Introduction
The association between cancer and metabolism has
been extensively studied (1). While
there are countless secondary characteristics, cancer cells are
primarily characterized by aerobic glycolysis as opposed to
oxidative respiration in normal cells, an event called ‘Warburg
effect’ (2–5). Several drugs, including metformin,
sulfonylureas and thiazolidinediones, have been shown to affect
morbidity and cancer prognosis (5,6). The study
of the therapeutic use of metformin in head and neck squamous cell
carcinoma (HNSCC) treatment represents a new paradigm of clinical
medicine (7). In addition, various
studies have evaluated the use of metformin in combination with
certain anti-cancer therapies (8–13).
Metformin (1,1-dimethylbiguanide hydrochloride) is a
widely used drug for the treatment of type 2 diabetes mellitus
(14,15), and it is currently considered one of
the most widely prescribed drugs in the world (16). Only few adverse effects have been
associated with its administration (17–20).
Metformin use in diabetic patients has been associated with
decreased cancer incidence and mortality (21–23). This
effect seems to result from a reduction in circulating insulin
levels (24), but there are also data
indicating direct anti-tumor effects of metformin. The reduction in
the growth of tumor cells in response to metformin is mediated, in
part, by the inhibition of mammalian target of rapamycin complex 1
(mTORC1) (25), which is activated by
adenosine monophosphate-activated protein kinase (AMPK) (25–27). The
mTOR pathway plays a key role in the control of cell growth and
metabolism, which are important in cancer progression (28). Carcinogenesis in HNSCC is driven by
diverse signaling pathways, including epidermal growth factor
receptor (EGFR), p53, p16, insulin growth factor (IGF) receptor,
cyclin D1, human papillomavirus (HPV)/E6/E7, phosphoinositide
3-kinase (PI3K)/AKT/mTOR, nuclear factor kappa B and
hypoxia-inducible factor 1 alpha (29). The associations between signaling
pathways and metabolism have been extensively studied; however,
little is known about the role of metabolism in HNSCC
carcinogenesis, treatment failure and recurrence risk (30).
Mouth and pharynx cancers together are the sixth
most common type of cancer in the world, with high incidence
particularly in the South and Southeast of Asia, parts of Western
and Eastern Europe and parts of South America (31). The main risk factors for HNSCC are
alcohol intake and tobacco smoking (32,33).
Advancements in the management of HNSCC have included improved
clinical care for these patients. The ultimate goal is to have
therapies individually tailored to the specific genetic components
of each patient (34). Therefore, the
purpose of the present systematic review is to summarize the
available literature about the in vitro anti-tumor effects
of metformin on HNSCC.
Materials and methods
Protocol and registration
The present systematic review adheres as closely as
possible to the Preferred Reporting Items for Systematic Reviews
and Meta-Analyses checklist (35).
The protocol could not be registered as it is a systematic review
of in vitro studies.
Eligibility criteria
Inclusion criteria
Only in vitro studies comparing the effects
of metformin on the treatment of HNSCC cell lines were selected.
The cell lines were established from different body parts affected
by HNSCC, including the lips, oral cavity, pharynx, larynx, nasal
cavity and paranasal sinuses (36).
Studies on nasopharyngeal tumors were excluded due to differences
in cancer etiology, epidemiology and therapeutic options. The
population, intervention, comparison, outcome and study design
format was adjusted to elucidate clinical questions based on the
following inclusion criteria: i) Population, cells from HNSCC; ii)
intervention, metformin; iii) comparison, cells that received a
control treatment but not metformin treatment; iv) outcome, cell
viability, apoptosis, cell cycle arrest and regulation of protein
expression levels; and v) study design, studies with the presence
or absence of a comparable baseline (in vitro studies), and
randomized and non-randomized controlled trials (in vivo
animal studies).
Exclusion criteria
i) Studies that did not treat cancer cells with
metformin; ii) studies that did not establish an association
between metformin and HNSCC; iii) previous reviews of the
literature, letters, case reports, personal opinions, conference
abstracts and book chapters; and iv) clinical studies were excluded
from the present meta-analysis.
Information sources and search strategies
Studies to be considered for inclusion were
identified by searching through multiple databases, including
Cochrane Library (http://www.cochranelibrary.com), Embase (https://www.embase.com), LILACS (http://lilacs.bvsalud.org), MEDLINE (http://ovidsp.txovid.ez54.periodicos.capes.gov.br/sp)
and PubMed (https://www.ncbi.nlm.nih.gov/pubmed/). The search
strategy for PubMed included the following terms: ‘Oral cancer’ OR
‘oral carcinoma’ OR ‘head and neck cancer’ OR ‘head and neck
carcinoma’ OR ‘head cancer’ OR ‘head carcinoma’ OR ‘neck cancer’ OR
‘neck carcinoma’ OR ‘squamous cell carcinoma’ AND metformin. Cited
literature of the included studies was also checked. The search was
performed on June 2, 2015. The references were managed manually and
duplicate hits were discarded.
Study selection
Articles were selected in two phases. In phase 1,
two authors (D.F.R. and S.T.E.) independently reviewed the titles
and abstracts of all the references. These authors selected
articles that appeared to meet the inclusion criteria based on
their abstracts. In phase 2, two authors (D.F.R. and S.T.E.) read
all full-text articles and excluded those that were not in
agreement with the inclusion criteria. The same two authors
independently reviewed all full-text articles. Any disagreement
between the authors in the first and second phases was resolved by
means of discussion. In cases where a consensus could not be
reached, a third author (E.N.S.G.) made a final decision.
Data collection process and data items
One author (D.F.R.) collected the required
information from the selected articles, including author names,
year of publication, country, study design, assays, cell line,
treatment used, results, main conclusions and clinical applications
(Table I). A second author (S.T.E.)
cross-checked the information. Any disagreement was resolved by
means of discussion, and a third author (E.N.S.G.) was involved,
when required, in making a final decision.
 | Table I.Summary of the descriptive
characteristics of the included articles (n=11). |
Table I.
Summary of the descriptive
characteristics of the included articles (n=11).
|
|
|
| Methods |
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Author, year
(ref) | Country | Study design | Assays | Cell line | Treatment | Results | Main
conclusion | Clinical
application |
|---|
| Lin et al,
2014 (8) | Taiwan | In vitro and in
vivo | MTT assay, flow
cytometry and calculation of synergism between metformin and
dasatinib and SEXTM | Ca9-22, HSC3, SAS
and FaDu | Metformin+
dasatinib; DMSO (control) | Metformin and
dasatinib for 48 h caused cellular growth inhibition; metformin
enhanced dasatinib-induced apoptosis | Metformin enhanced
dasatinib-induced apoptosis in sensitive HNSCC cells, while
activation of AMPK by metformin potentiated dasatinib-induced
endoplasmic reticulum stress, EGFR degradation and anti-tumor
effect in vivo | 1 |
| Luo et al,
2012 (38) | China | in vitro and
in vivo | Cell proliferation
and clonogenic assay, cell cycle and apoptosis analysis, WB, IHC,
TUNEL and in vivo anti-tumor activity | CAL27, WSU-HN6 and
SCC25 | Metformin; PBS
(control) | Proportion of cells
in the G0/G1 phase: 69.70 vs. 50.86% in CAL27, 77.96 vs. 56.54% in
WSU-HN6 and 64.03 vs. 43.51% in SCC25 cells; colony formation was
reduced >90% compared with the untreated controls | Metformin inhibited
the growth of OSCC cells by blocking cell cycle progression at the
G0/G1 phase and inducing apoptosis; metformin was associated with
the activation of the AMPK pathway and suppression of mTOR and S6K
activation, and markedly decreased the expression of cyclin D1 and
increased the number of apoptotic cells in a xenograft model | 1 |
| Ma et al,
2012 (9) | Canada | in
vitro | WB and MTT
assay | SCC9 and SCC25 | Metformin;
metformin+ gefitinib | The combination of
metformin and gefitinib induced co-operative cytotoxicity that was
limited to the LKB1- expressing cell lines SCC9 and SCC25. In the
SCC9 cell line, similar combinations of metformin or lovastatin
with gefitinib displayed synergy when in combination | Metformin enhanced
gefitinib cytotoxicity only in LKB1-expressing SCC lines | 1 |
| Madera et
al, 2015 (40) | USA | in vitro and
in vivo | RNAi, OCT3
knockdown, IHC and WB | CAL27, CAL33 and
UMSCC47 | Metformin | Metformin inhibited
mTOR signaling and tumor growth in HNSCC cells expressing mutated
PIK3CA and HPV oncogenes, which required OCT3 expression | Metformin reduced
the proliferation in vitro of HNSCC cells harboring
mutations in PIK3CA or derived from HPV+ HNSCC lesions, which are
frequent events in oral malignancies. Metformin was highly
effective in reducing HNSCC tumor growth in vivo | 1 |
| Patel et al,
2013 (10) | USA | in
vitro | Cell viability,
RNAi and WB | HN4, HN13 and
Hep2 | Metformin;
metformin+ corticosterone | OCT3 is highly
expressed in oral dysplastic lesions and well to moderately
differentiated HNSCC tumors. Therefore, metformin was unable to
induce AMPK | The impact of OCT3
on metformin action was defined a novel chemopreventive oncologic
agent of head and neck cancer activation or inhibit the mTORC1
pathway | 1 |
| Sandulache et
al, 2012 (11) | USA | in
vitro | Metabolic studies,
clonogenic assay and ROS measurement | HN30 and HN31 | Metformin+ 2-DG;
metformin+ 2-DG+XRT | In combination with
2-DG, metformin resulted in potentiation of XRT toxicity. Metformin
triggered phosphorylation of AMPK but did not induce an increase in
the levels of pAMPK | Inhibition of
respiration using metformin increased glycolytic dependence in wt
TP53-expressing cells and potentiated the effects of glycolyic
inhibition on radiation toxicity | 1 |
| Sandulache et
al, 2011 (12) | USA | in
vitro | Soft agar
growth | FaDu, HN30, OSC19,
HN31, SQCCY1, PCI13, UMSCC17A, UMSCC22B, UMSCC17B, MDA1586, SCC61
and UMSCC25 | Metformin+
2-DG | Addition of
metformin (a glucose sensitizer) resulted in substantial
potentiation of 2-DG effects in multiple cell lines, independently
of p53 mutation status | Metformin greatly
potentiated the effects of glycolytic inhibition irrespective of
p53 status | 1 |
| Sikka et al,
2012 (39) | USA | in
vitro | Cell viability
assay, cell cycle analysis and WB | FaDu and D562 | Metformin; DMEM
(control) | Metformin inhibited
cell growth and cell cycle progression, decreased the protein
levels of CDKs, CDKIs, cyclins and oncogenic proteins SKP2 and
β-TrCP, decreased 4E-BP1 phosphorylation, and increased EF2 and
AMPK phosphorylation | Metformin
suppressed cell growth through targeting global translational
regulators in two different human HNSCC cell lines | 1 |
| Skinner et
al, 2012 (13) | USA | in vitro and
in vivo | Clonogenic assay,
immuno fluorescence, ROS measurement, WB, cell cycle analysis and
p21 transcription orthotopic mouse model | HN30, UMSCC and
UMSCC17A | Metformin | Metformin
selectively radiosensitized cells with disruptive TP53 mutations,
partially due to altered senescence | Metformin could
serve as a radiosensitizer for HNSCC with disruptive TP53
mutations | 1 |
| Vitale-Cross et
al, 2012 (7) | USA | in vitro and
in vivo | Cell proliferation
and viability assay, ATP assay, WB, experimental animal model,
plasma levels of IGF1 and insulin, IHC, immuno fluorescence, T-cell
proliferation assay and flow cytometry | CAL27, HN12, HN13
and Hep2 | Metformin;
rapamycin (control) | Metformin treatment
inhibited HNSCC cell proliferation, downregulated mTORC1 pathway
activity through an AMPK-independent mechanism and prevented HNSCC
development by significantly reducing the size and number of
carcinogen- induced oral tumoral lesions and by preventing their
spontaneous conversion to squamous cell carcinomas | Metformin could
become an attractive chemopreventive agent to hamper the
progression of premalignant lesions highly dependent on mTORC1
activity | 1 |
| Wang et al,
2014 (41) | China | in
vitro | MTT assay, flow
cytometry and WB | KB | Metformin | Metformin inhibited
HNSCC cell proliferation and KB clone colony formation. Metaformin
promoted the apoptosis and increased the expression of GRP78 and
caspase-3 protein in the oral cancer KB cell line | Metformin
significantly inhibited the proliferation of human oral cancer KB
cells, and induced apoptosis, the mechanism of activation of the
mitochondrial apoptotic pathway and excessive endoplasmic reticulum
stress. This finding suggests that metformin may be used as a novel
adjuvant and used to treat cancer | 1 |
Risk of bias in individual studies
The authors methodically appraised all the selected
studies according to the Grading of Recommendations Assessment,
Development and Evaluation (GRADE) method (37) to judge the quality of evidence. Two
authors (D.F.R. and S.T.E.) categorized the included articles as
‘high’, ‘moderate’, ‘low’ or ‘very low’ quality, according to their
analysis of each study. When the above authors did not reach a
consensus regarding the quality of a particular study, a third
author (E.N.S.G.) made a final decision.
Summary measures
Cell viability, apoptosis, cell cycle arrest and
changes in protein expression levels in HNSCC were the main
evaluated outcomes of metformin treatment.
Synthesis of results
A meta-analysis was planned if the data from the
included studies was considered relatively homogeneous.
Risk of bias across studies
Analysis of the risk of bias across studies was only
applied if a meta-analysis was possible.
Results
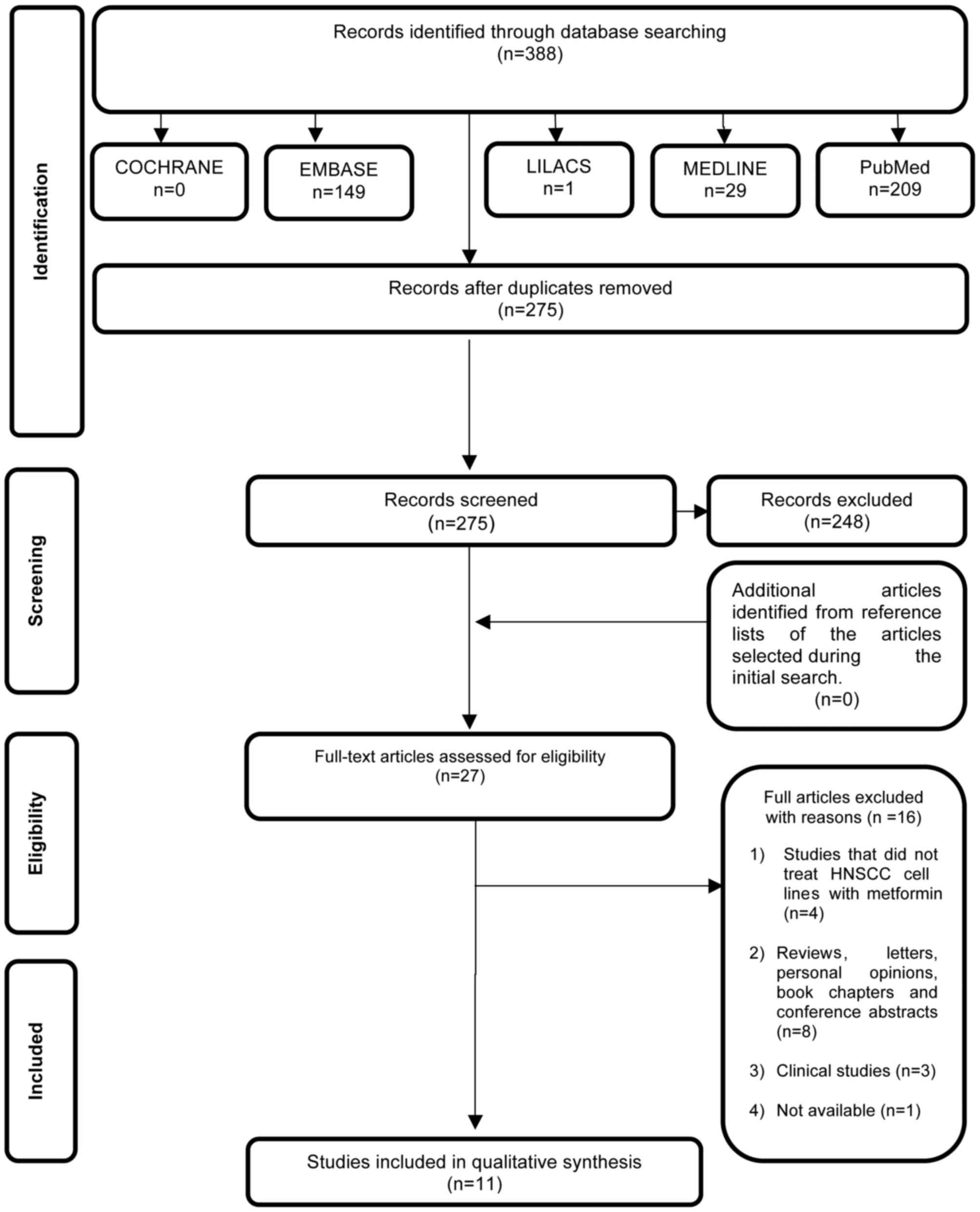
Study selection
In phase 1 of study selection, 388 citations were
identified across the aforementioned electronic databases.
Following the removal of duplicate articles, 275 citations
remained. Comprehensive evaluation of the abstracts was completed,
and 248 articles were excluded. No additional studies from the
reference lists were identified. The remaining 27 articles were
retrieved to conduct a full text review. This process led to the
exclusion of 16 studies (data not shown), resulting in the
selection of 11 articles (7–13,30–41). A
flow chart detailing the process of identification, inclusion and
exclusion of studies is shown in Fig.
1.
Study characteristics
All included studies were published between 2011 and
2015, demonstrating that the use of metformin in HNSCC is a new
concept. Nine of the articles were published in English (7,10–13,39,40) and
one in Chinese (41). The studies
were conducted in four different countries: Canada (9), China (38,41),
Taiwan (8) and USA (7,10–13,39,40). All
selected studies were performed in vitro (7–13,38–41), and
five of them involved in vivo animal experiments (7,8,13,38,41). A
summary of the included studies is presented in Table I. The studies were compared according
to the baselines for Randomized Controlled Trials (42), similarly to the work of Xiao et
al published in 2013 (43), using
the GRADE method (37). A summary of
cell viability tests in HNSCC cell lines treated with metformin is
shown in Table II.
 | Table II.Interventions used to test head and
neck carcinoma cell lines viability in cell culture. |
Table II.
Interventions used to test head and
neck carcinoma cell lines viability in cell culture.
|
| Population | Intervention |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Author, year
(ref) | Cell
linea | Treatment | Time (h) | Dose | C | O | S | V |
|---|
| Lin et al,
2014 (8) | Ca9-22, HSC3, SAS
and FaDu | Metformin;
dasatinib; metformin+dasatinib; DMSO (control) | 48 | 0.5–10 mM
metformin; 0.05–1 µM dasatinib | √ | √ | √ | 1 |
| Luo et al,
2012 (38) | CAL27, SCC25 and
WSU-HN6 | Metformin; PBS
(control) | 24,48 and 72 | 0–20 mM
metformin | √ | √ | √ | 1 |
| Ma et al,
2012 (9) | SCC9 and SCC25 | Metformin,
lovastatin; gefitinib; lovastatin+ gefitinib; ethanol
(control) | 24,48 and 72 | 0–20 mM metformin;
0–100 µM lovastatin; 0–100 µM gefitinib | √ | √ | √ | 1 |
| Madera et
al, 2015 (40) | CAL27, CAL33 and
UMSCC47 | Metformin | 4 | 0–03 mM
metformin | √ | √ | √ | 2 |
| Patel et al,
2013 (10) | HN4, HN13 and
Hep2 | Metformin | 72 | 3 mM metformin | √ | √ | √ | 2 |
| Sandulacheet
al, 2012 (11) | HN30 and HN31 | Metformin+2-DG;
metformin+2-DG+XRT | 16 | 5 mM metformin; 5
mM 2-DG; 2 Gy XRT | √ | √ | √ | 2 |
| Sandulache et
al, 2011 (12) | HN30 and HN31 | Metformin; 2-DG;
metformin+2-DG | 72 | 1 mM metformin;
0.5–8 mM 2-DG | √ | √ | √ | 2 |
| Sikka et al,
2012 (39) | FaDu and D562 | Metformin | 24 and 72 | 5–20 mM
metformin | √ | √ | √ | 1 |
| Skinner et
al, 2012 (13) | HN30, UMSCC and
UMSCC17A | Metformin+XRT; PBS
(control) | 24 | 5–10 µmol/l
metformin; 4 Gy XRT | √ | √ | √ | 1 |
| Vitale-Cross et
al, 2012 (7) | HN12 | Metformin;
rapamycin (control) | 24 and 96 | 2–100 mM
metformin | √ | √ | √ | 2 |
| Wang et al,
2014 (41) | KB | Metformin | 24,48 and 72 | 1.25–20 mmol/l
metformin | √ | √ | √ | 1 |
Risk of bias within studies
The GRADE method was used to assess the quality of
the included studies (37). Two
studies were assigned a moderate quality (11,41). All
others were considered high quality of evidence (7–12,13,38–40). One
of these studies (11) did not
describe the statistical methods used, and was considered
inconsistent. Wang et al (41)
used only one cell line (KB cells) to test the effect of metformin
in HNSCC. Furthermore, the authors did not present a statistical
analysis. Therefore, the results of the study were considered
inconclusive (Table III).
 | Table III.Judgment of the quality of evidence
for intervention. |
Table III.
Judgment of the quality of evidence
for intervention.
|
| Grading of
Recommendations Assessment, Development and Evaluation factors |
|---|
|
|
|
|---|
| Author, year
(ref) | Study design | Study
limitation | Inconsistency | Indirectness | Imprecision | Publication
bias | Moderate/large
effect size | Dose effect | Overall
quality |
|---|
| Lin et al,
2014 (8) | With comparable
baseline (in vitro) | √ | √ | √ | √ | √ | Present | Present | ++++ |
| Luo et al,
2012 (38) | With comparable
baseline (in vitro) RCT (animal) | √ | √ | √ | √ | √ | Present | Present | ++++ |
| Ma et al,
2012 (9) | With comparable
baseline (in vitro) RCT (animal) | √ | √ | √ | √ | √ | Present | Present | ++++ |
| Madera et
al, 2015 (40) | With comparable
baseline (in vitro) RCT (animal) | √ | √ | √ | √ | √ | Present | Present | ++++ |
| Patel et al,
2013 (10) | With comparable
baseline (in vitro) | √ | √ | √ | √ | √ | Present | Present | ++++ |
| Sandulache et
al, 2012 (11) | With comparable
baseline (in vitro) | √ | X (No statistical
analysis for all results) | √ | √ | √ | Present | Present | +++ |
| Sandulache et
al, 2011 (12) | With comparable
baseline (in vitro) | √ | √ | √ | √ | √ | Present | Present | ++++ |
| Sikka et al,
2012 (40) | With comparable
baseline (in vitro) | √ | √ | √ | √ | √ | Present | Present | ++++ |
| Skinner et
al, 2012 (13) | With comparable
baseline (in vitro) | √ | √ | √ | √ | √ | Present | Present | ++++ |
| Vitale-Cross et
al, 2012 (7) | With comparable
baseline (in vitro) | √ | √ | √ | √ | √ | Present | Present | ++++ |
| Wang et al,
2014 (41) | With comparable
baseline (in vitro) | √ | √ | √ | X (Only one cell
line used; no statistical analysis) | √ | Present | Present | +++ |
The evidences describing a possibility of clinical
application of metformin were classified as: i) 1, showing a
potential effect following HNSCC treatment; ii) 2, inconclusive; or
iii) 3, non-supportive of using metformin to treat HNSCC (Table I).
Cell viability
The cytotoxicity of metformin in HNSCC cells lines
was evaluated using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (7–10,38–41).
Several studies monitored colony development (11,13). Six
studies (8,9,38–41) showed that metformin alone is cytotoxic
to HNSCC cells, reducing cell viability by >50% in a
dose-dependent manner (Table II).
Other studies involved co-administration of metformin with another
drug, and demonstrated an adjuvant effect of metformin (8,10–12). Lin et al (8) observed that metformin enhanced dasatinib
efficacy through activation of AMPK. Sandulache et al
(11) demonstrated the
anti-tumorigenic activity of 2-deoxyglucose (2-DG) when combined
with metformin, resulting in the potentiation of radiation
toxicity, despite minimal single agent effects. Sandulache et
al (12) showed in multiple cell
lines that this process was independent of tumor protein p53
status. Ma et al (9) compared
the ability of metformin or lovastatin to enhance the cytotoxicity
of gefitinib in various liver kinase B1 (LKB1)-deficient cell
lines, and demonstrated that metformin increased the cytotoxic
effects of gefitinib in the LKB1-expressing cell lines SCC9 and
SCC25, which were derived from tongue squamous cell carcinoma.
Specifically, the authors observed >90% cell death in SCC9 cells
treated with both metformin and gefitinib. Madera et al
(40) observed that metformin
significantly reduced cell proliferation in CAL27, CAL33 and
UMSCC47 cell lines. The HPV+ UMSCC47 cell line exhibited the
strongest sensitivity to metformin, with a decrease in cell
proliferation at lower than CAL33 metformin concentrations, in
3H-thymidine incorporation and colony forming assays. It was
reported that metformin significantly decreased colony size in
colony forming assay (40). Two
studies (11,13) demonstrated beneficial effects of
metformin when combined with radiotherapy.
Cell cycle regulation and
apoptosis
Three studies (8,38,39) demonstrated accumulation of cells in
the G0/G1 phase upon treatment with metformin, as evidenced by flow
cytometry assays of HNSCC cell lines. Lin et al (8) demonstrated metformin-dependent
enhancement in the apoptotic activity of dasatinib in Ca9-22 and
HSC3 cells, suggesting that metformin could potentiate the
dasatinib-induced anti-cancer effect. Luo et al (38) showed that metformin increased the
proportion of cells in the G0/G1 phase in three HNSCC-derived cell
lines, compared with control cells (69.70 vs. 50.86% in CAL27,
77.96 vs. 56.54% in WSU-HN6 and 64.03 vs. 43.51% in SCC25 cells).
In addition, metformin induced a significant increase in the
proportion of apoptotic tumor cells 48 h after treatment in CAL27,
WSU-HN6 and SCC25 cells (25.4, 24.4 and 43.7%, respectively,
compared with 11.4, 8.4 and 15.5% of apoptotic cells at 24 h after
treatment, respectively). Sikka et al (39) reported a dose- and time-dependent
increase in G1-phase cell population in FaDu and D562 cell lines
(P<0.001) following treatment with metformin.
Regulation of protein expression
level
Lin et al (8)
demonstrated metformin-dependent enhanced effects of dasatinib on
the phosphorylation of AMPK and elongation factor 2, in addition to
down-regulation of EGFR, in sensitive HSC3 tumor cells. According
to Sikka et al (39),
metformin affects the expression of cyclins, cyclin-dependent
kinases (CDKs) and CDK inhibitors (CDKIs) in human HNSCC cells 24
and 48 h after treatment, causing a strong and dose-dependent
decrease in the expression levels of cyclins D1 and E in FaDu and
D562 cell lines. Luo et al (38) confirmed the role of metformin in the
expression of related cell cycle regulatory proteins (including
AMPK, mTOR, S6 kinase, cyclin D1, retinoblastoma protein, CDKs 4
and 6, p21 and p27) in arrested and proliferating oral squamous
cell carcinoma cells. The expression of the anti-apoptotic protein
B-cell lymphoma (Bcl)-extra large and the pro-apoptotic protein
Bcl2-associated × protein were also regulated by metformin. Patel
et al (10) reported the
abrogation of increased S6 and acetyl coenzyme A carboxylase
phosphorylation levels, as well as the inhibition of mTORC1 in
metformin-treated HN13 cells transfected with organic cation
transporter 3 (OCT3) small interfering RNA (siRNA), compared with
control siRNA-transfected cells. These findings indicate that, in
HNSCC cells, the uptake transporter OCT3 plays a key role in
mediating the intracellular effects of metformin on AMPK activation
and subsequent inhibition of mTORC1 activity. Sandulache et
al (11) demonstrated that, in
combination with 2-DG, metformin triggered the phosphorylation of
AMPK, whereas alone, metformin did not increase the levels of
phosphorylated AMPK. Vitale-Cross et al (7) observed that, in the absence of AMPK
activation, metformin treatment led to a marked decrease in mTORC1
activity and tumor cell proliferation. Madera et al
(40) revealed a reduction in
phosphorylated S6 levels upon treatment with metformin in xenograft
tumor models, whereas the non-phosphorylated fraction of 4E-binding
protein 1 (4E-BP1) increased, indicating a cumulative decrease of
4E-BP1. In alignment with their known activities, treatment with
metformin resulted in increased phosphorylated AMPK levels, while
both treatments diminished phosphorylated S6. Thus, metformin
downregulates the activity of the mTOR signaling pathway in HNSCC
cell lines in vitro. Similarly, the reduction of OCT3
diminishes the metformin effect on cell proliferation in
vitro and its anti-tumoral effect in vivo. According to
Wang et al (41), metformin
caused an increase in activated caspase-3 expression levels, as
well as an initial up-regulation followed by a down-regulation of
78 kDa glucose-regulated protein expression in KB cells.
Risk of bias across studies
The studies selected for the present analysis were
considered heterogeneous, and they did not have compatible data
that would allow a meta-analysis. In addition to the
non-comparability of the results of each study, a meta-analysis
could not be conducted due to a lack of clinical studies on the
subject of interest.
Discussion
Summary of evidence
The present systematic review evaluated the in
vitro and in vivo (animals) anti-tumor effects of
metformin in HNSCC. The majority of HNSCC cases arise in the oral
cavity, and continue to be a major public health concern (31). Despite the use of multiple treatments,
the prognosis for this aggressive solid tumor remains poor
(44). Surgery is the most well
established initial treatment for the majority of oral cancers;
however, radiotherapy is employed in conjunction with surgery
(45). Considering organ preservation
and survival, a multidisciplinary approach is strongly encouraged,
and concurrent chemo-radiotherapy has been recommended (46). Complete response rates subsequent to
induction chemotherapy combined with radiotherapy are usually
higher than following radiotherapy alone. In addition to high nodal
stage, molecular mechanisms should be identified and integrated in
order to elucidate markers of distant metastasis risk (47). The review from Busch et al
(47) highlights the recent
developments, including randomized trials, comparing radiotherapy
and induction chemotherapy, followed by definitive radiotherapy.
The review summarizes the developments in induction chemotherapy,
provides critical remarks of recent discoveries and discusses how
clinical trials such as those assessing induction chemotherapy
should be conducted in the future (46). There is a requirement for novel
perspectives and therapeutic approaches to provide a better
understanding and more successful treatment of HNSCC (47).
Metformin is a biguanide that has been used for its
insulin-sensitizing and glucose-lowering effects in type 2 diabetes
mellitus, as well as in gestational diabetes mellitus, polycystic
ovary syndrome and metabolic syndrome, and also for diabetes
prevention (48). This drug has shown
beneficial effects in suppressing the intestinal absorption of
glucose (17), reducing
cardiovascular risk (19), reducing
the risk of lactic acidosis compared with other biguanides
(20), in addition to anti-neoplastic
activity in vitro and in vivo (49,50).
Numerous studies have demonstrated the anti-tumor effect of
metformin in cancer progression (6,15,51–53). A
recent publication confirmed the association between decreased risk
of HNSCC and metformin use in clinical studies (54). Therefore, metformin, an old drug, is
now gaining increasing attention as an anti-cancer agent (55).
The mechanisms underlying the possible anti-cancer
effect of metformin have not yet been fully elucidated. Data from
clinical and pre-clinical studies suggest that amelioration of
insulin resistance/hyperinsulinemia and glucose lowering by
metformin may play a role, since both the insulin-IGF1 system
(56–58) and hyperglycemia (59–61) have
been associated with cancer risk. However, there has been mounting
evidence of metformin's direct effects on cancer cells, and
promising models have been proposed, which suggested the inhibition
of cell proliferation and progression of cancer, cell cycle arrest
and stimulation of apoptosis (62).
In the present systematic review, the in vitro effect of
metformin on HNSCC was studied, and 11 in vitro studies
addressing this topic were identified (7–13,38–41). To
evaluate the effects of metformin on HNSCC cell lines, the authors
used MTT assay (7–10,38–41) or
colony formation tests (11,13), and demonstrated that metformin alone
or in combination with other treatments could effectively reduce
cancer cell viability by >50%, in a time- and dose-dependent
manner (Table II). The effect of
metformin on HNSCC cells in combination with radiation was also
evaluated (11,13), and was demonstrated to sensitize the
tumor to the effect of radiotherapy.
Combination of metformin with other drugs inhibits
cancer cell proliferation (8,11). Metformin has been reported to improve
cancer responses to radiation therapy, probably via down-regulation
of the hyperactive PI3K/AKT/mTOR signaling pathway (63). Previous studies have reported that
mTOR plays a key role in controlling cell growth, proliferation and
metabolism, and mediates the PI3K/AKT signaling pathway, which is
frequently dysregulated in human cancers (64,65).
Multiple genetic changes leading to cancer
progression cause dysregulation of the G1 to S transition (66). The G1 phase of the cell cycle is
controlled by a dynamic interaction between cyclins D1 and E, CDKs
2, 4 and 6 and CDKIs, including members of the CDK interacting
protein/kinase inhibitory protein (Kip) and inhibitors of CDK4
(67). During the transition from G1
phase, the levels of Kip1/p27 decrease to allow the cyclin/CDK
complex to initiate the transcription of genes necessary for G1-S
progression (68). Lin et al
(8), Luo et al (38) and Sikka et al (39) demonstrated accumulation of cells in
the G0/G1 phase when HNSCC cell lines were treated with metformin.
Activation of AMPK and subsequent inhibition of mTORC1 signaling,
reduction of cyclin D1 levels and dephosphorylation of AKT (at
Ser473) have been shown to participate in metformin-induced
apoptotic process (69).
In summary, the present study is the first
systematic review of the in vitro anti-tumor effects of
metformin in HNSCC cell lines, and the first review to summarize
the evidence of a positive association between the decrease of
HNSCC cell viability and metformin. The present authors have
previously published a systematic review about the clinical effects
of metformin on HNSCC patients (55),
which suggested that metformin appears to improve the overall
survival of HNSCC patients. Considering the context, multiple
mechanisms of metformin action should therefore be clarified, and
the current review summarizes the potential effects of metformin on
such important pathways of HNSCC carcinogenesis as cell
proliferation, cell cycle progression and protein expression.
Limitations
While the present review has methodological
limitations, which should be considered, its main strength lies in
the description of several studies that tested metformin in
combination with other drugs. Despite the fact that the included
studies were aimed at understanding the in vitro effects of
metformin on the expression of proteins involved in the process of
carcinogenesis of HNSCC, only three studies described the effect of
metformin in G0/G1 cell cycle arrest and apoptosis. Therefore,
further investigations are warranted to validate these findings.
For the quality assessment of in vitro studies, no standard
assessment was used for basic studies; however, a method to assess
the quality of all articles was defined.
In conclusion, the present systematic review reveals
that preclinical evidence supports the potential use of metformin
as an adjuvant agent in chemotherapy and/or radiotherapy approaches
routinely used in the management of HNSCC. The aforementioned
studies demonstrated that metformin is important in the inhibition
of cell proliferation, G0/G1 cell cycle arrest, apoptosis and
regulation of various proteins involved in cancer pathways, thus
corroborating its potential in vitro and in vivo
(animals) anti-tumor effects. Based on these data and its favorable
safety profile, the present authors suggest the future use of
metformin in both molecular and clinical trial studies.
References
|
1
|
Devic S: Warburg effect - a consequence or
the cause of carcinogenesis? J Cancer. 7:817–822. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heiden MG Vander, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He XX, Tu SM, Lee MH and Yeung SC:
Thiazolidinediones and metformin associated with improved survival
of diabetic prostate cancer patients. Ann Oncol. 22:2640–2645.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He X, Esteva F, Ensor J, Hortobagyi G, Lee
MH and Yeung SC: Metformin and thiazolidinediones are associated
with improved breast cancer-specific survival of diabetic women
with HER2+ breast cancer. Ann Oncol. 23:1771–1780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vitale-Cross L, Molinolo AA, Martin D,
Younis RH, Maruyama T, Patel V, Chen W, Schneider A and Gutkind JS:
Metformin prevents the development of oral squamous cell carcinomas
from carcinogen-induced premalignant lesions. Cancer Prev Res
(Phila). 5:562–573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin YC, Wu MH, Wei TT, Lin YC, Huang WC,
Huang LY, Lin YT and Chen CC: Metformin sensitizes anticancer
effect of dasatinib in head and neck squamous cell carcinoma cells
through AMPK-dependent ER stress. Oncotarget. 5:298–308.
2014.PubMed/NCBI
|
|
9
|
Ma L, Niknejad N, GornHondermann I, Dayekh
K and Dimitroulakos J: Lovastatin induces multiple stress pathways
including LKB1/AMPK activation that regulate its cytotoxic effects
in squamous cell carcinoma cells. PLoS One. 7:e460552012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patel H, Younis RH, Ord RA, Basile JR and
Schneider A: Differential expression of organic cation transporter
OCT3 in oral premalignant and malignant lesions: Potential
implications in the antineoplastic effects of metformin. J Oral
Pathol Med. 42:250–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sandulache VC, Skinner HD, Ow TJ, Zhang A,
Xia X, Luchak JM, Wong LJ, Pickering CR, Zhou G and Myers JN:
Individualizing antimetabolic treatment strategies for head and
neck squamous cell carcinoma based on TP53 mutational status.
Cancer. 118:711–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sandulache VC, Ow TJ, Pickering CR,
Frederick MJ, Zhou G, Fokt I, DavisMalesevich M, Priebe W and Myers
JN: Glucose, not glutamine, is the dominant energy source required
for proliferation and survival of head and neck squamous carcinoma
cells. Cancer. 117:2926–2938. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Skinner HD, Sandulache VC, Ow TJ, Meyn RE,
Yordy JS, Beadle BM, Fitzgerald AL, Giri U, Ang KK and Myers JN:
TP53 disruptive mutations lead to head and neck cancer treatment
failure through inhibition of radiation-induced senescence. Clin
Cancer Res. 18:290–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Knowler WC, BarrettConnor E, Fowler SE,
Hamman RF, Lachin JM, Walker EA and Nathan DM: Diabetes Prevention
Program Research Group: Reduction in the incidence of type 2
diabetes with lifestyle intervention or metformin. N Engl J Med.
346:393–403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giovannucci E, Harlan DM, Archer MC,
Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG and
Yee D: Diabetes and cancer: A consensus report. CA Cancer J Clin.
60:207–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ben Sahra I, Le Marchand-Brustel Y, Tanti
JF and Bost F: Metformin in cancer therapy: A new perspective for
an old antidiabetic drug? Mol Cancer Ther. 9:1092–1099. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Czyzyk A, Tawecki J, Sadowski J,
Ponikowska I and Szczepanik Z: Effect of biguanides on intestinal
absorption of glucose. Diabetes. 17:492–498. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hermann LS: Metformin: A review of its
pharmacological properties and therapeutic use. Diabetes Metab.
5:233–245. 1979.
|
|
19
|
Boussageon R, Supper I, BejanAngoulvant T,
Kellou N, Cucherat M, Boissel JP, Kassai B, Moreau A, Gueyffier F
and Cornu C: Reappraisal of metformin efficacy in the treatment of
type 2 diabetes: A meta-analysis of randomised controlled trials.
PLoS Med. 9:e10012042012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sogame Y, Kitamura A, Yabuki M, Komuro S
and Takano M: Transport of biguanides by human organic cation
transporter OCT2. Biomed Pharmacother. 67:425–430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noto H, Goto A, Tsujimoto T and Noda M:
Cancer risk in diabetic patients treated with metformin: A
systematic review and meta-analysis. PLoS One. 7:e334112012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Franciosi M, Lucisano G, Lapice E,
Strippoli GF, Pellegrini F and Nicolucci A: Metformin therapy and
risk of cancer in patients with type 2 diabetes: Systematic review.
PLoS One. 8:e715832013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sandulache VC, Hamblin JS, Skinner HD,
Kubik MW, Myers JN and Zevallos JP: Association between metformin
use and improved survival in patients with laryngeal squamous cell
carcinoma. Head Neck. 36:1039–1043. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pollak M: Insulin and insulin-like growth
factor signalling in neoplasia. Nat Rev Cancer. 8:915–928. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pollak M: Metformin and other biguanides
in oncology: Advancing the research agenda. Cancer Prev Res
(Phila). 3:1060–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zakikhani M, Dowling R, Fantus IG,
Sonenberg N and Pollak M: Metformin is an AMP kinase-dependent
growth inhibitor for breast cancer cells. Cancer Res.
66:10269–10273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dowling RJ, Zakikhani M, Fantus IG, Pollak
M and Sonenberg N: Metformin inhibits mammalian target of
rapamycin-dependent translation initiation in breast cancer cells.
Cancer Res. 67:10804–10812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shaw RJ: LKB1 and AMP-activated protein
kinase control of mTOR signalling and growth. Acta Physiol (Oxf).
196:65–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmitz S and Machiels JP: Molecular
biology of squamous cell carcinoma of the head and neck: Relevance
and therapeutic implications. Expert Rev Anticancer Ther.
10:1471–1484. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Curry JM, Tuluc M, WhitakerMenezes D, Ames
JA, Anantharaman A, Butera A, Leiby B, Cognetti DM, Sotgia F,
Lisanti MP and Martinez-Outschoorn UE: Cancer metabolism, stemness
and tumor recurrence: MCT1 and MCT4 are functional biomarkers of
metabolic symbiosis in head and neck cancer. Cell Cycle.
12:1371–1384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Petti S: Lifestyle risk factors for oral
cancer. Oral Oncol. 45:340–350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Warnakulasuriya S: Causes of oral
cancer-an appraisal of controversies. Br Dent J. 207:471–475. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Belcher R, Hayes K, Fedewa S and Chen AY:
Current treatment of head and neck squamous cell cancer. J Surg
Oncol. 110:551–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. Ann Intern Med.
151:264–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th. Wiley-Blackwell;
New York, NY: 2009
|
|
37
|
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist
G, Brozek J, Norris S, FalckYtter Y, Glasziou P, DeBeer H, et al:
GRADE guidelines: 1. Introduction-GRADE evidence profiles and
summary of findings tables. J Clin Epidemiol. 64:383–394. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo Q, Hu D, Hu S, Yan M, Sun Z and Chen
F: In vitro and in vivo anti-tumor effect of metformin as a novel
therapeutic agent in human oral squamous cell carcinoma. BMC
Cancer. 12:5172012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sikka A, Kaur M, Agarwal C, Deep G and
Agarwal R: Metformin suppresses growth of human head and neck
squamous cell carcinoma via global inhibition of protein
translation. Cell Cycle. 11:1374–1382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Madera D, VitaleCross L, Martin D,
Schneider A, Molinolo AA, Gangane N, Carey TE, McHugh JB, Komarck
CM, Walline HM, et al: Prevention of tumor growth driven by PIK3CA
and HPV oncogenes by targeting mTOR signaling with metformin in
oral squamous carcinomas expressing OCT3. Cancer Prev Res (Phila).
8:197–207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang F, Xu J, Xia F, Liu Z, Zhao S, Liu H
and Jiang Z: Effects of metformin on human oral cancer KB cell
proliferation and apoptosis in vitro. Nan Fang Yi Ke Da Xue Xue
Bao. 34:159–163. 2014.(In Chinese). PubMed/NCBI
|
|
42
|
Goodkind JR, Amer S, Christian C, Hess JM,
Bybee D, Isakson BL, Baca B, Ndayisenga M, Greene RN and Shantzek
C: Challenges and innovations in a community-based participatory
randomized controlled trial. Health Educ Behav. May 13–2016.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xiao Z, Li CW, Shan J, Luo L, Feng L, Lu
J, Li SF, Long D and Li YP: Interventions to improve chronic
cyclosporine A nephrotoxicity through inhibiting renal cell
apoptosis: A systematic review. Chinese Med J (Engl).
126:3767–3774. 2013.
|
|
44
|
Pignon JP, le Maître A, Maillard E and
Bourhis J: MACH-NC Collaborative Group: Meta-analysis of
chemotherapy in head and neck cancer (MACH-NC): An update on 93
randomised trials and 17,346 patients. Radiother Oncol. 92:4–14.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shah JP and Gil Z: Current concepts in
management of oral cancer-and Surgery. Oral Oncol. 45:394–401.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Salama JK, Haddad RI, Kies MS, Busse PM,
Dong L, Brizel DM, Eisbruch A, Tishler RB, Trotti AM and Garden AS:
Clinical practice guidance for radiotherapy planning after
induction chemotherapy in locoregionally advanced head-and-neck
cancer. Int J Radiat Oncol Biol Phys. 75:725–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Busch CJ, Tribius S, Schafhausen P and
Knecht R: The current role of systemic chemotherapy in the primary
treatment of head and neck cancer. Cancer Treat Rev. 41:217–221.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kundu SK and Nestor M: Targeted therapy in
head and neck cancer. Tumour Biol. 33:707–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cicero AF, Tartagni E and Ertek S:
Metformin and its clinical use: New insights for an old drug in
clinical practice. Arch Med Sci. 8:907–917. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Segal ED, Yasmeen A, Beauchamp MC,
Rosenblatt J, Pollak M and Gotlieb WH: Relevance of the OCT1
transporter to the antineoplastic effect of biguanides. Biochem
Biophys Res Commun. 414:694–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Appleyard MV, Murray KE, Coates PJ,
Wullschleger S, Bray SE, Kernohan NM, Fleming S, Alessi DR and
Thompson AM: Phenformin as prophylaxis and therapy in breast cancer
xenografts. Br J Cancer. 106:1117–1122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee JH, Kim TI, Jeon SM, Hong SP, Cheon JH
and Kim WH: The effects of metformin on the survival of colorectal
cancer patients with diabetes mellitus. Int J Cancer. 131:752–759.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Suissa S and Azoulay L: Metformin and the
risk of cancer: Time-related biases in observational studies.
Diabetes Care. 35:2665–2673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Niraula S, Pond G, De Wit R, Eisenberger
M, Tannock IF and Joshua AM: Influence of concurrent medications on
outcomes of men with prostate cancer included in the TAX 327 study.
Can Urol Assoc J. 7:E74–E81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rêgo DF, Pavan LM, Elias ST, De Luca Canto
G and Guerra EN: Effects of metformin on head and neck cancer: A
systematic review. Oral Oncol. 51:416–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Novosyadlyy R and LeRoith D:
Hyperinsulinemia and type 2 diabetes. Impact on cancer. Cell Cycle.
9:1449–1450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ferguson RD, Novosyadlyy R, Fierz Y,
Alikhani N, Sun H, Yakar S and Leroith D: Hyperinsulinemia enhances
c-Myc-mediated mammary tumor development and advances metastatic
progression to the lung in a mouse model of type 2 diabetes. Breast
Cancer Res. 14:R82012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Clayton PE, Banerjee I, Murray PG and
Renehan AG: Growth hormone, the insulin-like growth factor axis,
insulin and cancer risk. Nat Rev Endocrinol. 7:11–24. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Stattin P, Björ O, Ferrari P, Lukanova A,
Lenner P, Lindahl B, Hallmans G and Kaaks R: Prospective study of
hyperglyce-mia and cancer risk. Diabetes Care. 30:561–567. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Johnson JA and Bowker SL: Intensive
glycaemic control and cancer risk in type 2 diabetes: A
meta-analysis of major trials. Diabetologia. 54:25–31. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chocarro-Calvo A, García-Martínez JM,
Ardila-González S, De la Vieja A and García-Jiménez C:
Glucose-induced β-catenin acetylation enhances Wnt signaling in
cancer. Mol Cell. 49:474–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Würth R, Barbieri F and Florio T: New
molecules and old drugs as emerging approaches to selectively
target human glioblastoma cancer stem cells. Biomed Res Int.
2014:1265862014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pernicova I and Korbonits M:
Metformin-mode of action and clinical implications for diabetes and
cancer. Nat Rev Endocrinol. 10:143–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Song CW, Lee H, Dings RP, Williams B,
Powers J, Santos TD, Choi BH and Park HJ: Metformin kills and
radiosensitizes cancer cells and preferentially kills cancer stem
cells. Sci Rep. 2:3622012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Martini M, Ciraolo E, Gulluni F and Hirsch
E: Targeting PI3K in Cancer: Any good news? Front Oncol. 3:1082013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Foster DA, Yellen P, Xu L and Saqcena M:
Regulation of G1 cell cycle progression: Distinguishing the
restriction point from a nutrient-sensing cell growth
checkpoint(s). Genes Cancer. 1:1124–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hamilton E and Infante JR: Targeting
CDK4/6 in patients with cancer. Cancer Treat Rev. 45:129–138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chu IM, Hengst L and Slingerland JM: The
Cdk inhibitor p27 in human cancer: Prognostic potential and
relevance to anticancer therapy. Nat Rev Cancer. 8:253–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhao L, Wen ZH, Jia CH, Li M, Luo SQ and
Bai XC: Metformin induces G1 cell cycle arrest and inhibits cell
proliferation in nasopharyngeal carcinoma cells. Anat Rec
(Hoboken). 294:1337–1343. 2011. View Article : Google Scholar : PubMed/NCBI
|