroshima,
Japan), between April 1991 and March 2000 or Hiroshima University
Hospital (HUH; Hiroshima, Japan) between April 1991 and March
2000.
The HRCHABSH cohort included 192 GC samples, all
from atomic bomb survivors in Hiroshima treated at HRCHABSH. As
these patients were not LSS cohort members, the atomic bomb
radiation doses were not estimated. The HRCHABSH cohort patients
included directly exposed patients and those not present in
Hiroshima city at the time of bombing, but who entered the city
soon following the bombing (within 2 weeks). These patients were
classified into two groups: The exposed at a short distance group
(directly exposed patients, exposure distance from the hypocenter
of ≤4 km) and the exposed at a long distance group (patients not
present in Hiroshima city at the time of bombing, but who entered
the region ≤4 km from the hypocenter within 2 weeks of the
explosion).
The HUH cohort included 86 GC samples, all from
atomic bomb survivors in Hiroshima treated at HUH. The HUH cohort
of 86 patients with GC were also LSS cohort members, and therefore
atomic bomb radiation doses were estimated using the DS02 system
(20). These patients were classified
into two groups according to the levels of exposed radiation dose:
The high dose-exposed group (≥5 mGy) and the low dose-exposed group
(<5 mGy).
Tumor staging was performed according to the
tumor-node-metastasis classification system (21). Histologic classification of GC was
performed according to the Lauren classification system (22). The study was approved by The Ethics
Committee for Human Genome Research of Hiroshima University
(Hiroshima, Japan).
Immunohistochemistry
One or two representative tumor blocks, including
the tumor center, invading front and tumor-associated
non-neoplastic mucosa, from each patient were examined by
immunohistochemistry. In cases of large, late-stage tumors, two
varied sections were examined to include representative areas of
the tumor center, in addition to the lateral and deep tumor
invasive front. Immunohistochemical analysis was performed using
the Dako EnVision+ Mouse Peroxidase Detection system (Dako,
Glostrup, Denmark). Antigen retrieval was performed by microwave
heating in citrate buffer (pH 6.0) for 30 min. Peroxidase activity
was blocked with 3% H2O2-methanol for 10 min
at room temperature, and sections were incubated with normal goat
serum (Dako) for 20 min at room temperature to block nonspecific
antibody binding sites. Sections were incubated with a mouse
monoclonal anti-fukutin antibody (#ab131280; dilution, 1:50; Abcam,
Cambridge, UK) for 1 h at room temperature, followed by incubation
with Envision+ anti-rabbit peroxidase for 1 h at room temperature.
For the color reaction, sections were incubated with Liquid DAB+
Substrate Chromogen Solution (Dako) for 10 min. Sections were
counterstained with 0.1% hematoxylin, and negative controls were
created by omission of the primary antibody.
Expression of fukutin was scored in all tumors as
positive or negative. Immunostaining for fukutin was considered
positive when >10% of tumor cells were stained. Using these
definitions, two surgical pathologists (Hiroshima University),
without knowledge of the clinical and pathological parameters or
the patients' outcomes, independently reviewed immunoreactivity in
each specimen. Interobserver differences were resolved by consensus
review at a double-headed microscope following independent
review.
Phenotypic analysis of GC
GCs were classified into four phenotypes: Gastric
(G) type, intestinal (I) type, gastric and intestinal mixed (GI)
type and unclassified (N) type. For phenotypic expression analysis
of GC, immunohistochemical analysis was performed (as described
above) with four antibodies: Anti-Mucin (MUC) 5AC (#NCL-MUC-5AC;
dilution, 1:50; Novocastra; Leica Biosystems, Nussloch, Germany) as
a marker of gastric foveolar epithelial cells, anti-MUC6
(#NCL-MUC-6; dilution, 1:50; Novocastra; Leica Biosystems) as a
marker of pyloric gland cells, anti-MUC2 (#PA0155; dilution, 1:50;
Novocastra; Leica Biosystems) as a marker of goblet cells in the
small intestine and colorectum and anti-CD10 (#PA0270; dilution,
1:50; Novocastra; Leica Biosystems) as a marker of microvilli of
absorptive cells in the small intestine and colorectum. The
criteria for the classification of G type and I type GCs were as
follows (17): GCs in which >10%
of cells in the section expressed at least one gastric epithelial
cell marker (MUC5AC or MUC6) or intestinal epithelial cell marker
(MUC2 or CD10) were classified as G type or I type cancers,
respectively; sections that exhibited gastric and intestinal
phenotypes were classified as GI type; and those that lacked the
gastric and intestinal phenotypes were classified as N type.
Statistical analysis
SPSS version 23.0 was used for all statistical
analyses (IBM SPSS, Armonk, NY, USA). Associations between
clinicopathological parameters and fukutin protein expression were
analyzed using Fisher's exact test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of fukutin in the HRCHABSH
cohort
Expression of fukutin was first analyzed in the
HRCHABSH cohort. All 192 patients were atomic bomb survivors in
Hiroshima, who developed GC following the bombing. This cohort was
subdivided into two groups, which comprised of 117 patients in the
group exposed at a short distance and 75 patients in the group
exposed at a long distance. Patient characteristics, including age
at diagnosis, gender, tumor stage and Lauren classification, did
not statistically differ between the two groups (data not shown).
As reported previously (18), in
non-neoplastic gastric mucosa, immunohistochemical analysis
exhibited weak staining of fukutin in intestinal metaplastic cells,
but not in the normal gastric epithelial glands. By contrast, GC
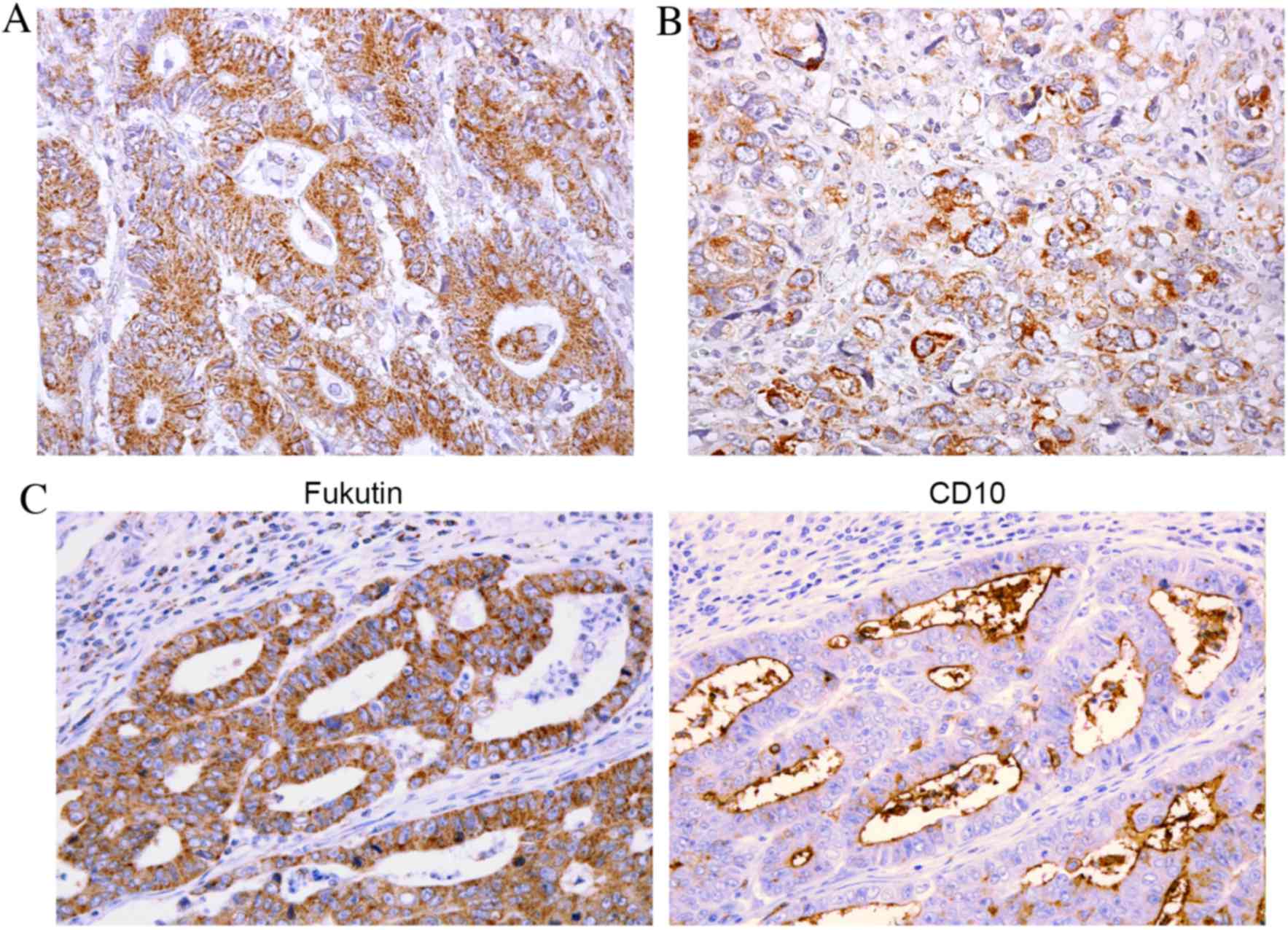
tissue exhibited stronger, more extensive staining of fukutin.
Cytoplasmic granular staining of fukutin was observed in GC cells,
from the superficial layer to the deep layer, particularly in
intestinal type GC (Fig. 1A). Certain
cells of diffuse type GC also demonstrated fukutin staining
(Fig. 1B). It has been previously
noted that positive fukutin expression is frequently observed in
CD10-positive GC cases (18).
Therefore, the association between fukutin expression and CD10
expression was subsequently analyzed, and confirmed that positive
fukutin expression was frequently observed in CD10-positive GC
cases (Fig. 1C). Numerous GC cases
demonstrated heterogeneous fukutin staining and the percentage of
fukutin-stained GC cells ranged from 0–80%. Staining of >10% of
tumor cells was considered to be positive for fukutin. In total,
102 (53%) of 192 GC cases were positive for fukutin.
Following this, the associations between fukutin
expression and clinicopathological characteristics were examined
(Table I). Positive fukutin
expression was observed more frequently in intestinal type GC cases
than in diffuse type GC cases (P=0.0009, Fisher's exact test;
Table I). In addition, positive
fukutin expression was frequently observed in CD10-positive GC
cases (P=0.0001; Table I). By
contrast, fukutin expression was not associated with exposure
status (Table I) or with
gastric/intestinal mucin phenotype (data not shown).
 | Table I.Clinicopathological characteristics of
patients and radiation exposure status in the HRCHABSH cohort. |
Table I.
Clinicopathological characteristics of
patients and radiation exposure status in the HRCHABSH cohort.
|
| Fukutin
expression |
|
|---|
|
|
|
|
|---|
| Characteristic | Positive, n (%) | Negative, n (%) | P-value |
|---|
| Age, years |
|
| 0.0527 |
| ≤65 | 20 (41) | 29 (59) |
|
|
>65 | 82 (57) | 61 (43) |
|
| Gender |
|
| 0.9405 |
| Male | 64 (53) | 56 (47) |
|
|
Female | 38 (53) | 34 (47) |
|
| CD10 expression |
|
| 0.0001 |
|
Positive | 30 (83) | 6
(17) |
|
|
Negative | 72 (46) | 84 (54) |
|
| Exposure status |
|
| 0.8025 |
| Exposed
in short distance group | 63 (54) | 54 (46) |
|
| Exposed
in long distance group | 39 (52) | 36 (48) |
|
| Stage |
|
| 0.4470 |
| Stage
I/II | 71 (55) | 58 (45) |
|
| Stage
III/IV | 31 (49) | 32 (51) |
|
| Lauren
classification |
|
| 0.0009 |
|
Intestinal | 68 (76) | 21 (24) |
|
|
Diffuse | 34 (33) | 69 (67) |
|
Expression of fukutin in the HUH
cohort
In the HRCHABSH cohort, it was demonstrated that
fukutin expression was associated with Lauren classification and
CD10 expression. However, there was no association between fukutin
expression and exposure status. Therefore, analysis of fukutin
expression levels in the HUH cohort was performed, as these
patients were LSS cohort members, in which atomic bomb radiation
doses were estimated correctly by the DS02 system (20). All 86 patients were atomic bomb
survivors in Hiroshima and were comprised of 44 high dose-exposed
and 42 low dose-exposed patients, who developed GC following the
bombing. Patient characteristics, including age at diagnosis, sex,
tumor stage and Lauren classification, did not statistically differ
between the high dose-exposed and the low dose-exposed groups (data
not shown). As with the HRCHABSH cohort, cytoplasmic granular
staining of fukutin was observed in the GC cells, from the
superficial layer to the deep layer, particularly in intestinal
type GC (data not shown). A number of GC cases exhibited
heterogeneous fukutin staining and the percentage of
fukutin-stained GC cells ranged from 0–80%. A total of 58 (67%) of
86 GC cases were positive for fukutin.
As with the HRCHABSH cohort, positive fukutin
expression was observed more frequently in intestinal type GC cases
than in diffuse type GC cases (P=0.0160; Table II). In this cohort, fukutin
expression was associated with exposure status (P=0.0001), but not
with CD10 expression (Table II). No
association was noted between fukutin expression levels and mucin
phenotype (data not shown).
 | Table II.Clinicopathological characteristics of
patients and radiation exposure status in the HUH cohort. |
Table II.
Clinicopathological characteristics of
patients and radiation exposure status in the HUH cohort.
|
| Fukutin
expression |
|
|---|
|
|
|
|
|---|
| Characteristic | Positive, n (%) | Negative, n (%) | P-value |
|---|
| Age, years |
|
| 0.4297 |
| ≤65 | 13 (59) | 9
(41) |
|
|
>65 | 45 (70) | 19 (30) |
|
| Gender |
|
| 0.8202 |
|
Male | 29 (69) | 13 (31) |
|
|
Female | 29 (66) | 15 (34) |
|
| CD10
expression |
|
| 0.7911 |
|
Positive | 15 (71) | 6
(29) |
|
|
Negative | 43 (66) | 22 (34) |
|
| Exposure
status |
|
| 0.0001 |
|
High-dose group | 21 (48) | 23 (52) |
|
|
Low-dose group | 37 (88) | 5
(12) |
|
| Stage |
|
| 0.2307 |
| Stage
I/II | 40 (73) | 15 (27) |
|
| Stage
III/IV | 18 (58) | 13 (42) |
|
| Lauren
classification |
|
| 0.0160 |
|
Intestinal | 43 (77) | 13 (23) |
|
|
Diffuse | 15 (50) | 15 (50) |
|
Discussion
As the use of radiation in medical settings
increases, particularly in nuclear medicine for diagnosis or in
treatment as radiotherapy, understanding the effect of radiation on
human health and particularly radiation-associated cancer becomes
critical. However, studies and conclusive evidence regarding
radiation exposure are lacking. In the present study two cohorts,
including 278 patients who are all atomic bomb survivors, were
examined. Immunohistochemistry was used to determine the expression
and localization of fukutin in GC in relation to radiation exposure
status.
Although the positive expression of fukutin was not
associated with radiation exposure status in the first cohort from
HRCHABSH, the second cohort from HUH exhibited a significant
association between these two variables. As the HRCHABSH patients
were not LSS cohort members, atomic bomb radiation doses may not be
correctly estimated. The HRCHABSH patients were classified into a
group exposed at a short distance and a group exposed at a long
distance according to the distance from the hypocenter of the
bombing. Precise estimation of radiation dose in the LSS cohort
required a complicated system with numerous criteria (20). The LSS cohort from the RERF is based
on the DS02 system, which was established by the Joint US-Japan
Working Group, a collaboration among American, German and Japanese
universities together with national laboratories (20). This group revised the gamma ray and
neutron fluence calculations of source terms from the previous DS86
system (20,23,24). It
may be hypothesized that the difference in estimation strategies
for radiation exposure led to the discrepancy between the two
cohorts. In addition, as fukutin expression was associated with
CD10 expression in the HRCHABSH cohort in the present study, the
association between fukutin expression and radiation exposure may
be affected by CD10 expression.
The HUH cohort with LSS members (and radiation dose
estimation by the DS02 system) demonstrated a statistically
significant association between fukutin expression and radiation
exposure in the current study. These results suggest that the
immunohistochemical analysis of fukutin from surgically resected GC
samples is useful to identify radiation-associated GC. Previous
studies have demonstrated that high dose-exposed groups exhibit
lower levels of versican and osteonectin in tumor-associated stroma
(13) and higher expression levels of
miR-24 (14), compared with low
dose-exposed groups. This suggests that a combination of these
markers may help successfully identify radiation-associated GC.
Fukutin is hypothesized to be involved in basement
membrane formation. Knockdown of FKTN reduces the binding
activity of α-dystroglycan for ligands, including laminin, agrin or
neurexin (25). This indicates that
the loss of fukutin function results in defective glycosylation of
α-dystroglycan, a key element of the dystrophin-glycoprotein
complex, resulting in disruption of the linkage between the
cytoskeleton and the basal lamina (25). This suggests that alteration of
glycosylation may occur in GC in atomic bomb survivors.
In the present study, the expression of fukutin in
the LSS cohort demonstrated a significant association with
radiation dose exposure; however, the sample size of 86 GC cases
may not be sufficient for statistical confirmation. Therefore,
further studies with larger sample sizes from the LSS cohort with
appropriate radiation dose estimation are required.
In conclusion, several studies have been performed
on atomic bomb survivor cohorts for improved understanding of the
effects of radiation on human health. The present study detected
the expression of fukutin in the low dose-exposed group (LSS
cohort), but did not observe an association between fukutin
expression levels and radiation exposure status in the HRCHABSH
cohort. To clarify whether fukutin may be a potential biomarker to
define radiation-induced GC in atomic bomb survivors, future
studies in a larger cohort with precise radiation dose estimation
are required.
Acknowledgements
The authors would like to thank Mr. Shinichi
Norimura (Hiroshima University, Hiroshima, Japan) for technical
assistance and advice. This work was supported by Grants-in-Aid for
Scientific Research (grant nos. 25460417 and 15H04713) from the
Japan Society for the Promotion of Science.
References
|
1
|
Thompson DE, Mabuchi K, Ron E, Soda M,
Tokunaga M, Ochikubo S, Sugimoto S, Ikeda T, Terasaki M and Izumi
S: Cancer incidence in atomic bomb survivors. Part II: Solid
tumors, 1958–1987. Radiat Res. 137:(2 Suppl). S17–S67. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pierce DA, Shimizu Y, Preston DL, Vaeth M
and Mabuchi K: Studies of the mortality of atomic bomb survivors.
Report 12, Part I. Cancer: 1950–1990. 1996. Radiat Res. 146:61–87.
2012. View
Article : Google Scholar
|
|
3
|
Ozasa K, Shimizu Y, Suyama A, Kasagi F,
Soda M, Grant EJ, Sakata R, Sugiyama H and Kodama K: Studies of the
mortality of atomic bomb survivors, Report 14, 1950–2003: An
overview of cancer and noncancer diseases. Radiat Res. 177:229–243.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakata R, Grant EJ, Furukawa K, Misumi M,
Cullings H, Ozasa K and Shore RE: Long-term effects of the rain
exposure shortly after the atomic bombings in Hiroshima and
Nagasaki. Radiat Res. 182:599–606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Global Burden of Disease Cancer
Collaboration, . Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G and Woodbrook R:
The global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yasui W, Sentani K, Sakamoto N, Anami K,
Naito Y and Oue N: Molecular pathology of gastric cancer: Research
and practice. Pathol Res Pract. 207:608–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Preston DL, Shimizu Y, Pierce DA, Suyama A
and Mabuchi K: Studies of mortality of atomic bomb survivors.
Report 13: Solid cancer and noncancer disease mortality: 1950–1997.
2003. Radiat Res. 178:AV146–AV172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Preston DL, Ron E, Tokuoka S, Funamoto S,
Nishi N, Soda M, Mabuchi K and Kodama K: Solid cancer incidence in
atomic bomb survivors: 1958–1998. Radiat Res. 168:1–64. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeshima Y, Seyama T, Bennett WP, Akiyama
M, Tokuoka S, Inai K, Mabuchi K, Land CE and Harris CC: p53
mutations in lung cancers from non-smoking atomic-bomb survivors.
Lancet. 342:1520–1521. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi K, Eguchi H, Arihiro K, Ito R,
Koyama K, Soda M, Cologne J, Hayashi Y, Nakata Y, Nakachi K and
Hamatani K: The presence of BRAF point mutation in adult papillary
thyroid carcinomas from atomic bomb survivors correlates with
radiation dose. Mol Carcinog. 46:242–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwamoto KS, Mizuno T, Tokuoka S, Mabuchi K
and Seyama T: Frequency of p53 mutations in hepatocellular
carcinomas from atomic bomb survivors. J Natl Cancer Inst.
90:1167–1168. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oue N, Sentani K, Sakamoto N, Motoshita J,
Nishisaka T, Fukuhara T, Matsuura H, Sasaki H, Nakachi K and Yasui
W: Characteristic gene expression in stromal cells of gastric
cancers among atomic-bomb survivors. Int J Cancer. 124:1112–1121.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Naito Y, Oue N, Pham TT, Yamamoto M,
Fujihara M, Ishida T, Mukai S, Sentani K, Sakamoto N, Hida E, et
al: Characteristic miR-24 expression in gastric cancers among
atomic bomb survivors. Pathobiology. 82:68–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anami K, Oue N, Noguchi T, Sakamoto N,
Sentani K, Hayashi T, Hinoi T, Okajima M, Graff JM and Yasui W:
Search for transmembrane protein in gastric cancer by the
Escherichia coli ampicillin secretion trap: Expression of DSC2 in
gastric cancer with intestinal phenotype. J Pathol. 221:275–284.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oo HZ, Sentani K, Sakamoto N, Anami K,
Naito Y, Oshima T, Yanagihara K, Oue N and Yasui W: Identification
of novel transmembrane proteins in scirrhous-type gastric cancer by
the Escherichia coli ampicillin secretion trap (CAST) method:
TM9SF3 participates in tumor invasion and serves as a prognostic
factor. Pathobiology. 81:138–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oue N, Sentani K, Sakamoto N and Yasui W:
Clinicopathologic and molecular characteristics of gastric cancer
showing gastric and intestinal mucin phenotype. Cancer Sci.
106:951–958. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oo HZ, Sentani K, Mukai S, Hattori T,
Shinmei S, Goto K, Sakamoto N, Naito Y, Anami K, Trang PT, et al:
Fukutin, identified by the Escherichia coli ampicillin secretion
trap (CAST) method, participates in tumor progression in gastric
cancer. Gastric Cancer. 19:443–452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kobayashi K, Nakahori Y, Miyake M,
Matsumura K, KondoIida E, Nomura Y, Segawa M, Yoshioka M, Saito K,
Osawa M, et al: An ancient retrotransposal insertion causes
Fukuyama-type congenital muscular dystrophy. Nature. 394:388–392.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Preston DL, Pierce DA, Shimizu Y, Cullings
HM, Fujita S, Funamoto S and Kodama K: Effect of recent changes in
atomic bomb survivor dosimetry on cancer mortality risk estimates.
Radiat Res. 162:377–389. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sobin LH and Compton CC: TNM seventh
edition: What's new, what's changed: Communication from the
international union against cancer and the American Joint Committee
on Cancer. Cancer. 116:5336–5339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
23
|
Cullings HM, Fujita S, Funamoto S, Grant
EJ, Kerr GD and Preston DL: Dose estimation for atomic bomb
survivor studies: Its evolution and present status. Radiat Res.
166:219–254. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Straume T, Rugel G, Marchetti AA, Rühm W,
Korschinek G, McAninch JE, Carroll K, Egbert S, Faestermann T, Knie
K, et al: Measuring fast neutrons in Hiroshima at distances
relevant to atomic-bomb survivors. Nature. 424:539–542. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saito F, Masaki T, Saito Y, Nakamura A,
Takeda S, Shimizu T, Toda T and Matsumura K: Defective peripheral
nerve myelination and neuromuscular junction formation in
fukutin-deficient chimeric mice. J Neurochem. 101:1712–1722. 2007.
View Article : Google Scholar : PubMed/NCBI
|