Introduction
Tumor protein 53-inducible nuclear protein 1
(TP53INP1) is an apoptotic protein involved in cell stress
responses (1). It increases in
abundance in response to inflammatory stress and stress-inducing
agents such as heat shock, ultraviolet rays, ethanol and mutagens
(2–4).
TP53INP1 was first identified in a screen of stress-activated
pancreatic genes in mice with acute pancreatitis (4). TP53INP1 localizes to human chromosome
8q22 (5) and encodes two nuclear
isoforms, TP53INP1 and TP53INP1β. Both isoforms are related to
homeodomain-interacting protein kinase-2, and regulate p53-mediated
transcriptional activation of the p53-inducible gene 3,
BCL2-associated X protein and p21 promoters (6).
TP53INP1 expression is downregulated in numerous
human cancers, including esophageal carcinomas (7), poorly differentiated stomach
adenocarcinomas (8), primary breast
carcinomas (9) and pancreatic ductal
adenocarcinomas (10). TP53INP1
messenger RNA (mRNA) levels were reduced in 35–59% of melanoma cell
lines compared with melanocytes (11). TP53INP1 expression is enhanced in
certain cancers. Ito et al (12) detected elevated TP53INP1 expression in
anaplastic thyroid carcinomas, and Giusiano et al (13) reported increased TP53INP1 expression
in prostate cancers. The reason why TP53INP1 is upregulated in
certain cancers and downregulated in others is not clear. In
addition, its expression and prognostic value in hepatocellular
carcinoma (HCC) have not been reported to date.
HCC is one of the most common cancers in the world
(14). It is the third major cause of
cancer-associated mortalities (15)
and accounts for 75–90% of all malignant tumors in adult livers
(16). The aim of the present study
was to analyze the expression patterns of TP53INP1 in a large
series of human HCCs in order to i) identify the possible
variations of TP53INP1 expression; ii) investigate its correlation
with clinicopathological parameters; and iii) evaluate its
prognostic value.
Materials and methods
Patient management and tissue
samples
The present study was performed in accordance with
the reporting recommendations for tumor marker prognostic studies
guidelines (17). The institutional
ethics committee of Southern Hospital (Guangzhou, China) approved
the protocol, and all enrolled subjects provided written informed
consent.
Fresh HCC tissue samples and matched adjacent
non-tumorous tissues were collected from 65 HCC patients who
underwent resection at the Digestive Disease Research Institute of
Southern Hospital between March 2008 and March 2011. The enrolled
patients i) had a conclusive pathologic diagnosis of HCC; ii) had
received curative resection, which was defined as macroscopically
complete removal of the tumor; and iii) had available detailed
clinicopathological data. Patients were excluded if they had
received adjuvant chemotherapy or radiotherapy prior to surgery, or
if there was evidence of other malignancies. The detailed
clinicopathological characteristics of the HCC patients included in
the current study are presented in Table
I.
 | Table I.Clinicopathological features of 65
patients with hepatocellular carcinoma. |
Table I.
Clinicopathological features of 65
patients with hepatocellular carcinoma.
| Variables | Value |
|---|
| Median age (range),
years | 49.9 (18–83) |
| Gender, n |
|
| Male | 59 |
|
Female | 6 |
| HBsAg expression,
n |
|
|
Positive | 56 |
|
Negative | 9 |
| AFP levels, n |
|
| >400
ng/ml | 30 |
| ≤400
ng/ml | 35 |
| Liver cirrhosis,
n |
|
|
Absent | 40 |
|
Present | 25 |
| Vascular invasion,
n |
|
|
Absent | 11 |
|
Present | 54 |
| Intrahepatic
metastasis, n |
|
|
Absent | 53 |
|
Present | 12 |
| Tumor size, n |
|
| ≤5
cm | 23 |
| >5
cm | 42 |
| Tumor number, n |
|
|
Single | 55 |
|
Multiple | 10 |
| Tumor
differentiation, n |
|
| Well | 23 |
|
Moderate | 31 |
| Poor | 11 |
| AJCC stage, n |
|
| I/II | 56 |
|
III/IV | 9 |
Patients were followed up until August 31, 2014.
Among the 65 patients, 9 (13.8%) were lost to follow-up. Tumor
recurrence confirmation was based on typical appearances on
magnetic resonance imaging and/or computed tomography scans, as
well as elevated α-fetoprotein protein (AFP) levels. The median
follow-up period was 31 months (range, 1–71 months). Tumor
differentiation was based on the criteria proposed by Edmondson and
Steiner (18). Tumor stage was
defined according to the American Joint Committee on Cancer
(AJCC)/International Union against Cancer tumor node metastasis
classification system (19).
Immunohistochemistry assay
Immunostaining was performed on 4-µm sections of
paraffin-embedded tissue specimens. The sections were
deparaffinized with xylene and rehydrated in a graded alcohol
series. Antigen retrieval was carried out in a microwave oven in a
sodium citrate solution (pH 8.0). Endogenous peroxidase was
inactivated by incubating the samples in 3% H2O2 at room
temperature for 20 min. Upon blocking with goat serum (Wuhan Boster
Biological Engineering Co., Ltd.) at room temperature for 30 min,
the samples were incubated with rabbit polyclonal anti-TP53INP1
antibody (catalog no. AP11890b; 1:50; Abgent, Inc., San Diego, CA,
USA) at 4°C overnight in a moist chamber. They were then washed
thoroughly with PBS and incubated with secondary antibodies
(catalog no. HSP0007; 1:200; Shanghai Mjol Biological Technology
Co., Ltd.) at 37°C for 30 min, conjugated to peroxidase (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China).
Staining (which was brown-colored) was visualized using a
3,3′-diaminobenzidine kit (Zhongshan Golden Bridge Biotechnology
Co., Ltd.). Upon counterstaining with hematoxylin, the samples were
dehydrated in a graded alcohol series and mounted. Negative
controls were prepared in the absence of primary antibody.
Immunohistochemical staining was evaluated by two
independent observers who were blinded to the clinical data.
Concordance was achieved in 94% of the cases, and disagreements
were resolved by consensus (20).
Each sample was scored according to the intensity of the staining
(no staining=0, weak staining=1, moderate staining=2 and strong
staining=3) and the percentage of stained cells (<5%=0, 5–25%=1,
26–50%=2, 51–75%=3 and 76–100%=4). The percentage of cells at each
intensity was multiplied by the corresponding intensity value to
obtain an immunostaining score ranging from 0 to 12. The scores
were combined to obtain an overall mean score. Using this
assessment system, the optimal cutoff values were as follows: 0–3
(low) and 4–12 (high).
Western blot analysis
Proteins were extracted in radioimmunoprecipitation
buffer (EMD Millipore, Billerica, MA, USA). Protein concentration
was determined using a Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Samples with equal amounts of
total protein were separated on 12% SDS-PAGE and electrotransferred
to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Upon blocking in TBS/Tween-20 containing 5%
non-fat milk powder at room temperature for 5 min with agitation,
the membranes were incubated for 1 h with anti-TP53INP1 rabbit
polyclonal antibody (catalog no. AP11890b; 1:250; Abgent, Inc.) and
anti-β-actin mouse monoclonal antibody (catalog no. CW0096;
1:1,000; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)
at 4°C overnight. Upon incubation of the membranes with secondary
antibodies (catalog no. HSP0007; 1:200; Shanghai Mjol Biological
Technology Co., Ltd.) at room temperature for 3 h, immunoreactive
bands were visualized by enhanced chemilumiscence using a GeneGnome
HR Bioimaging System (Syngene, Frederick, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from tissues using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.), and complementary DNA
libraries were generated from total RNA using a High-Capacity cDNA
Archive kit according to the manufacturer's protocol (Applied
Biosystems; Thermo Fisher Scientific, Inc.). RT-qPCR was performed
in triplicate using the SYBR-Green system on a LightCycler 480
Real-Time PCR System (Roche Diagnostics GmbH, Mannheim, Germany).
Relative mRNA levels were calculated according to the
quantification cycle (Cq) values corrected for GAPDH expression
using the 2-ΔΔCq method as follows: ΔΔCq=ΔCq (treatment)-ΔCq
(control) or ΔCq=Cq (target genes)-Cq (GAPDH). The primer sequences
were as follows: TP53INP1, 5′-GCACCCTTCAGTCTTTTCCTGTT-3′ (forward)
and 5′-GAGAAAGCAGGAATCACTTGTATC-3′ (reverse); and GAPDH,
5′-GAAGGTGAAGGTCGGAGT-3′ (forward) and 5′-GAAGATGGTGATGGGATTTC-3′
(reverse).
Statistical analysis
All statistical analyses were carried out using SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA). Differences
between two independent groups were analyzed using the Student's
t-test. The clinicopathological features of HCC were analyzed using
the Pearson's χ2 test. Recurrence-free survival (RFS) and overall
survival (OS) were calculated using the Kaplan-Meier method, and
significance was assessed using the log-rank test. RFS was defined
as the interval between the date of surgery and the date of
detection of a recurrent tumor. OS was defined as the interval
between the date of surgery and the date of mortality or last
follow-up. Independent prognostic factors for OS and RFS were
identified using the Cox proportional hazards regression model.
Data are presented as the mean ± standard error of the mean.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TP53INP1 expression is downregulated
in HCC tissues
TP53INP1 expression was significantly decreased in
HCC tissues compared with adjacent non-tumorous tissues, as
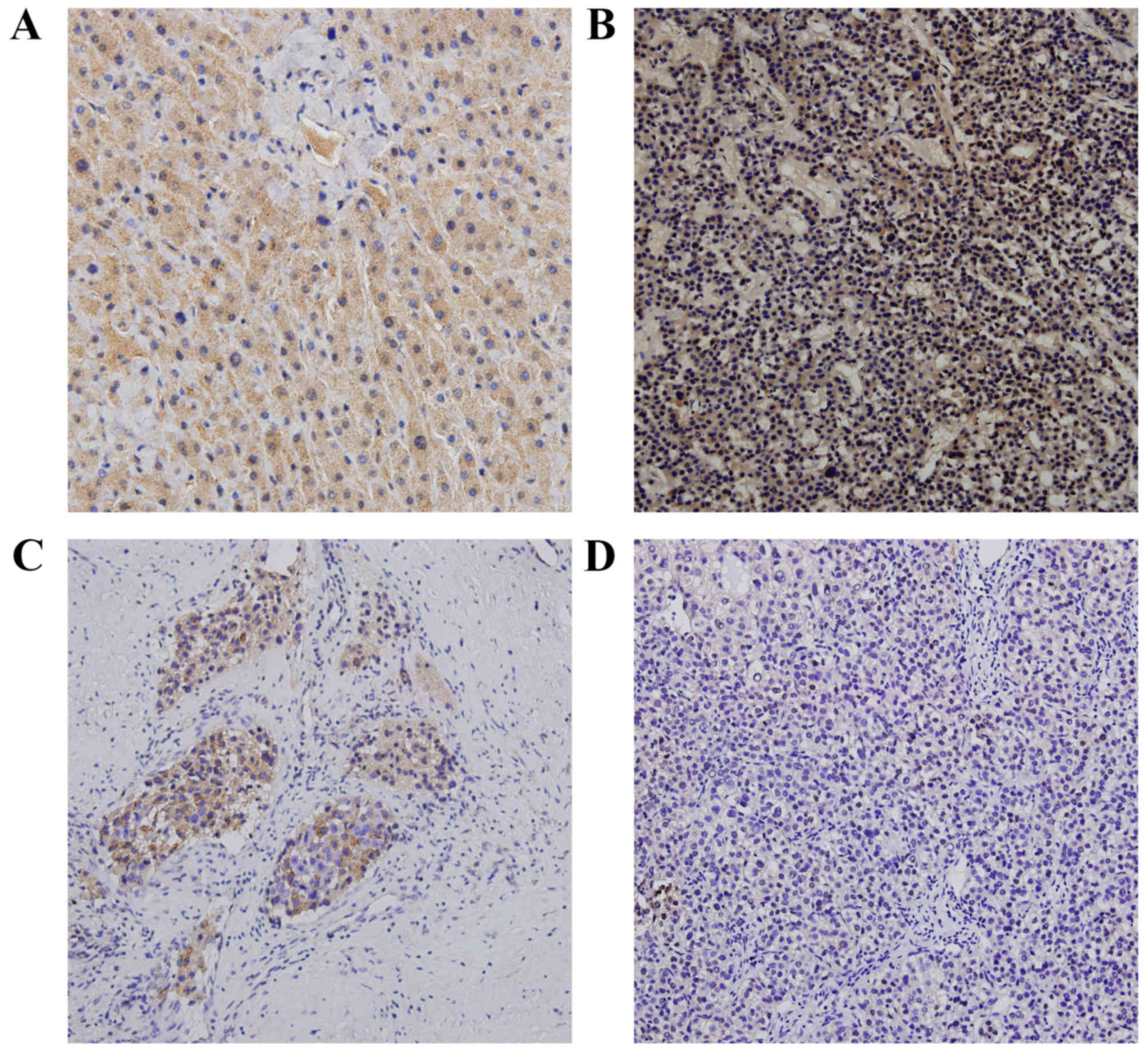
determined via western blotting and immunohistochemistry. TP53INP1
was predominantly localized in the cytoplasm of hepatic cells, with
little staining was present in the nuclei, as visualized via
immunohistochemistry. Heavy TP53INP1 staining was observed in the
epithelial cells in normal-appearing mucosa adjacent to HCC cells,
whereas TP53INP1 staining in HCC cells was faint or absent
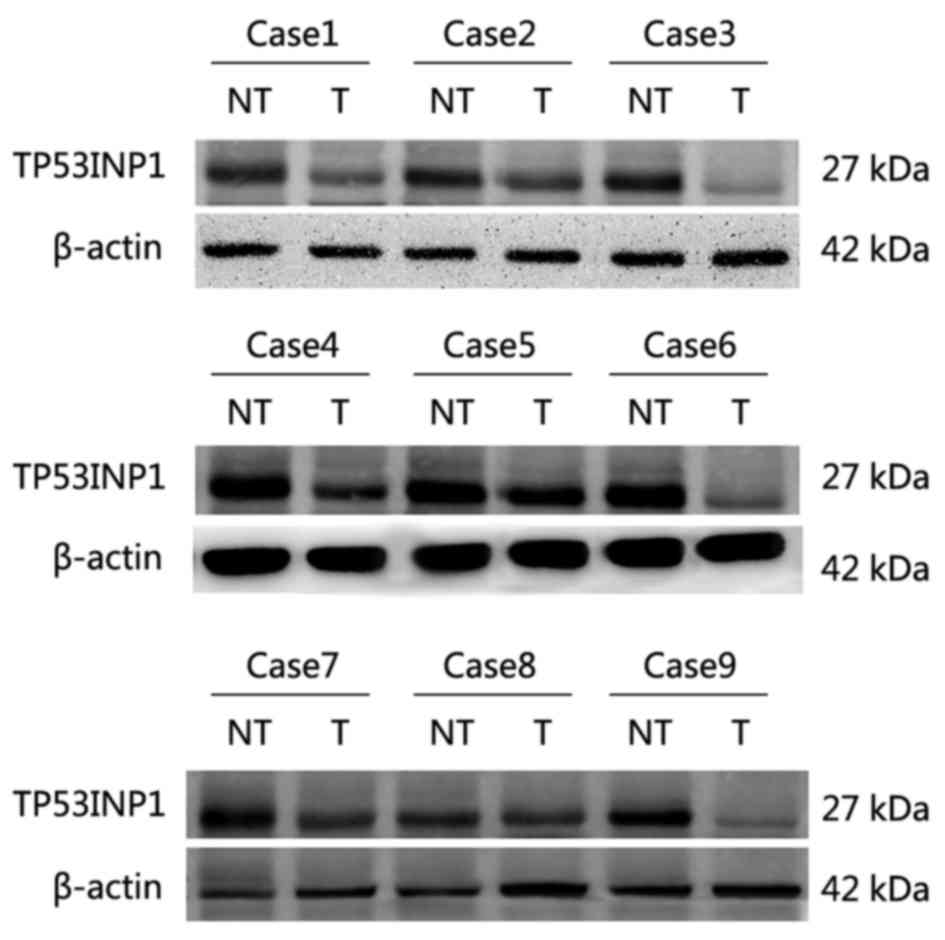
(Fig. 1). In western blot analyses,
TP53INP1 expression was lower in 8 of 9 HCC tissue samples than in
matched adjacent non-tumorous tissue (Fig. 2).
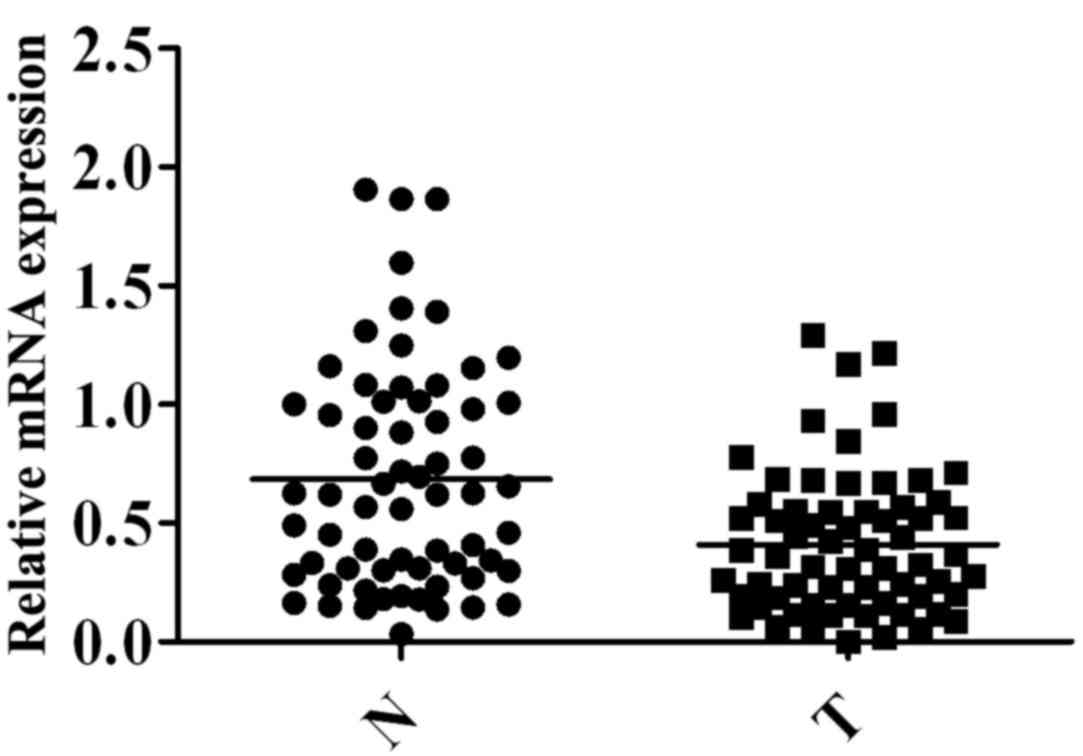
To further examine TP53INP1
expression, several sample sets were analyzed via RT-qPCR
Notably, TP53INP1 mRNA expression was significantly
lower in HCC tissues (0.4103±0.03674) than in adjacent non-tumorous
tissues (0.6851±0.05825, P=0.0001) (Fig.
3).
Expression of TP53INP1 and its clinicopathological
relevance in hepatic tissues. To investigate the significance of
TP53INP expression in HCC, the association between TP53INP mRNA
levels and the clinicopathological characteristics of 65 HCC
patients were evaluated in the present study. TP53INP1 mRNA
expression was categorized as high or low. As shown in Table II, low expression of TP53INP1 mRNA
closely correlated with AJCC stage (P=0.014) and vascular invasion
(P=0.024). There were no significant differences in other clinical
characteristics between the high and low expression groups.
 | Table II.Association of TP53INP1 messenger RNA
expression with clinicopathological characteristics of 65 patients
with hepatocellular carcinoma. |
Table II.
Association of TP53INP1 messenger RNA
expression with clinicopathological characteristics of 65 patients
with hepatocellular carcinoma.
|
|
| TP53INP1
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | Patients, n | Low (n=32) | High (n=33) | P-value |
|---|
| Age, years |
|
|
| 0.897 |
|
<50 | 34 | 17 | 17 |
|
| ≥50 | 31 | 15 | 16 |
|
| Gender |
|
|
| 0.968 |
| Male | 59 | 29 | 30 |
|
|
Female | 6 | 3 | 3 |
|
| HBsAg expression |
|
|
| 0.304 |
|
Positive | 56 | 29 | 27 |
|
|
Negative | 9 | 3 | 6 |
|
| AFP levels,
ng/ml |
|
|
| 0.702 |
|
>400 | 30 | 16 | 14 |
|
|
≤400 | 35 | 18 | 17 |
|
| Liver
cirrhosis |
|
|
| 0.875 |
|
Absent | 25 | 12 | 13 |
|
|
Present | 40 | 20 | 20 |
|
| Vascular
invasion |
|
|
| 0.024 |
|
Absent | 11 | 2 | 9 |
|
|
Present | 54 | 30 | 24 |
|
| Intrahepatic
metastasis |
|
|
| 0.223 |
|
Absent | 53 | 28 | 25 |
|
|
Present | 12 | 8 | 4 |
|
| Tumor size, cm |
|
|
| 0.056 |
| ≤5 | 23 | 15 | 8 |
|
|
>5 | 42 | 17 | 25 |
|
| Tumor number |
|
|
| 0.110 |
|
Single | 54 | 29 | 25 |
|
|
Multiple | 42 | 17 | 25 |
|
| Tumor
differentiation |
|
|
| 0.644 |
|
Well | 23 | 12 | 11 |
|
|
Moderate | 31 | 16 | 15 |
|
|
Poor | 11 | 4 | 7 |
|
| AJCC stage |
|
|
| 0.014 |
|
I/II | 56 | 31 | 25 |
|
|
III/IV | 9 | 1 | 8 |
|
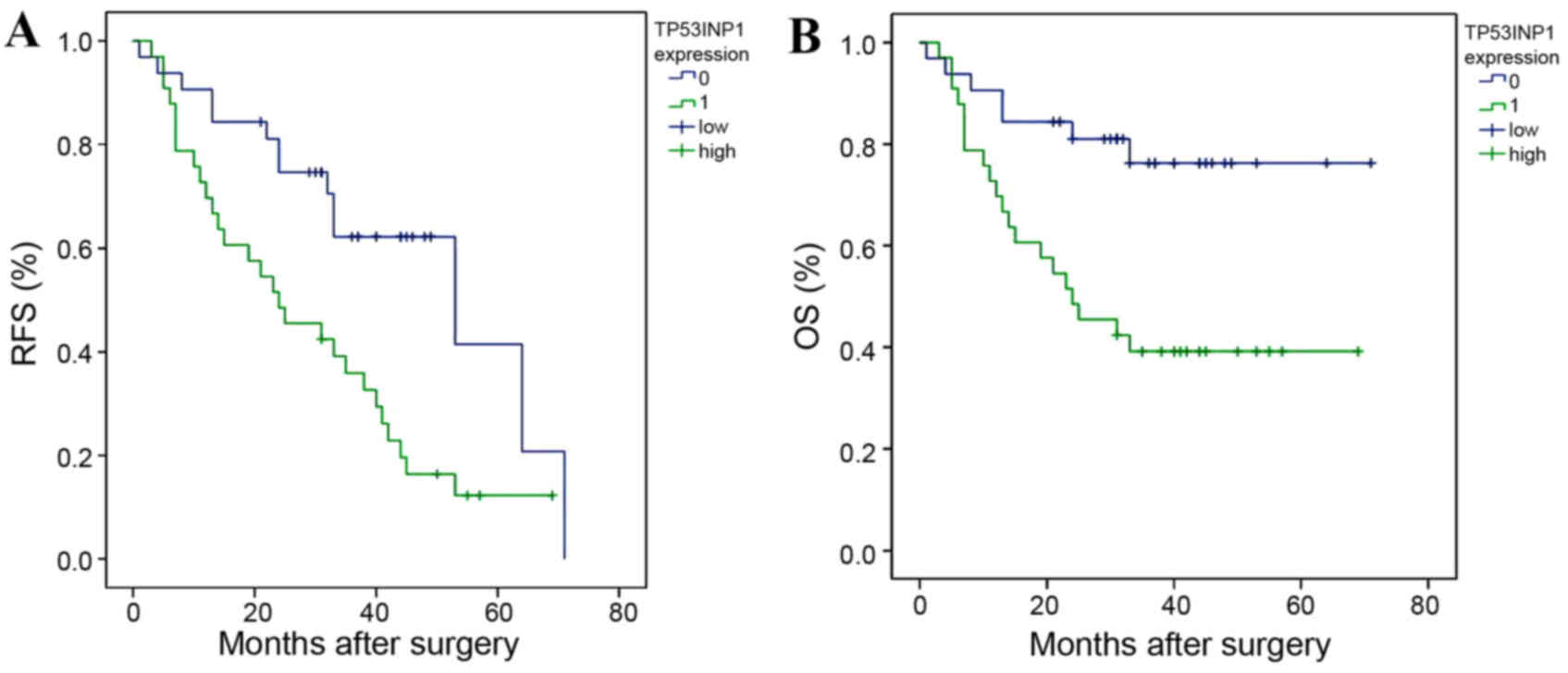
Low expression of TP53INP1 predicts
poor prognosis in HCC patients
The potential association between TP53INP1
expression and survival (RFS and OS) was retrospectively evaluated
via Kaplan-Meier analysis. RFS (Fig.
4A) and OS (Fig. 4B) were
significantly worse in HCC patients expressing low TP53INP1 levels
compared with those expressing high TP53INP1 levels, with median
survival times of 10 and 38 months, respectively (P=0.003).
Twelve clinicopathological variables were included
in a univariate analysis, including age, gender, serum hepatitis B
surface antigen (HBsAg) levels, serum AFP levels, liver cirrhosis,
vessel invasion, intrahepatic metastasis, tumor size, tumor number,
tumor differentiation, AJCC stage and TP53INP1 expression. Of
these, tumor differentiation, AJCC stage, vascular invasion and
TP53INP1 expression were significant prognostic factors of RFS and
OS in univariate analysis (Table
III). In addition, multivariate analysis (Table IV) revealed that TP53INP1 was also an
independent prognostic factor for both RFS [hazard ratio
(HR)=2.284, 95% confidence interval (CI)=1.157–4.511, P=0.017] and
OS (HR=2.680, 95% CI=1.087–6.608, P=0.032) (Table III). Thus, low expression of
TP53INP1 may serve as a prognostic indicator for patients with
HCC.
 | Table III.Univariate analysis of factors
associated with RFS and OS in patients with hepatocellular
carcinoma. |
Table III.
Univariate analysis of factors
associated with RFS and OS in patients with hepatocellular
carcinoma.
|
| RFS | OS |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years (<50
vs. ≥50) | 1.111
(0.600–2.059) | 0.737 | 0.667
(0.310–1.438) | 0.302 |
| Gender (male vs.
female) | 1.200
(0.468–3.075) | 0.704 | 1.223
(0.368–4.067) | 0.743 |
| HBsAg expression
(negative vs. positive) | 1.420
(0.555–3.632) | 0.464 | 1.656
(0.498–5.505) | 0.411 |
| AFP levels, ng/ml
(≤400 vs. >400) | 1.816
(0.972–3.391) | 0.061 | 2.812
(1.281–6.169) | 0.010 |
| Liver cirrhosis
(absent vs. present) | 0.902
(0.478–1.703) | 0.751 | 1.118
(0.512–2.442) | 0.780 |
| Vascular invasion
(absent vs. present) | 3.004
(1.416–6.373) | 0.004 | 4.275
(1.848–9.889) | 0.001 |
| Intrahepatic
metastasis (absent vs. present) | 1.833
(0.895–3.753) | 0.098 | 1.759
(0.709–4.363) | 0.223 |
| Tumor size, cm (≤5
vs. >5) | 1.447
(0.737–2.841) | 0.283 | 1.377
(0.602–3.150) | 0.448 |
| Tumor number
(single vs. multiple) | 1.729
(0.821–3.641) | 0.150 | 1.744
(0.702–4.328) | 0.231 |
| Differentiation
(poor/moderate vs. well) | 0.521
(0.338–0.801) | 0.003 | 0.432
(0.251–0.744) | 0.002 |
| AJCC stage (I/II
vs. III/IV) | 2.224
(1.016–4.869) | 0.045 | 2.943
(1.182–7.327) | 0.020 |
| TP53INP1 expression
(low vs. high) | 2.604
(1.345–5.039) | 0.005 | 3.403
(1.436–8.063) | 0.005 |
 | Table IV.Multivariate analysis of factors
associated with RFS and OS in patients with hepatocellular
carcinoma. |
Table IV.
Multivariate analysis of factors
associated with RFS and OS in patients with hepatocellular
carcinoma.
|
| RFS | OS |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years (<50
vs. ≥50) | 2.063
(0.971–4.387) | 0.060 | 1.227
(0.499–3.017) | 0.656 |
| Gender (male vs.
female) | 1.500
(0.468–4.802) | 0.495 | 1.635
(0.400–6.674) | 0.493 |
| HBsAg expression
(negative vs. positive) | 1.833
(0.561–5.994) | 0.316 | 1.565
(0.375–6.537) | 0.539 |
| AFP levels, ng/ml
(≤400 vs. >400) | 1.587
(0.838–3.004) | 0.156 | 2.512
(1.135–5.560) | 0.023 |
| Liver cirrhosis
(absent vs. present) | 1.060
(0.419–2.681) | 0.902 | 1.398
(0.460–4.248) | 0.554 |
| Vascular invasion
(absent vs. present) | 2.310
(1.065–5.012) | 0.034 | 2.841
(1.172–6.884) | 0.021 |
| Intrahepatic
metastasis (absent vs. present) | 2.043
(0.558–7.482) | 0.281 | 1.842
(0.379–8.944) | 0.449 |
| Tumor size, cm (≤5
vs. >5) | 2.214
(0.839–5.839) | 0.108 | 1.889
(0.589–6.064) | 0.285 |
| Tumor number
(single vs. multiple) | 1.354
(0.462–3.967) | 0.580 | 1.350
(0.388–4.697) | 0.637 |
| Differentiation
(poor/moderate vs. well) | 1.584
(0.575–4.236) | 0.672 | 1.736
(0.458–5.872) | 0.413 |
| AJCC stage (I/II
vs. III/IV) | 0.695
(0.220–2.199) | 0.536 | 0.867
(0.219–3.438) | 0.839 |
| TP53INP1 expression
(low vs. high) | 2.284
(1.157–4.511) | 0.017 | 2.680
(1.087–6.608) | 0.032 |
Discussion
TP53INP1 is a stress-induced protein that serves a
role in p53-mediated apoptosis and cell cycle arrest (2,4,21). Its expression is downregulated in
stomach (8), pancreatic (10) and inflammation-mediated colic
carcinomas (22), but upregulated in
medullary thyroid carcinomas (23)
and prostate cancers (24).
Therefore, it is possible that TP53INP1 can act either as a tumor
suppressor or an oncoprotein depending on the tumor
microenvironment or the tissue type. To date, TP53INP1 expression
and its prognostic value in HCC have been unclear.
The present study systematically examined the
expression of TP53INP1 in HCC tissue samples. TP53INP1 protein
levels were measured via western blotting and immunohistochemistry,
and TP53INP1 mRNA levels were quantitated via RT-qPCR. All analyses
revealed significantly lower expression of TP53INP1 in HCC tissue
samples than in paired samples of non-tumorous adjacent regions. To
the best of our knowledge, the present study is the first to report
TP53INP1 downregulation in HCC.
TP53INP1 expression is high in HCC adjacent
non-tumorous tissues and low in HCC tissue, suggesting that loss of
TP53INP1 maybe contribute to HCC progression. Seux et al
(25) observed that TP53INP1
silencing increased the migration of mouse embryonic fibroblasts
and pancreatic cancer cells. Seillier et al (26) demonstrated that TP53INP1 interacted
with autophagy-related 8 proteins to induce autophagy-dependent
cell death in U2OS cells. Future studies should focus on clarifying
the mechanisms of TP53INP1 expression in HCC.
Jiang et al (8)
reported that reductions in TP53INP1 expression in gastric
adenocarcinomas were associated with poor prognosis. Conversely,
Giusiano et al (24) observed
that TP53INP1 overexpression was an unfavorable prognostic factor
in prostate cancer. The present study is the first to reveal a
positive impact of TP53INP1 expression on survival in HCC. In
addition to confirming that the expression of TP53INP1 was
downregulated in HCC tissues, the present results further revealed
that low TP53INP1 expression significantly correlated with advanced
AJCC stage and vascular invasion, and that decreased expression of
TP53INP1 predicted poor prognosis in patients with HCC following
hepatectomy. Lastly, TP53INP1 expression was a prognostic indicator
of RFS and OS, independently of other clinicopathological
variables, in a multivariate analysis.
In conclusion, the present study identified for the
first time the downregulation of TP53INP1 in human HCC tissues,
which was closely associated with AJCC stage and vascular invasion
in patients with HCC. Our findings also suggest that low expression
of TP53INP1 may serve as a potent prognostic marker for patients
with HCC.
References
|
1
|
Ringer L, Sirajuddin P, Tricoli L, Waye S,
Choudhry MU, Parasido E, Sivakumar A, Heckler M, Naeem A,
Abdelgawad I, et al: The induction of the p53 tumor suppressor
protein bridges the apoptotic and autophagic signaling pathways to
regulate cell death in prostate cancer cells. Oncotarget.
5:10678–10691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okamura S, Arakawa H, Tanaka T, Nakanishi
H, Ng CC, Taya Y, Monden M and Nakamura Y: p53DINP1, a
p53-inducible gene, regulates p53-dependent apoptosis. Mol Cell.
8:85–94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomasini R, Samir AA, Pebusque MJ, Calvo
EL, Totaro S, Dagorn JC, Dusetti NJ and Iovanna JL: P53-dependent
expression of the stress-induced protein (SIP). Eur J Cell Biol.
81:294–301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tomasini R, Samir AA, Vaccaro MI, Pebusque
MJ, Dagorn JC, Iovanna JL and Dusetti NJ: Molecular and functional
characterization of the stress-induced protein (SIP) gene and its
two transcripts generated by alternative splicing. SIP induced by
stress and promotes cell death. J Biol Chem. 276:44185–44192. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nowak J, Depetris D, Iovanna JL, Mattei MG
and Pebusque MJ: Assignment of the tumor protein p53 induced
nuclear protein 2 (TP53INP2) gene to human chromosome band 20q11.2
by in situ hybridization. Cytogenet Genome Res.
108:3622005.PubMed/NCBI
|
|
6
|
Tomasini R, Samir AA, Carrier A, Isnardon
D, Cecchinelli B, Soddu S, Malissen B, Dagorn JC, Iovanna JL and
Dusetti NJ: TP53INP1s and homeodomain-interacting protein kinase-2
(HIPK2) are partners in regulating p53 activity. J Biol Chem.
278:37722–37729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weng W, Yang Q, Huang M, Qiao Y, Xie Y, Yu
Y, jing A and Li Z: c-Myc inhibits TP53INP1 expression via promoter
methylation in esophageal carcinoma. Biochem Biophys Res Commun.
405:278–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang PH, Motoo Y, Garcia S, Iovanna JL,
Pébusque MJ and Sawabu N: Down-expression of tumor protein
p53-induced nuclear protein 1 in human gastric cancer. World J
Gastroenterol. 12:691–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ito Y, Motoo Y, Yoshida H, Iovanna JL,
Takamura Y, Miya A, Kuma K and Miyauchi A: Decreased expression of
tumor protein p53-induced nuclear protein 1 (TP53INP1) in breast
carcinoma. Anticancer Res. 26:4391–4395. 2006.PubMed/NCBI
|
|
10
|
Gironella M, Seux M, Xie MJ, Cano C,
Tomasini R, Gommeaux J, Garcia S, Nowak J, Yeung ML, Jeang KT, et
al: Tumor protein 53-induced nuclear protein 1 expression is
repressed by miR-155, and its restoration inhibits pancreatic tumor
development. Proc Natl Acad Sci USA. 104:16170–16175. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bonazzi VF, Irwin D and Hayward NK:
Identification of candidate tumor suppressor genes inactivated by
promoter methylation in melanoma. Genes Chromosomes Cancer.
48:10–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ito Y, Motoo Y, Yoshida H, Iovanna JL,
Nakamura Y, Kuma K and Miyauchi A: High level of tumour protein
p53-induced nuclear protein 1 (TP53INP1) expression in anaplastic
carcinoma of the thyroid. Pathology. 38:545–547. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giusiano S, Baylot V, Andrieu C, Fazli L,
Gleave M, Iovanna JL, TarangerCharpin C, Garcia S and Rocchi P:
TP53INP1 as new therapeutic target in castration-resistant prostate
cancer. Prostate. 72:1286–1294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li WG and Wang HQ: Inhibitory effects of
Silibinin combined with doxorubicin in hepatocellular carcinoma; an
in vivo study. J BUON. 21:917–924. 2016.PubMed/NCBI
|
|
15
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Center MM and Jemal A: International
trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers
Prev. 20:2362–2368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
European association for the study of the
liver; European organisation for research and treatment of cancer,
. EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pinder SE, Brown JP, Gillett C, Purdie CA,
Speirs V, Thompson AM and Shaaban AM: Translational Subgroup of the
NCRI Breast Clinical Studies Group: The manufacture and assessment
of tissue microarrays: Suggestions and criteria for analysis, with
breast cancer as an example. J Clin Pathol. 66:169–177. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carrier A, Nguyen C, Victorero G,
Granjeaud S, Rocha D, Bernard K, Miazek A, Ferrier P, Malissen M,
Naquet P, et al: Differential gene expression in CD3epsilon- and
RAG1-deficient thymuses: Definition of a set of genes potentially
involved in thymocyte maturation. Immunogenetics. 50:255–270. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gommeaux J, Cano C, Garcia S, Gironella M,
Pietri S, Culcasi M, Pébusque MJ, Malissen B, Dusetti N, Iovanna J,
et al: Colitis and colitis-associated cancer are exacerbated in
mice deficient for tumor protein 53-induced nuclear protein 1. Mol
Cell Biol. 27:2215–2228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taïeb D, Giusiano S, Sebag F, Marcy M, de
Micco C, Palazzo FF, Dusetti NJ, Iovanna JL, Henry JF, Garcia S, et
al: Tumor protein p53-induced nuclear protein (TP53INP1) expression
in medullary thyroid carcinoma: A molecular guide to the optimal
extent of surgery? World J Surg. 34:830–835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giusiano S, Garcia S, Andrieu C, Dusetti
NJ, Bastide C, Gleave M, TarangerCharpin C, Iovanna JL and Rocchi
P: TP53INP1 overexpression in prostate cancer correlates with poor
prognostic factors and is predictive of biological cancer relapse.
Prostate. 72:117–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seux M, Peuget S, Montero MP, Siret C,
Rigot V, Clerc P, Gigoux V, Pellegrino E, Pouyet L, N'Guessan P, et
al: TP53INP1 decreases pancreatic cancer cell migration by
regulating SPARC expression. Oncogene. 30:3049–3061. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seillier M, Peuget S, Gayet O, Gauthier C,
N'Guessan P, Monte M, Carrier A, Iovanna JL and Dusetti NJ:
TP53INP1, a tumor suppressor, interacts with LC3 and ATG8-family
proteins through the LC3-interacting region (LIR) and promotes
autophagy-dependent cell death. Cell Death Differ. 19:1525–1535.
2012. View Article : Google Scholar : PubMed/NCBI
|