Introduction
In patients with metastatic solid tumors treated
with systemic chemotherapy, targeted therapy or both,
oligoprogression is a relatively new term used to describe the
clinical scenario in which a solitary or a few (≤5) metastatic
tumors progress, while all other sites of disease are stable or
responding to the current regimen (1). The concept of oligoprogression is based
in the biological principle of branched cancer evolution. Tumor
biopsies and cadaveric studies have shown significant genetic
heterogeneity between the primary tumor mass and metastatic
lesions, and also amongst different metastatic lesions in the same
patient (2–4). This genetic heterogeneity is believed to
explain the mixed response to systemic therapy often observed in
clinical practice.
One of the main factors that restricts the
effectiveness of chemotherapy is acquired resistance. Drug
resistance is a complex issue, as a number of factors affect drug
sensitivity, including drug activation and inactivation,
accelerated drug efflux, drug target alterations, processing of
drug-induced damage, DNA methylation and apoptotic evasion
(5). Chemotherapy-induced genetic
events lead to the development of new drug resistance and the
standard of care in this clinical scenario is typically to change
the systemic therapy. This approach introduces new problems,
including added expense, different toxicities, dose adjustments,
different supportive care treatments, patient treatment schedule
changes and potentially, decreased efficacy.
Stereotactic body radiotherapy (SBRT) is a highly
conformal radiation technique that delivers high-dose radiation to
a tumor while sparing much of the nearby normal organs in a timely
manner and with excellent tolerance. Compared with conventional
palliative radiation therapy, which is delivered over 10 to 15 days
with the intent to control pain but not disease progression, SBRT
is commonly delivered in 3 to 10 treatments with the intent of
disease ablation. There is evidence that SBRT achieves local
control rates in excess of 90%. A number of studies also boast
long-term disease-free survival and overall survival (6–8). SBRT has
become the standard treatment in numerous institutions for
oligometastatic disease presentation (1–5 metastases) in
coordination with definitive local therapy.
For oligoprogressive cancers, the goal of the
present study was to increase the duration until out of field tumor
progression, indicating prolonged efficacy of the chemotherapeutic
regimen. The primary outcome was to evaluate the time from SBRT to
the initiation of subsequent chemotherapy. Finally, the study also
hoped to shed light on the hypothesis that SBRT may overcome
treatment resistance through sterilization of the few tumor clones
that evade otherwise effective systemic therapy.
Patients and methods
Patients
Patients eligible for treatment presented with
metastatic disease in the thorax, liver or adrenals on
chemotherapy, with 5 or fewer progressive metastases smaller than 5
cm in axial diameter. The patients were amenable to SBRT with other
sites of disease remaining stable on the current systemic therapy.
All patients were enrolled on an institutional review
board-approved prospective outcomes tracking protocol in the
Department of Radiation Oncology at the University of Florida
(Gainesville, FL, USA). All cases were thoroughly reviewed by the
Department of Pathology at the University of Florida, and the
diagnoses were clear. The majority of the treated lesions were not
biopsied beyond the primary diagnosis.
Chemotherapy
Systemic treatment was cancer histology-specific.
The details of the individual regimens are presented with the
patients' results, including the sequencing of the chemotherapy
with SBRT.
Radiation therapy
All patients received SBRT with ablative intent
concurrently to each of the progressive metastatic sites.
Immobilization occurred in a vacuum cushion. The gross tumor volume
(GTV) and internal target volume (ITV) were defined using
4-dimensional (4D) computed tomography (CT) simulation with
Pinnacle Software (Phillips, Andover, MA, USA). The GTV was defined
in the 50% phase of the 4D CT and the ITV was expanded to account
for breathing motion. There was no clinical target volume expansion
and a 5-mm planning target volume (PTV) was isometrically expanded
from the ITV. The dose specified to the GTV D95% ensured that, at
minimum, 95% of the PTV was covered by the 80% isodose line. Target
localization was achieved using on-board image guidance with kV
cone-beam CT and computer-assisted image registration for each
fraction, then confirmed by the treating physician and a medical
physicist. Treatment couch repositioning was applied for all
translational corrections and rotational corrections were not
performed if <3 degrees in each axis. Treatment was delivered
using conformal arcs or multiple fixed coplanar beams shaped with
multileaf collimators. The dose per fraction and total dose were
determined using the dose-volume histogram of the organs at risk;
individual patient doses are described in the Results section.
Endpoint
The primary endpoint was the progression-free
interval, defined as the time from oligoprogression treated with
SBRT to the initiation of a different systemic drug or regimen.
Disease progression requiring a treatment change was based on
radiographic or clinical progression. Patients with dosage
adjustments or treatment breaks with re-initiation of the same
chemotherapy were not considered to have changed chemotherapy.
Results
Summary of results
A total of 5 patients were treated with SBRT for
oligoprogressive disease and followed until the initiation of a new
systemic therapy regimen. The group consisted of 2 patients with
colon cancer, 2 with rectal cancer and 1 with pancreatic cancer.
Patient characteristics are shown in Table I. At the time of SBRT, 4 patients were
on second-line and 1 patient was on third-line chemotherapy. The
median time from diagnosis to SBRT was 35 months (range, 11–72
months). The mean and median time to chemotherapy change from first
SBRT treatment was 11.7 and 10.6 months, respectively. The median
follow-up time was 10 months (range, 3–17 months). During the
interval from oligoprogression to chemotherapy change, 2 patients
received 2 separate courses of SBRT, while 3 patients were treated
with SBRT once. After the second course of SBRT, the time to
chemotherapy change was 3 and 5 months in the 2 patients,
respectively. All treatments met the aforementioned criteria
defined for oligometastatic disease and chemotherapy was held
during SBRT. The median time from SBRT to mortality or last
follow-up was 10 months (range, 5–27 months) and the time from
diagnosis to mortality or last follow-up was 41 months (range,
21–90 months). There were no grade 3 or higher toxicities
associated with SBRT per the Common Terminology Criteria for
Adverse Events, version 4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/About.html). All
patients were staged using the American Joint Committee on Cancer's
Cancer Staging Manual, 7th edition (9).
 | Table I.Patient characteristics and outcomes
for 5 patients treated with SBRT for oligoprogression. |
Table I.
Patient characteristics and outcomes
for 5 patients treated with SBRT for oligoprogression.
| Patient no. | Age at first SBRT,
years/gender | Time from diagnosis
to first SBRT, months | Primary site and
histology | Chemotherapy agents
at the time of SBRT | First SBRT total dose
and fraction size, Gy | Site of first SBRT
(number of metastases) | Months from first
SBRT to changes in chemotherapy | Second SBRT total
dose and fraction size, Gy | Site of second SBRT
(number of metastases) | Months from second
SBRT to changes in chemotherapy regimen |
|---|
| 1 | 62/male | 11 | Pancreatic
adenocarcinoma | Gemcitabine,
nab-paclitaxel |
50/10 | Liver (1) | 10 | N/A | N/A | N/A |
| 2 | 48/male | 73 | Rectal
adenocarcinoma | Bevacizumab,
leucovorin, 5-FU |
50/10 | Lung (1) | 10 | N/A | N/A | N/A |
| 3 | 49/female | 15 | Colon
adenocarcinoma | Irinotecan,
aflibercept | 30/6 | Right hilum (1) | 17 | 30/6 | Left hilum (1) | 4 |
| 4 | 60/male | 63 | Rectal
adenocarcinoma | Capecitabine,
bevacizumab | 50/5 | Anterior mediastinum
(1) | 17 | 50/5 | Lung (5) | 5 |
| 5 | 68/male | 35 | Colon
adenocarcinoma | FOLFIRI,
bevacizumab | 40/8 | Anterior mediastinum
(1) | 3 | N/A | N/A | N/A |
| All patients |
|
| Mean
(range) |
|
|
|
| 44 (30–50) | 1 (1) | 11.7 | 40 | 3.5 | 4.6 |
| Median
(range) |
|
|
|
| 50 (30–50) | 1 (1) | 10 (3–17) | 40 (30–50) | 3.5 (1–5) | 4 |
Patient 1
The patient was a 62-year-old male at the time of
SBRT and had been diagnosed with stage III (T4N1M0) adenocarcinoma
of the pancreas. An exploratory laparotomy was performed 10 months
prior to SBRT and unresectable cancer was found invading into the
origin of the gastroduodenal artery and proper hepatic artery. A
FOLFIRINOX chemotherapy regimen was started consisting of 180
mg/m2 irinotecan, 2,400 mg/m2 5-fluorouracil
(5-FU) via continuous intravenous infusion (CIVI) and 85
mg/m2 oxaliplatin, every 2 weeks, and 4 cycles were
completed.
Restaging imaging after 3 months showed an
unresectable, poorly-defined mass encasing the common hepatic
artery. This case was discussed at the University of Florida
Gastrointestinal Interdisciplinary tumor board conference (GI TB)
with recommendations to continue with systemic chemotherapy for
locally advanced pancreatic cancer and withhold radiation therapy
until the patient became symptomatic. Due to the disease
progression, the patient was started on a new systemic chemotherapy
consisting of 1,000 mg/m2 gemcitabine and 125
mg/m2 protein-bound paclitaxel (on days [D]1, 8 and 15)
every 28 days. Repeat imaging after 3 cycles showed stable disease
except for one ill-defined 1.3-cm liver lesion. Chemotherapy was
continued, and following cycle 5, imaging showed another 2.2-cm
lesion in the left hepatic lobe, with stable disease elsewhere,
including the primary site and 1.3-cm liver mass.
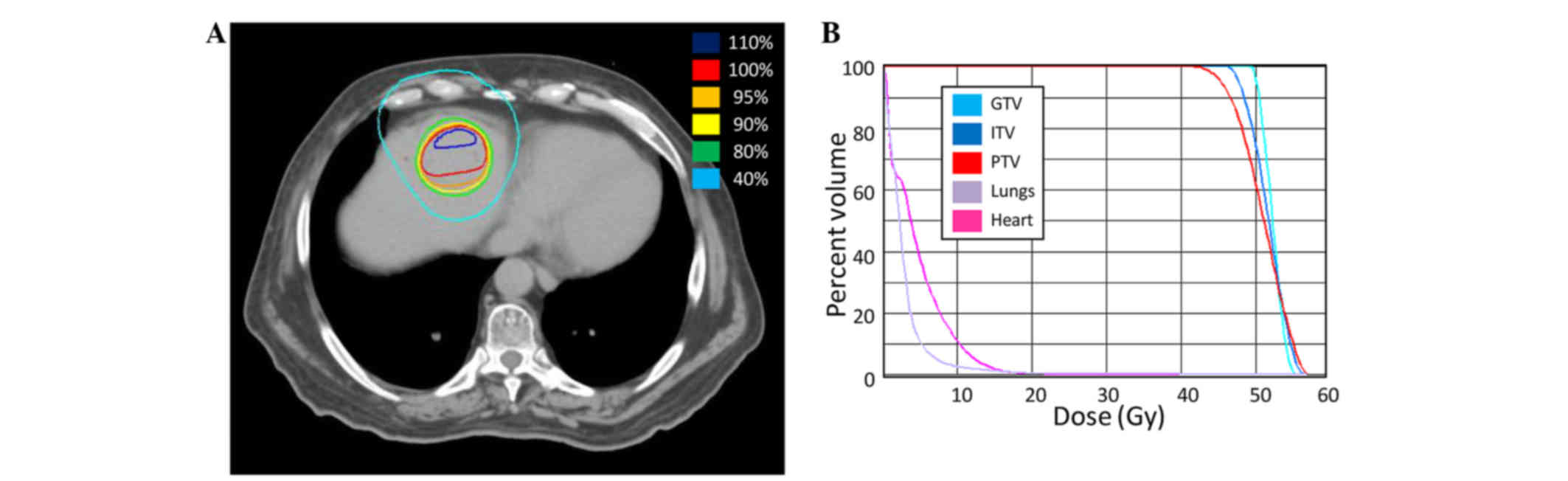
SBRT to the oligoprogressive liver metastases was
delivered at a total dose of 50 Gy over 5 fractions of 10 Gy
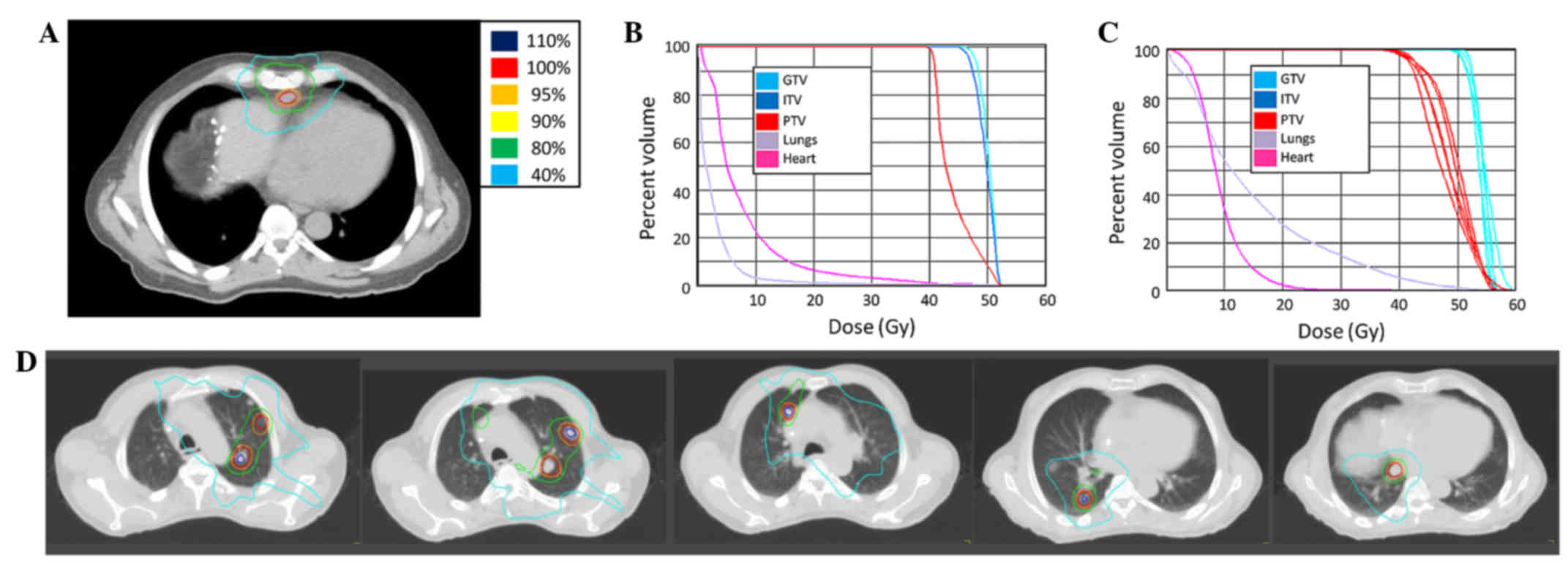
(Fig. 1). During radiation treatment,
chemotherapy was withheld.
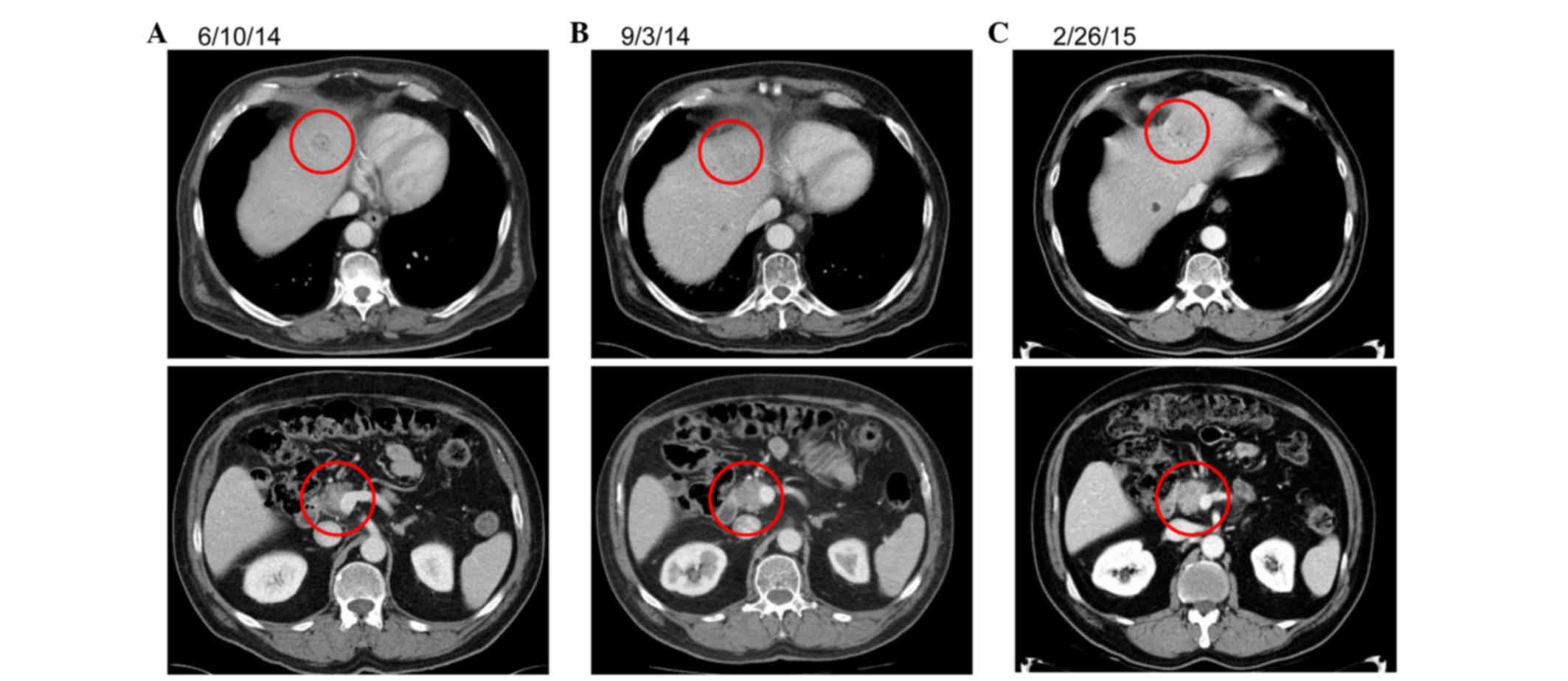
Following SBRT, the patient requested a chemotherapy
break and has since been off of systemic therapy for 10 months,
with stable disease on serial CT scans. Representative imaging is
presented in Fig. 2.
Patient 2
The patient was a 48-year-old male at the time of
SBRT and had been diagnosed with stage III (T3N1M0) invasive
moderately-differentiated adenocarcinoma of the rectum, with an
8.7×5-cm rectal mass, 6 years prior to SBRT. The patient underwent
a diverting colostomy, 6 weeks of concurrent neoadjuvant
chemoradiation with 5-FU CIVI D1-5, which was delivered at an
external institution, and then a resection that confirmed locally
invasive adenocarcinoma. Adjuvant mFOLFOX6 (400 mg/m2
5-FU D1, 2,400 mg/m2 5-FU CIVI and oxaliplatin 85
mg/m2) was then administered at our center every 2 weeks
for 3 months. The patient was under active surveillance for 3 years
when imaging showed a new 17-mm right lower lobe lung nodule, a
6-mm right apical lung nodule and a 32×26-mm mesenteric lymph node
mass at the level of the aortic bifurcation. Lung biopsy confirmed
metastatic rectal adenocarcinoma. The patient was started on
FOLFIRI-bevacizumab (2,400 mg/m2 5-FU CIVI, 200
mg/m2 5-FU, 180 mg/m2 irinotecan and 5 mg/kg
bevacizumab). Irinotecan was discontinued after 2 years due to a
parastomal abscess and thrombocytopenia. This regimen was continued
until the patient was found to have oligoprogression in the lungs
with a progressing single lung metastasis measuring 21×19 mm, and
multiple other <10 mm metastases throughout each lung, which
were stable.
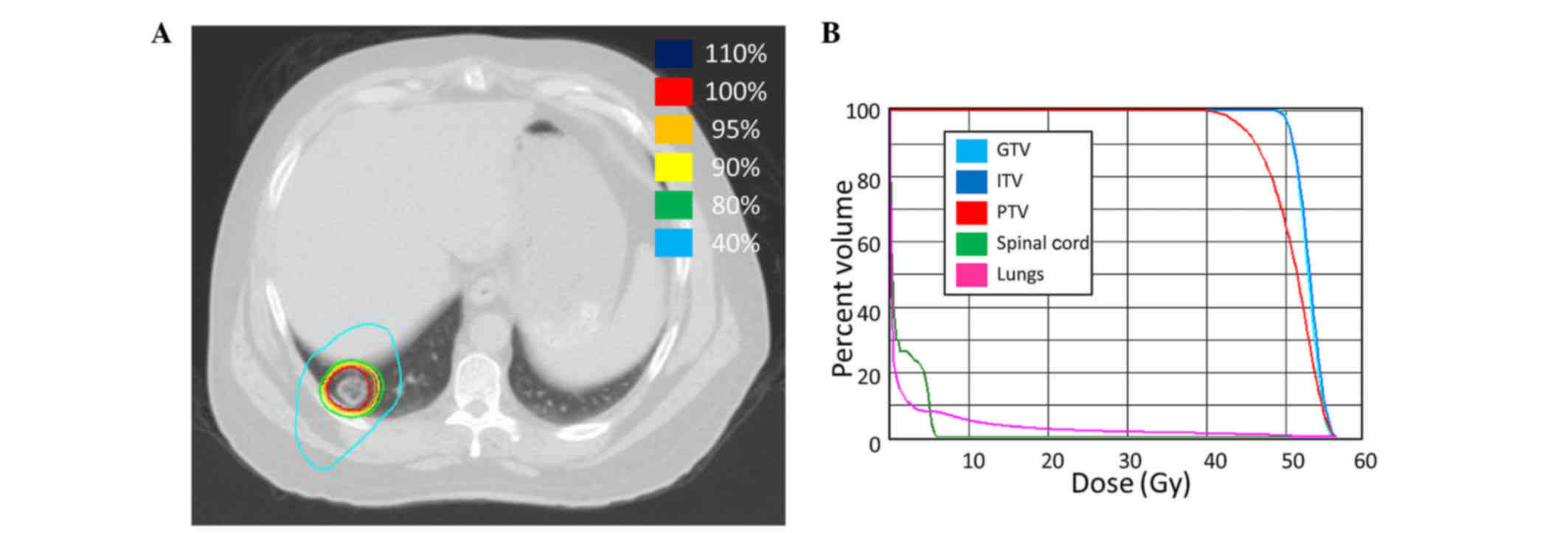
SBRT to the large lung lesion was delivered 6 years
after diagnosis at a total dose of 50 Gy in 5 fractions of 10 Gy
(Fig. 3). Chemotherapy was held
during SBRT, and then 5-FU/bevacizumab was resumed. The patient
continues to otherwise have stable disease 10 months after SBRT
with no change in systemic chemotherapy.
Patient 3
The patient was a 47-year-old female who was
initially diagnosed with metastatic adenocarcinoma of the colon,
with biopsy-proven liver metastases and image-diagnosed bilateral
lung involvement, 15 months prior to the first course of SBRT. A
left hemicolectomy was performed with 3/15 positive lymph nodes and
KRAS proto-oncogene, GTPase mutation. The patient was started on
chemotherapy with mFOLFOX6 (similar to patient 1) and 5 mg/kg
bevacizumab. Restaging imaging after 10 months showed a progressive
liver metastasis. Systemic chemotherapy was changed to FOLFIRI (135
mg/m2 irinotecan, 400 mg/m2 5-FU IV bolus and
2,400 mg/m2 5-FU CIVI) with 4 mg/kg ziv-aflibercept
every 3 weeks for 3 cycles. Follow-up imaging showed disease
progression in the right hilum.
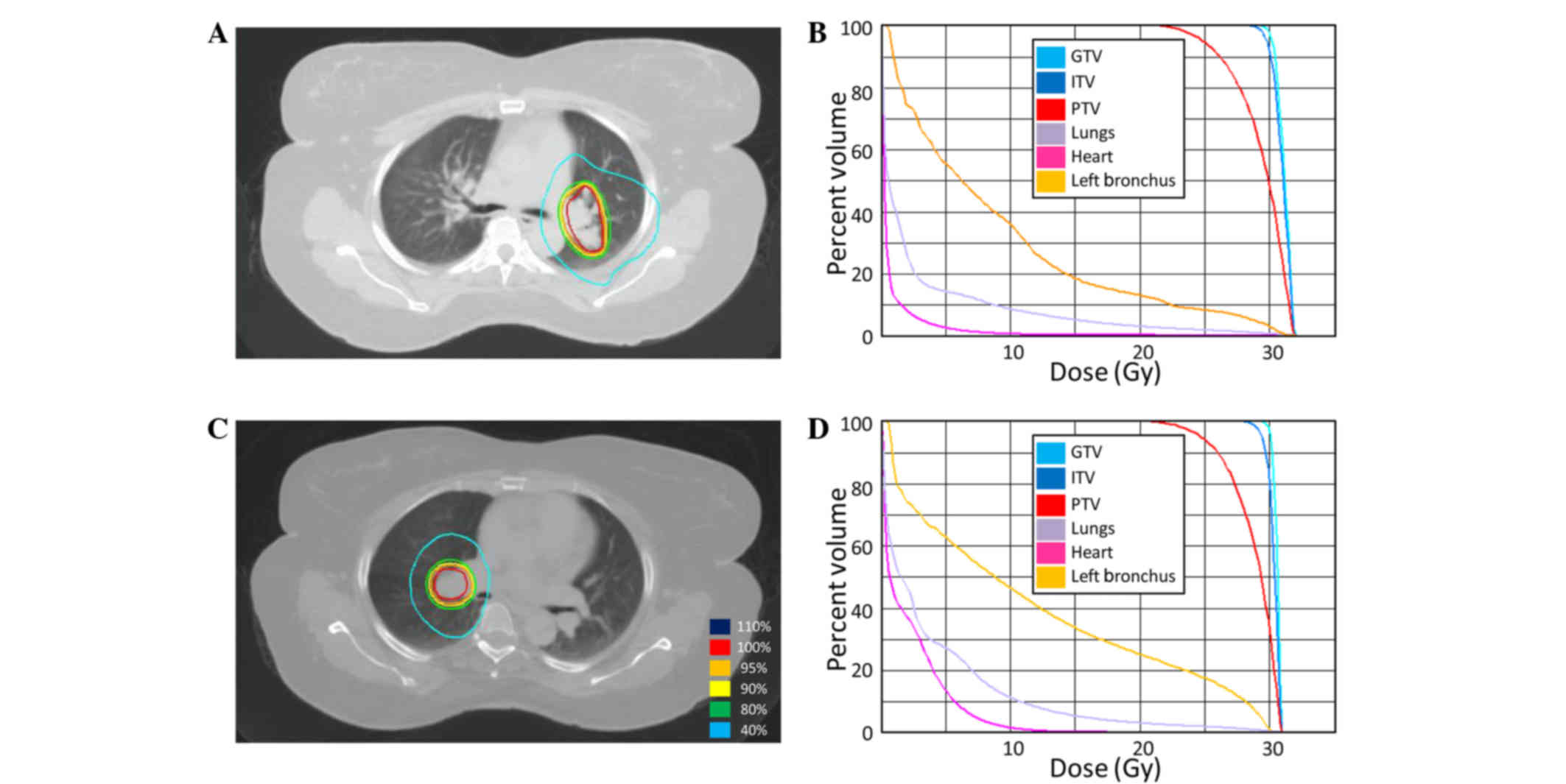
SBRT was delivered to the lung metastasis in the
right hilum consisting of a total dose of 30 Gy in 5 fractions of 6
Gy (Fig. 4). A repeat positron
emission tomography (PET)/CT scan 1 month after SBRT showed mild
progression of the multiple liver and lung metastases, and the
patient was restarted on FOLFIRI/ziv-aflibercept. The disease
responded well with the carcinoembryonic antigen level decreasing
from 450 to 75 ng/ml. The patient was maintained on this regimen
with surveillance for 1 year when repeat imaging showed progression
in a single pulmonary nodule. The case was discussed at the GI TB
and the recommendation was to continue the same systemic
chemotherapy regimen and pursue SBRT to the left hilum.
SBRT was delivered to the left lung hilum metastasis
at 13 months after the first course using a total of 30 Gy in 5
fractions of 6 Gy (Fig. 4). Following
SBRT, the current chemotherapy regimen was re-initiated.
At 16 months after the first course and 3 months
after the second course of SBRT, the patient was enrolled in a
clinical trial due to disease progression. The systemic disease
continues to progress despite additional changes to the treatment
plan.
Patient 4
The patient was a 60-year-old male diagnosed with
stage I (T2N0M0) adenocarcinoma of the rectum 5 years prior to
SBRT. The patient was treated initially with 825 mg/m2
neoadjuvant capecitabine twice daily and concurrent radiation
therapy to 50.4 Gy in 1.8-Gy fractions. A transanal resection was
performed and the pathological findings were consistent with
adenocarcinoma. Surveillance was continued for 1 year when 4 liver
metastases were found. Chemotherapy with mFOLFOX6-bevacizumab (400
mg/m2 5-FU D1, 2,400 mg/m2 5-FU CIVI, 85
mg/m2 oxaliplatin and 5 mg/kg bevacizumab) was initiated
with a good response on follow-up imaging. After 11 months, a right
partial hepatectomy and left wedge resection were performed for
isolated recurrence, which was followed by a 14-month disease-free
period on surveillance imaging.
The patient was then found to have a new liver
metastasis, which was also resected. Surveillance was continued for
8 months when PET/CT showed a thickened rectal wall and enlarged
perirectal lymph node, as well as a pericardial lymph node that had
increased in size since a prior exam. His case was presented at the
GI TB and the consensus was for mediastinal biopsy and endoscopic
rectal ultrasound for the recurrent disease at the primary site.
Following a mediastinal biopsy confirming adenocarcinoma, the
patient was started on systemic chemotherapy with 200
mg/m2 oral capecitabine administered twice daily, plus
7.5 mg/kg intravenous bevacizumab every day for 21 days. The
patient was maintained on this regimen with stable disease on
follow-up imaging for 11 months when CT imaging indicated
progression of the anterior mediastinal nodal disease.
SBRT was delivered at a total dose of 50 Gy in 10
fractions of 5 Gy (Fig. 5). Systemic
chemotherapy was continued with continued control of the disease
for 11 months. Several lung nodules then developed, each <1 cm
on CT imaging. SBRT to 5 progressive lung metastases was performed
to a total of 50 Gy in 10 fractions of 5 Gy (Fig. 5). Following the second round of SBRT,
the patient was continued on chemotherapy with serial imaging for
15 months. CT imaging indicated worsening pulmonary metastases with
a large right pleural effusion and progression of the anterior
mediastinal nodal metastasis. The systemic chemotherapy was changed
18 months after the first round of SBRT to single-agent panitumumab
(6 mg/kg every 2 weeks), with irinotecan (180 mg/m2)
subsequently added due to continued progression. The patient was
subjected to other treatments, and succumbed to progressive
systemic disease 2 years and 2 months from the first SBRT.
Patient 5
The patient was a 68-year-old male who was
originally diagnosed with a stage IIIC (pT3N2bM0) proximal
transverse colon adenocarcinoma 3 years prior to SBRT. Staging
imaging was notable for subcentimeter pulmonary nodules, but no
definitive evidence of metastatic disease. The patient underwent a
laparoscopic right colectomy followed by adjuvant systemic
chemotherapy with standard-dose mFOLFOX-6 (85 mg/m2
oxaliplatin, 400 mg/m2 leucovorin, 400 mg/m2
5-FU bolus and 2,400 mg/m2 5-FU over 46 h) repeated
every 2 weeks. Although several dose reductions were required,
there was no evidence of disease progression on follow-up imaging.
After a year, surveillance imaging demonstrated two enlarging
mesenteric soft-tissue nodules. Chemotherapy was changed to
FOLFIRI-bevacizumab (280 mg/m2 5-FU, 2,400
mg/m2 5-FU, 180 mg/m2 irinotecan and 5 mg/kg
bevacizumab), with a 50% dose reduction of the 5-FU bolus. The
patient had two breaks from chemotherapy due to non-cancer-related
disease requiring vascular surgery and subsequent complications.
After 2 years, repeat imaging revealed interval increase in size of
a soft tissue mass in the mesentery with stable lung metastases,
and FOLFIRI with bevacizumab was restarted without a 5-FU bolus
(180 mg/m2 irinotecan, 2,400 mg/m2 5-FU CIVI
and 5 mg/kg bevacizumab) with interval response consisting of
stable imaging. After 4 months, surveillance imaging showed a new
left adrenal nodule, which was discussed during GI TB. The
consensus recommendation was for short-interval surveillance.
Follow-up imaging 3 months later revealed a slight increase in the
left adrenal nodule.
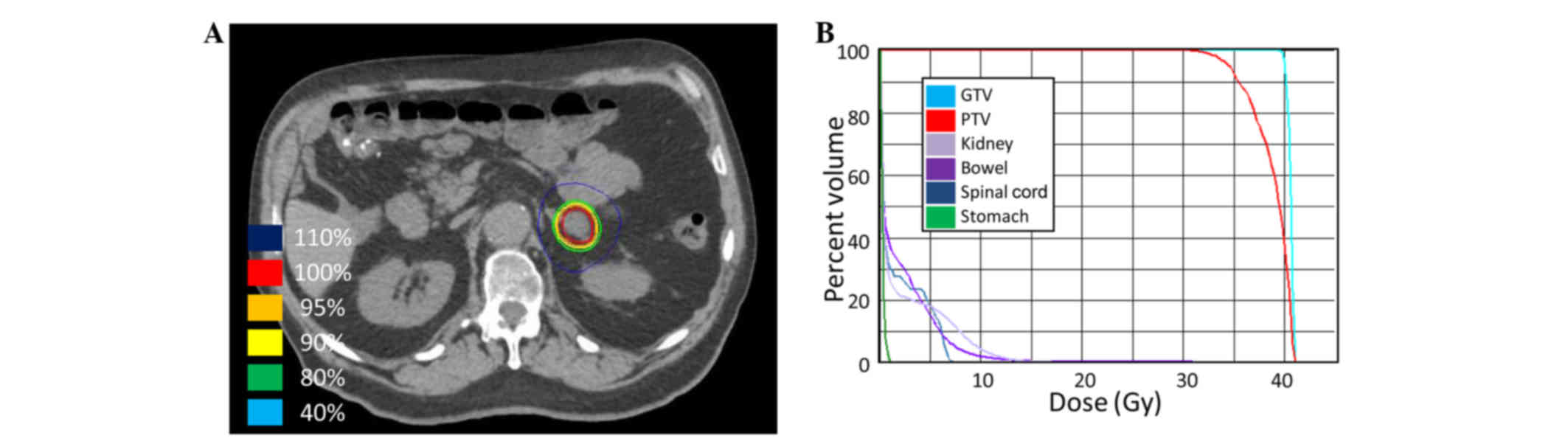
SBRT was delivered at a total of 40 Gy in 5
fractions of 8 Gy (Fig. 6) to the
left adrenal metastasis. Chemotherapy was continued during SBRT.
Ziv-alfibercept (4 mg/kg) replaced bevacizumab with FOLFIRI 4
months after completing the SBRT due to systemic disease
progression outside of the radiation field.
Discussion
The present study describes the cases of 5 patients
with metastatic cancer treated with SBRT for oligoprogressive
disease with the goal of delaying the requirement to transition to
subsequent lines of chemotherapy. This goal was achieved in 4 out
of 5 patients, each of whom was able to remain on pre-SBRT systemic
therapy beyond 7 months without progression warranting a change in
chemotherapy. SBRT was well-tolerated and there were no grade 3 or
higher toxicities from SBRT, enabling continuation of the same
systemic chemotherapy with sustained quality of life. Ongoing
disease control is being performed with the same systemic regimen
in 2 of the patients, and 1 patient has remained off chemotherapy
for 8 months. Additionally, 2 of the patients developed disease
progression after 16 and 18 months, 1 of whom has since succumbed.
Furthermore, 1 patient was found to have widespread disease
progression at the 3-month follow-up imaging.
Biologically, this approach appears to be intuitive
since treatment resistance results from cancer adaptations and
mutations, which induce subsequent clonal selection (10). However, systemic treatment changes to
account for local clonal evolution creates new challenges. Systemic
therapeutic options are finite and further mutations accumulate
after each line of therapy, as new selection forces are applied
even to clones that previously were static and responding to
previous treatment. By eradicating resistant, oligoprogressive
sub-clones, prematurely discontinuing otherwise effective systemic
therapy may be avoided. Significant clinical benefit may be
obtained if the duration of each of line of systemic therapy is
extended prior to changing to a new regimen. Historically, surgical
debulking has been the only way to provide durable disease control
for oligometastatic disease, but for a number of patients this
carries some morbidity and requires recovery time off of systemic
therapy, which allows progression of otherwise stable metastatic
disease. Additionally, the surgical recovery of hepatic
metastasectomy may stimulate otherwise stable growth factors
potentially accelerating disease progression. In the modern era,
SBRT has become a novel approach to achieve non-invasive debulking
or ablation and has been reported in patients treated with targeted
therapy to control their widely metastatic disease (1,11–13).
In a retrospective series (sequential publications
including the same patients), Gan et al (11) and Weickhardt et al (1) described the cases of 33 patients
administered crizotinib for metastatic non-small cell lung cancer
with anaplastic lymphoma kinase-positive tumors. In total, 14
patients exhibited extracranial progression in 4 or fewer tumor
sites that were treated with SBRT, yielding excellent local control
rates and an increased time to widespread progression. Those
patients who were able to continue taking crizotinib for over 12
months experienced significantly higher overall survival times.
Straka et al (13) described the case of a patient with
metastatic renal cell carcinoma who developed a solitary area of
progression in an adrenal metastasis whilst being administered
sunitinib. Rather than switching to a different systemic therapy,
SBRT was used to treat the progressing tumor. Sunitinib was then be
administered for another 8 months prior to the occurrence of more
widespread progression. A Canadian, multi-institutional,
single-arm, prospective, phase 2 trial (OZM-053; clinicaltrials.gov identifier NCT02019576) is
currently being conducted to study the use of SBRT in metastatic
renal cell carcinoma patients who develop oligoprogression whilst
being administered first-line sunitinib therapy. SBRT is being used
to treat ≤5 progressing tumors, with ≤3 progressing soft-tissue
metastases.
Finally, Iyengar et al (12) recently published a prospective phase
II trial employing SBRT in patients on second-line erlotinib to
debulk progressive metastatic disease. This study is subtly
different compared with the others in that every site of metastatic
disease was treated with SBRT prior to initiation of erlotinib
after the patients failed first-line chemotherapy. Nonetheless, an
increase in progression-free (14.7 months) and overall (20.4
months) survival times was shown compared with historical
benchmarks.
All these studies employed targeted biologically
active tyrosine kinase inhibitors, but to the best of our
knowledge, no previously published study has indicated that this
approach would be beneficial in patients on systemic chemotherapy.
Iyengar et al (12) used SBRT
following systemic treatment failure, but did not continue the
current regimen; they instead initiated a targeted agent once
debulking was accomplished.
There are several limitations of the present series,
including the small patient population treated in this manner and
the lack of a comparison group. Despite these limitations, this
study provides proof of principle and clinical rationale for
considering the thoughtful use of SBRT for treatment of
oligoprogressive disease in select situations where continuation of
an otherwise effective systemic chemotherapy is reasonable.
One of the greatest strengths of the present study
is the novel approach to evaluating the efficacy of the treatment
by measuring the time from the treatment with SBRT to the change in
chemotherapy. This is an innovative strategy to evaluate
progressive metastatic disease treatment efficacy and we advocate
for systemic change in this arena. Future studies will benefit from
this approach, as patients in the metastatic setting often
experience short overall survival times and the intent of the
treatment is to extend quality of life, which may be difficult to
prove significant, since it is difficult to objectively measure.
Furthermore, once patients enroll in a hospice and decide to
discontinue therapy, the endpoint of overall survival is no longer
relevant. Other endpoints such as health care resource utilization,
pharmacoeconomic analyses and patient-reported quality of life
should be considered as correlative endpoints in future
studies.
To summarize, the current study presents the cases
of 5 patients who achieved an extended, high quality of life
following SBRT delivered to increase the duration of effective
systemic chemotherapy, with no significant toxicity. We believe
that this strategy will improve the duration of effective
chemotherapy and allow for increased, high quality longevity in a
select group of patients with oligoprogression.
References
|
1
|
Weickhardt AJ, Scheier B, Burke JM, Gan G,
Lu X, Bunn PA Jr, Aisner DL, Gaspar LE, Kavanagh BD, Doebele RC and
Camidge DR: Local ablative therapy of oligoprogressive disease
prolongs disease control by tyrosine kinase inhibitors in
oncogene-addicted non-small-cell lung cancer. J Thorac Oncol.
7:1807–1814. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gerlinger M, Rowan AJ, Horswell S, Larkin
J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A,
Tarpey P, et al: Intratumor heterogeneity and branched evolution
revealed by multiregion sequencing. N Engl J Med. 366:883–892.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ding L, Ellis MJ, Li S, Larson DE, Chen K,
Wallis JW, Harris CC, McLellan MD, Fulton RS, Fulton LL, et al:
Genome remodelling in a basal-like breast cancer metastasis and
xenograft. Nature. 464:999–1005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yachida S, Jones S, Bozic I, Antal T,
Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al:
Distant metastasis occurs late during the genetic evolution of
pancreatic cancer. Nature. 467:1114–1117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilson TR, Longley DB and Johnston PG:
Chemoresistance in solid tumours. Ann Oncol. 17:(Suppl 10).
x315–x324. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Milano MT, Katz AW, Zhang H and Okunieff
P: Oligometastases treated with stereotactic body radiotherapy:
Long-term follow-up of prospective study. Int J Radiat Oncol Biol
Phys. 83:878–886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeda A, Sanuki N, Tsurugai Y, Oku Y and
Aoki Y: Stereotactic body radiotherapy for patients with
oligometastases from colorectal cancer: Risk-adapted dose
prescription with a maximum dose of 83–100 Gy in five fractions. J
Radiat Res. March 16–2016.(Epub ahead of print). View Article : Google Scholar
|
|
8
|
Filippi AR, Guerrera F, Badellino S,
Ceccarelli M, Castiglione A, Guarneri A, Spadi R, Racca P, Ciccone
G, Ricardi U and Ruffini E: Exploratory analysis on overall
survival after either surgery or stereotactic radiotherapy for lung
oligometastases from colorectal cancer. Clin Oncol (R Coll Radiol).
28:505–512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: American Joint Committee on CancerAJCC
Cancer Staging Manual. 8th. Springer; New York, NY: 2010
|
|
10
|
Talmadge JE: Clonal selection of
metastasis within the life history of a tumor. Cancer Res.
67:11471–11475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gan GN, Weickhardt AJ, Scheier B, Doebele
RC, Gaspar LE, Kavanagh BD and Camidge DR: Stereotactic radiation
therapy can safely and durably control sites of extra-central
nervous system oligoprogressive disease in anaplastic lymphoma
kinase-positive lung cancer patients receiving crizotinib. Int J
Radiat Oncol Biol Phys. 88:892–898. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iyengar P, Kavanagh BD, Wardak Z, Smith I,
Ahn C, Gerber DE, Dowell J, Hughes R, Abdulrahman R, Camidge DR, et
al: Phase II trial of stereotactic body radiation therapy combined
with erlotinib for patients with limited but progressive metastatic
non-small-cell lung cancer. J Clin Oncol. 32:3824–3830. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Straka C, Kim DW, Timmerman RD, Pedrosa I,
Jacobs C and Brugarolas J: Ablation of a site of progression with
stereotactic body radiation therapy extends sunitinib treatment
from 14 to 22 months. J Clin Oncol. 31:e401–e403. 2013. View Article : Google Scholar : PubMed/NCBI
|