Introduction
Laryngeal carcinoma is a common malignant tumor in
otolaryngology that seriously endangers physical and mental health
(1). The incidence of laryngeal
malignant tumors is ~1–5% of all whole body malignant tumors and is
ranked third of all otolaryngology malignant tumors. Laryngeal
squamous cell carcinoma (LSCC) accounts for 95% of laryngeal
carcinoma and tends to occur in middle-aged and older males
(2). Due to the early presence of
hoarseness (3), glottic carcinoma can
be detected timely. However, it brings challenges for the diagnosis
of LSCC owing to the unapparent symptoms in the early stage of
supraglottic and subglottic carcinomas (4). Early diagnosis and treatment of LSCC are
critical in improving the 5-year survival rate and quality of life
of patients (5–7).
The Hippo pathway was originally observed to be a
key pathway that is involved in the regulation of the size of
organs of Drosophila melanogaster by regulating cell
proliferation and apoptosis (8).
Previous studies on the regulation of the development and size of
mammals (9–11) indicated that the Hippo pathway was
quite conservative. It was also demonstrated that abnormal
functions of Hippo pathway were closely associated with the
occurrence, development and prognosis of tumors (12,13), which
has become a popular subject of cancer research.
The aim of the present study was to investigate the
expression and localization of YAP in LSCC and vocal cord polyps
tissues as well as the clinical significance thereof.
Materials and methods
Sample collection
Approval for the present study was obtained from the
Human Research Ethics Committee of Lihuili Hospital, Ningbo, China.
Prior to sample collection, the patients and their family members
were informed with regard to the process of the study and the
possible risks. Tissues were collected from 128 patients with LSCC
and 10 patients with vocal cord polyps between January 2009 and
January 2013 at the Ningbo University Affiliated Lihuili Hospital.
All patients signed informed consent. Patients were examined and
diagnosed by two experienced pathologists. LSCC was classified
using the American Joint Committee on Cancer 2002 (AJCC 2012).
Primary reagents
Primary reagents included rabbit anti-human YAP
polyclonal antibody (Abcam, Cambridge, UK), rabbit anti-human p-YAP
polyclonal antibody (Abcam), Envision™ Kit (Dako, Glostrup,
Denmark), citrate antigen repair solution (Beijing Zhongshan
Jinqiao Biological Technology Co., Ltd., China), 3% hydrogen
peroxide (Dako), sheep serum (Beijing Boshide Technology Co.,
Beijing, China), a diaminobenzidine tetrahydrochloride (DAB) kit
(Dako), hematoxylin (Sigma, Shanghai, China), phosphate
buffered-saline (PBS) (Beijing Zhongshan Jinqiao Biological
Technology Co., Ltd.), xylene (Anhui Ante Biochemistry Co., Ltd.,
China), absolute ethyl alcohol (Anhui Ante Biochemistry Co., Ltd.),
hydrochloric acid (Anhui Ante Biochemistry Co., Ltd.) and neutral
quick-drying glue (Beijing Zhongshan Jinqiao Biological Technology
Co., Ltd.).
Primary instruments
Instruments used included an RM2135 paraffin slicing
machine (Leica, Munich, Germany), HI1210 water bath-slide drier
(Leica), GNP-9050 water-prevented constant temperature incubator
(Shanghai Jinghong Laboratory Equipment Co., Ltd., Shanghai,
China), BX41 optical microscope (OLYMPUS, Tokyo, Japan),
microscopic imaging system (OLYMPUS), Milli-Q Reference pure water
filter (Millipore, Billerica, MA, USA), pressure cooker (ASD
Company, Conover, NC, USA) and BCD-268H refrigerator (Haier
Company, Shandong, China).
Immunohistochemistry
Paraffin blocks of normal tissues surrounding
carcinoma and laryngeal squamous cell carcinoma tissues, which were
verified by hemotoxylin and eosin staining, were selected and cut
into 2 µm sections. The slices were mounted on cationic glass
slides for 4 h baking at 60°C, followed by dewaxing and hydration.
Antigen repair was subsequently performed. Citrate buffer (pH 6.0)
in a concentration of 1 mol/l was added into a pressure cooker.
After the pressure valve was closed, the pressure cooker was heated
using an electric stove until air gush and the heating stopped
after 2 min boiling. The glass slides were removed following
natural cooling at room temperature, rinsed using distilled water
and finally washed by PBS three times for 2 min each time. The
slides were processed by freshly prepared 3%
H2O2 for 15 min at room temperature to
inactivate endogenous peroxidase. Following three washes with PBS
(2 min each time), the slices were incubated at room temperature
following the addition of 1% protein liquid. After 10 min, the
slices were washed with PBS and antibody binding reaction was
subsequently performed. Rabbit anti-human YAP polyclonal antibody
and rabbit anti-human p-YAP polyclonal antibody (1:200, diluted
with phosphate buffer solution (pH 7.4) (ZLI-9062, Beijing
Zhongshan Jinqiao Biotech Co., Ltd., Beijing, China)) were added to
cover all the specimens. The slices were placed in a wet box for 18
h incubation at 4°C and subsequently rinsed with PBS three times
for 2 min each time. Envision™ reagent (general type) was added to
cover all the specimens, followed by 20-min incubation at 37°C and
rinsing three times with PBS (2 min each time). One or two drops of
DAB were added to each slice for coloration. After 2 min, the
staining was observed under a microscope (Olympus BX41, Olympus
Corp, Japan), followed by full washing by tap water. Following
redye with hematoxylin, the slices were fully washed with water to
repeat staining for 1 min, and then were fully washed in water.
After dehydration, the slices were mounted by neural drying rubber
and observed under a microscope.
Determination of immunohistochemical
results
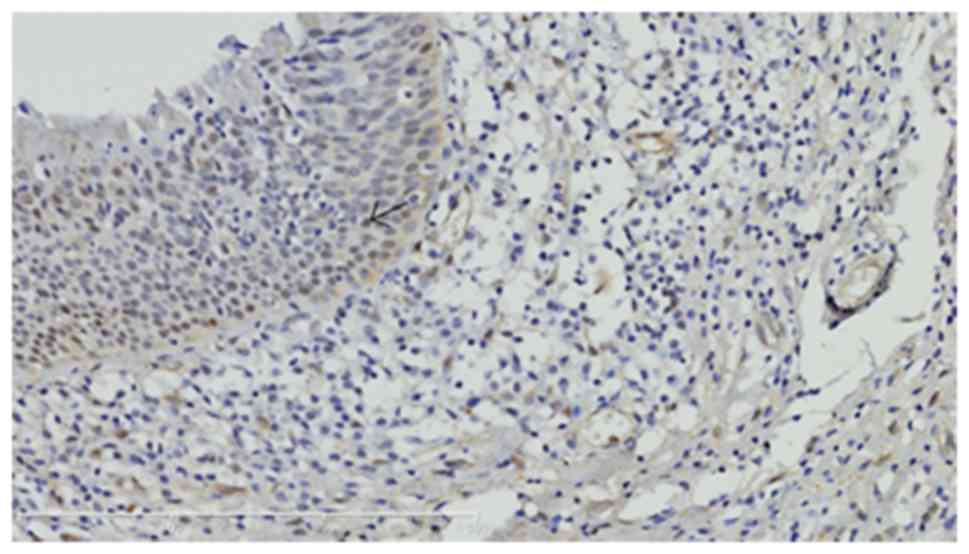
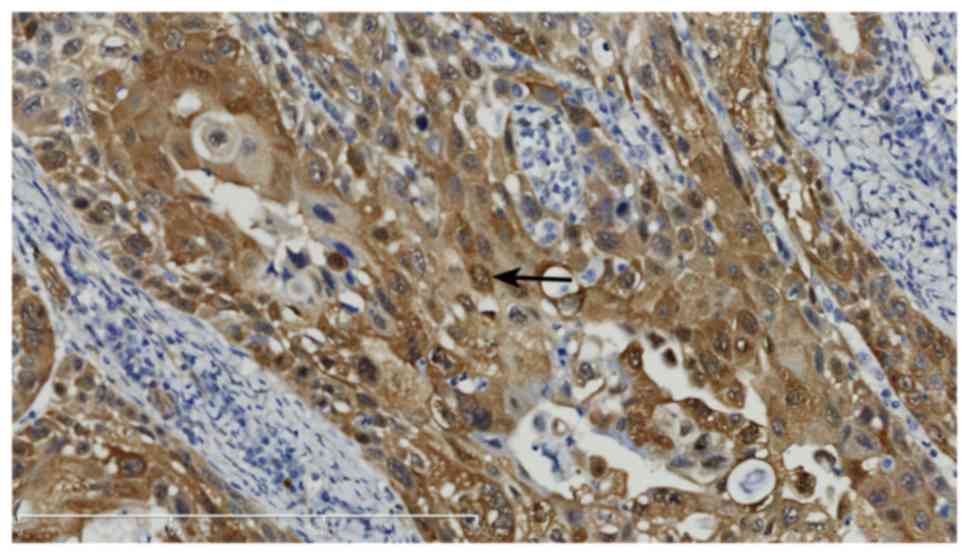
A positive expression of YAP in carcinoma tissues
presented as brown yellow or brown granules in the tumor cytoplasm
or cell membrane. The tumor solid area was selected under a ×100
lens and 5 views were selected under ×400 lenses. The result was
scored according to the staining intensity: 0, negative; 1, weak
positive; 2, moderate positive; and 3, strong positive. The number
of cells with positive staining results was graded as: ≤10%, i.e.,
0, negative; 11–30%, i.e., weak positive, for 1 point; 31–50%,
moderate positive, for 2 points; and > 50%, i.e., strong
positive, for 3 points. When the sum of these two scores was ≥3
points, the expression of YAP was determined as positive.
Statistical analysis
SPSS ver. 18.0 software (Chicago, IL, USA) was used
for statistical analysis. The correlation between a positive
expression rate of YAP and pathological stages was analyzed using
the χ2 test. Differences were considered statistically
significant when P<0.01.
Results
Expression level of YAP in LSCC and
vocal cord polyps
YAP and phosphorylated YAP (p-YAP) were expressed in
the nucleus and cytoplasm of LSCC cells, respectively, whereas YAP
was negatively expressed in vocal cord polyps (Figs. 1–5). In
addition, the intensity of YAP expression varied in LSCC with
different histological differentiation degrees and poorly
differentiated LSCC exhibited higher expression of YAP.
Correlation between YAP expression and
clinicopathological characteristics of LSCC patients
The correlation between YAP expression in LSCC and
the clinicopathological characteristics was analyzed using the
χ2 test. Table I
demonstrates that the expression of YAP was significantly
associated with tumor-node-metastasis stage (P<0.001), lymph
node metastasis (P<0.001) and pathological differentiation
(P<0.001). However, there was no correlation with age, smoking
history or gender.
 | Table I.Correlations of YAP and p-YAP with
different clinical factors. |
Table I.
Correlations of YAP and p-YAP with
different clinical factors.
|
| YAP |
| P-YAP |
|
|---|
|
|
|
|
|
|
|---|
| Group | − | + | ++ | +++ | P-value | − | + | ++ | +++ | P-value |
|---|
| Age (years) |
|
|
|
| 0.746 |
|
|
|
| 0.629 |
|
<59 | 1 | 31 | 27 | 7 |
| 3 | 29 | 24 | 10 |
|
|
>60 | 3 | 27 | 25 | 7 |
| 5 | 22 | 27 | 8 |
|
| Clinical stage |
|
|
|
|
<0.001a |
|
|
|
|
<0.001a |
| Tis | 4 | 5 | 0 | 0 |
| 7 | 2 | 0 | 0 |
|
| I | 21 | 11 | 1 | 0 |
| 1 | 22 | 9 | 1 |
|
| II | 0 | 11 | 10 | 0 |
| 0 | 11 | 10 | 0 |
|
| III | 0 | 14 | 16 | 6 |
| 0 | 10 | 19 | 7 |
|
| IV | 0 | 7 | 15 | 7 |
| 0 | 6 | 13 | 10 |
|
| Lymphatic
metastasis |
|
|
|
|
<0.001a |
|
|
|
|
<0.001a |
| Yes | 0 | 11 | 23 | 12 |
| 0 | 8 | 22 | 16 |
|
| No | 4 | 47 | 29 | 2 |
| 8 | 43 | 29 | 2 |
|
| Smoking history |
|
|
|
| 0.279 |
|
|
|
| 0.56 |
| Yes | 2 | 43 | 38 | 13 |
| 5 | 36 | 41 | 14 |
|
| No | 2 | 15 | 14 | 1 |
| 3 | 15 | 10 | 4 |
|
| Gender |
|
|
|
| 0.71 |
|
|
|
| 0.003 |
| Male | 3 | 56 | 51 | 14 |
| 6 | 50 | 50 | 18 |
|
|
Female | 1 | 2 | 1 | 0 |
| 2 | 1 | 1 | 0 |
|
| Pathological
type |
|
|
|
|
<0.001a |
|
|
|
|
<0.001a |
| Highly
differentiated | 4 | 52 | 11 | 0 |
| 8 | 45 | 14 | 0 |
|
|
Moderately differentiated | 0 | 6 | 38 | 8 |
| 0 | 6 | 34 | 12 |
|
| Lowly
differentiated | 0 | 0 | 3 | 6 |
| 0 | 0 | 3 | 6 |
|
Discussion
YAP determines the size of organs by regulating cell
proliferation and apoptosis. It is a transcriptional co-activator
that monitors cell behavior in the nucleus and is assisted by
transcription factors. The signaling pathway of the Hippo
kinase-mediated cascade is important in the phosphorylation of YAP
(14). It has been suggested that YAP
may be a tumor suppressor and an oncogene (15,16). For
example, levels of YAP mRNA in pancreatic cancer tissues were 2.5-
and 1.3-fold higher than those observed in normal pancreas tissues
and chronic pancreatitis tissues, respectively. Similar results
have been observed in gastric and liver cancer (17,18).
However, the role of YAP in the occurrence of tumors as an oncogene
has been questioned (19–21). The expression of YAP was observed to
be downregulated or even lost, YAP-deficit breast cancer cells
demonstrated stronger potentials of invasion and metastasis, and
results from in vivo studies indicated that tumors occurred
early and grew rapidly in YAP knockout nude mice (22,23).
However, the role of YAP in LSCC has yet to be elucidated.
In the present study, immunohistochemistry was used
to detect the expression level of YAP in vocal cord polyps and
laryngeal squamous cell carcinoma tissues. The results demonstrated
that YAP was overexpressed in LSCC and positively correlated with
the histological differentiation degree of tumors. The analysis of
the clinicopathological characteristics also demonstrated that YAP
expression was correlated with TNM stage, lymph node metastasis and
pathologic differentiation. These results suggest that YAP is an
independent prognostic biomarker of LSCC as an oncogene. The
results of the current study are consistent with a number of
previous studies reporting that YAP expression is linked with tumor
evolution and progression (24).
Thus, YAP is a potential treatment target for LSCC (25).
In conclusion, the present study has provided
evidence for the expression and localization of YAP in LSCC and
vocal cord polyps tissues. Additionally, YAP may be involved in the
occurrence and development of LSCC as an oncogene. However, further
studies are required to determine whether targeting YAP inhibits
the organ size of patients with tumors.
Acknowledgements
The present study was supported by grants from the
Zhejiang Provincial Natural Science Foundation of China (no.
LY14H160003), Ningbo Medical Science and Technology Plan (no.
2013A04), the Scientific Innovation Team Project of Ningbo (no.
2012B82019), Ningbo Social Developmental Key Research Project (no.
2012C5015), Ningbo Natural Science Foundation (no. 2012A610208),
Medical and Health Research Project of Zhejiang Province (no.
2012ZDA042), and Medical and Health Training Project of Zhejiang
Province (no. 2014PYA017).
References
|
1
|
Guerrier Y and Dejean Y: Cancer of larynx
and hypopharynx in women [M]UICC Manual of Clinical Oncology. 9.
John Wiley & Sons, Ltd.; pp. 542–558. 2015
|
|
2
|
Agra IM, Ferlito A, Takes RP, Silver CE,
Olsen KD, Stoeckli SJ, Strojan P, Rodrigo JP, Gonçalves Filho J,
Genden EM, et al: Diagnosis and treatment of recurrent laryngeal
cancer following initial nonsurgical therapy. Head Neck.
34:727–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saki N, Saki G, Abdoulhassan S, Rahim F,
Mostofi NE and Soheila N: Effect of smoking tobacco in the etiology
of cancer of larynx in Khuzestan. Jentashapir Journal of Health
Research. 2:1–9. 2011.
|
|
4
|
Xie ZN, Ji CY, Chen JC, Wang YN, Guan LQ,
Li HT, Zhang M and Yang JH: The influence of
pSilencer2.0-c-Met-siRNA on human cancer of larynx Hep-2 cells
growth in nude mice. J Otolaryngol Ophthalmol Shandong Univ.
23:5–7. 2009.
|
|
5
|
Tarnawski R, Skladowski K and Maciejewski
B: Prognostic value of hemoglobin concentration in radiotherapy for
cancer of supraglottic larynx. Int J Radiat Oncol Biol Phys.
38:1007–1011. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wynder EL, Bross IJ and Day E: A study of
environmental factors in cancer of the larynx. Cancer. 9:86–110.
1956. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moore I: Intrinsic cancer of the larynx
and the operation of Laryngo-Fissure, with a description of some
new instruments specially designed for improving the technique.
Multibody Syst Dyn. 31:191–240. 2013.
|
|
8
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai J, Zhang N, Zheng Y, de Wilde RF,
Maitra A and Pan D: The Hippo signaling pathway restricts the
oncogenic potential of an intestinal regeneration program. Genes
Dev. 24:2383–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramos A and Camargo FD: The Hippo
signaling pathway and stem cell biology. Trends Cell Biol.
22:339–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mo JS, Park HW and Guan KL: The Hippo
signaling pathway in stem cell biology and cancer. EMBO Rep.
15:642–656. 2014.PubMed/NCBI
|
|
12
|
Yue T, Tian A and Jiang J: The cell
adhesion molecule echinoid functions as a tumor suppressor and
upstream regulator of the Hippo signaling pathway. Dev Cell.
22:255–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwon Y, Vinayagam A, Sun X, Dephoure N,
Gygi SP, Hong P and Perrimon N: The Hippo signaling pathway
interactome. Science. 342:737–740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao PF, Cui PC, YanYan R, et al: Early
vocal cords cancer and precancerosis of larynx treated with Nd: YAP
laser under the laryngoscope and rigid endoscope. J Mod Oncol.
1:191–195. 2009.
|
|
15
|
Li D, You HH, Jia YJ, Guo JD and Du HL:
Association of C722T polymorphism in XRCC3 gene with larynx cancer:
A meta-analysis. Tumor Biol. 35:5427–5430. 2014. View Article : Google Scholar
|
|
16
|
Kim M, Kim M, Lee S, Kuninaka S, Saya H,
Lee H, Lee S and Lim DS: cAMP/PKA signalling reinforces the
LATS-YAP pathway to fully suppress YAP in response to actin
cytoskeletal changes. EMBO J. 32:1543–1555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang S, Li H, Wang G, Zhang T, Fu B, Ma M,
Quan Z and Chen G: Yes-associated protein (YAP) expression is
involved in epithelial-mesenchymal transition in hepatocellular
carcinoma. Clin Transl Oncol. 18:172–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu X, Xin Y, Xiao Y and Zhao J:
Overexpression of YAP1 is correlated with progression, metastasis
and poor prognosis in patients with gastric carcinoma. Pathol Oncol
Res. 20:805–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chan SW, Lim CJ, Chong YF, Pobbati AV,
Huang C and Hong W: Hippo pathway-independent restriction of TAZ
and YAP by angiomotin. J Biol Chem. 286:7018–7026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gilgenkrantz H: The world according to
YAP: A continuous cross-talk between Wnt and Hippo pathways. Med
Sci (Paris). 29:868–874. 2013.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma L: Abstract BS2-2: Targeting the
LIFR-Hippo-YAP pathway as an anti-metastatic strategy. Cancer Res.
75:BS2-2. 2015. View Article : Google Scholar
|
|
22
|
Chen D, Sun Y, Wei Y, Zhang P, Rezaeian
AH, TeruyaFeldstein J, Gupta S, Liang H, Lin HK, Hung MC and Ma L:
LIFR is a breast cancer metastasis suppressor upstream of the
Hippo-YAP pathway and a prognostic marker. Nat Med. 18:1511–1517.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hergovich A: YAP-Hippo signalling
downstream of leukemia inhibitory factor receptor: Implications for
breast cancer. Breast Cancer Res. 14:3262012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li HX, He N and Zheng H: Research progress
on correlation between YAP and tumor stem cells. Chin J Clin Oncol.
728–731. 2015.
|
|
25
|
Bohl CR, Harihar S, Denning WL, Sharma R
and Welch DR: Metastasis suppressors in breast cancers: Mechanistic
insights and clinical potential. J Mol Med (Berl). 92:13–30. 2014.
View Article : Google Scholar : PubMed/NCBI
|